10 Transdermal Drug Delivery Systems Contents I III





























































- Slides: 73

10. Transdermal Drug Delivery Systems 经皮给药系统

Contents I. III. IV. V. VI. VII. Factors affecting percutaneous absorption Percutaneous absorption enhancer Design features of transdermal drug delivery system Percutaneous absorption model Advantages and disadvantages of TDDSs Examples of transdermal drug deliver systems General clinical considerations in the use of TDDSs

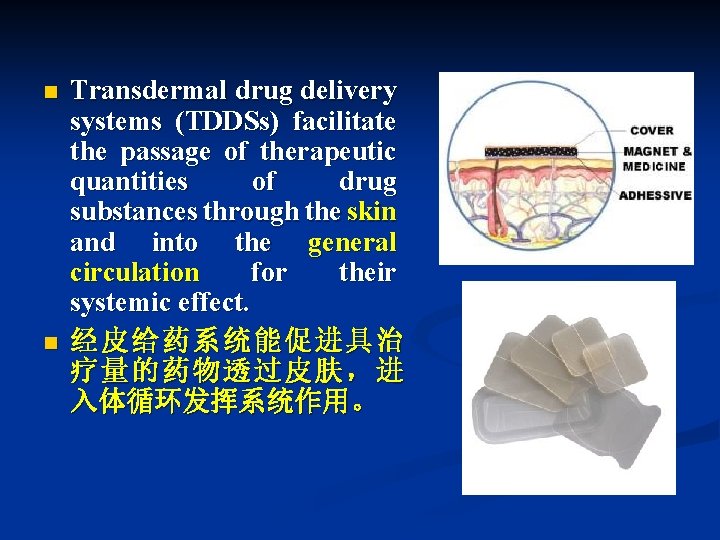
n n Transdermal drug delivery systems (TDDSs) facilitate the passage of therapeutic quantities of drug substances through the skin and into the general circulation for their systemic effect. 经皮给药系统能促进具治 疗量的药物透过皮肤,进 入体循环发挥系统作用。

n For transdermal drug delivery, it is considered ideal if the drug penetrates through the skin to the underlying blood supply without drug buildup in the dermal layers. n 理想的经皮给药系统时,药物 渗透入皮肤后,能够进入血液 而不在皮下蓄积。 (3 M Transdermal Drug Delivery http: //www. 3 M. com/DDS)




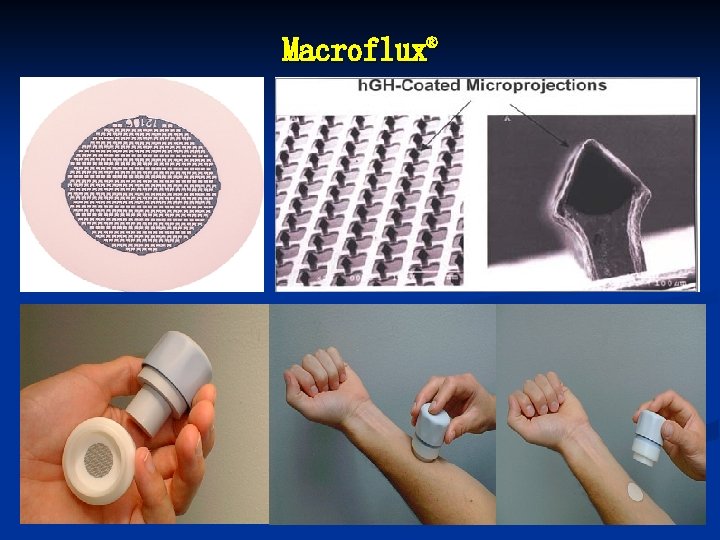
Macroflux®

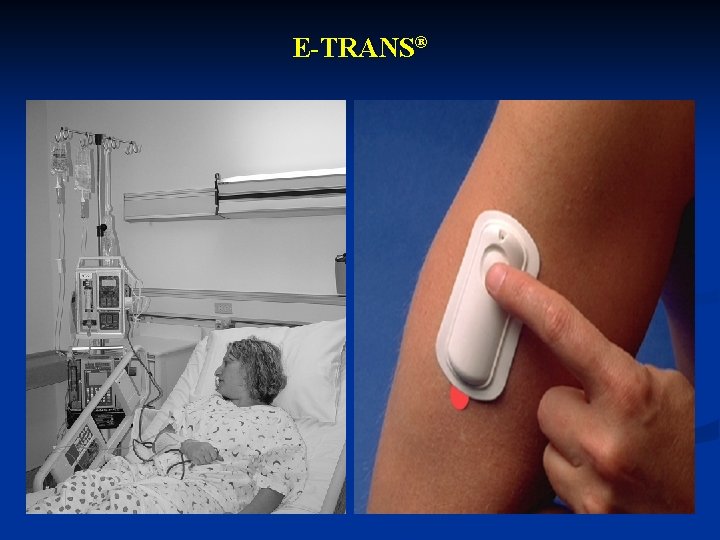
E-TRANS®

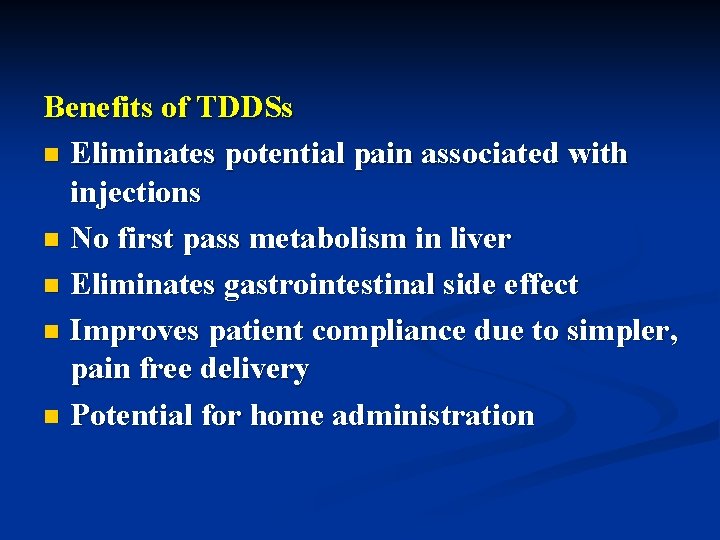
Benefits of TDDSs n Eliminates potential pain associated with injections n No first pass metabolism in liver n Eliminates gastrointestinal side effect n Improves patient compliance due to simpler, pain free delivery n Potential for home administration

I. Factors affecting percutaneous absorption 1. Drug concentration is an important factor. (药物浓度是一个重要因素。) 2. The larger the area of application, the more drug is absorbed. (当药物应用面积增大,经皮吸收的药物量 增加。)

3. The aqueous solubility of a drug determines the concentration presented to the absorption site, and the partition coefficient influences the rate of transport across the absorption site. 药物的水溶性决定了吸收部位的浓度,分配系 数影响吸收部位的药物转运速率。 Drugs generally penetrate the skin better in their un-ionized form. Nonpolar drugs tend to across the cell barrier through the lipid-rich regions, whereas the polar drugs favor transport between cells. 非解离型的药物透皮效果好。非极性药物易通 过富含脂质的部位跨越细胞屏障,而极性药物 则通过细胞转运。

4. Drugs with molecular weights of 100 to 800 and adequate lipid and aqueous solubility can permeate skin. The ideal molecular weight of a drug for transdermal drug delivery is believed to be 400 or less. 分子量在 100和800之间并且具有一定的脂 溶性和水溶性的药物能渗透入皮肤。理想 的经皮吸收系统的药物分子量应为 400或 更小。

5. Hydration of the skin generally favors percutaneous absorption. The TDDS acts as an occlusive moisture barrier through which sweat cannot pass, increasing skin hydration. 皮肤的水和作用通常有利于经皮吸收。TDDS 可以作为隔绝湿气的屏障(汗水不能通过), 使皮肤水合化程度增加。

6. Percutaneous absorption appears to be greater when the TDDS is applied to a site with a thin horny layer than with a thick one. TDDS在角质层薄的部位比角质层厚的部位经 皮吸收好。 7. Generally, the longer the medicated application is permitted to remain in contact with the skin, the greater is the total drug absorption. 一般而言,药物应用的时间越长,即与皮肤 接触时间越长,药物吸收总量越多。

II. Percutaneous absorption enhancers There is great interest among pharmaceutical scientists to develop chemical permeation enhancers and physical methods that can increase percutaneous absorption of therapeutic agents.

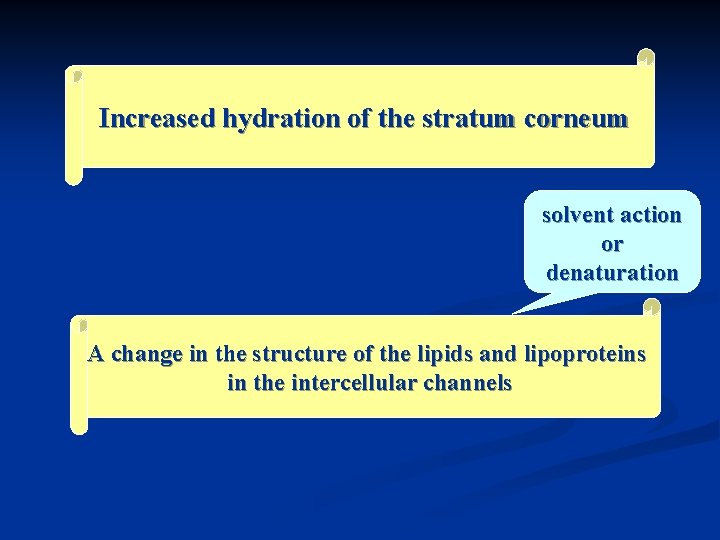
1. Chemical enhancers A chemical skin penetration enhancer increases skin permeability by reversibly damaging or altering the physicochemical nature of the stratum corneum to reduce its diffusional resistance. 化学吸收促进剂通过通过可逆地改变角质 层的理化状态,降低扩散阻力而提高皮肤 渗透率。

Increased hydration of the stratum corneum solvent action or denaturation A change in the structure of the lipids and lipoproteins in the intercellular channels

C C C C More than 275 chemical compounds have been cited in the literature as skin penetration enhancers; they include acetone, azone (氮酮), dimethyl acetamide (二甲基乙酰胺), dimethyl formamide(二甲基甲酰胺), dimethyl sulfoxide (DMSO), 二甲基亚砜 ethanol, oleic acid, 油酸 polyethylene glycol, propylene glycol (丙二醇), sodium lauryl sulfate (月桂醇硫酸钠).

The selection of a permeation enhancer should be based on n its efficacy in enhancing skin permeation n its dermal toxicity n its physicochemical and biologic compatibility with the system’s other components.

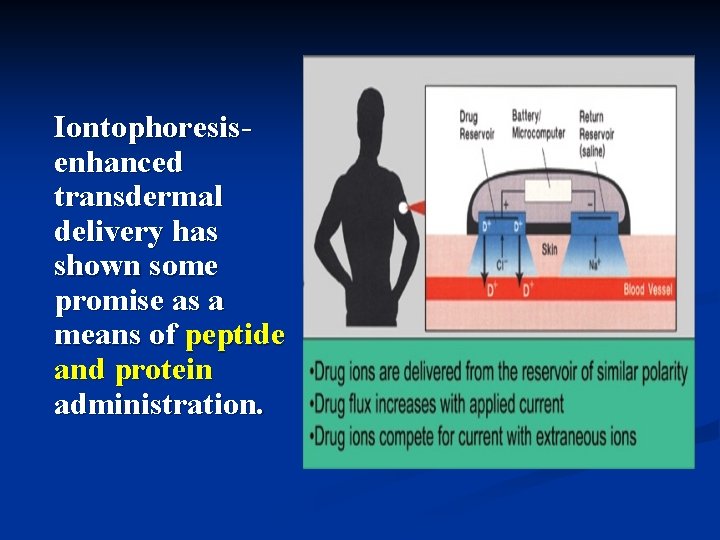
2. Iontophoresis and sonophoresis Iontophoresis is delivery of a charged chemical compound across the skin membrane using an electrical field. (离子导入法是指在电场 作用下,带电荷的化合 物导入皮肤粘膜的一种 方法。)

Iontophoresisenhanced transdermal delivery has shown some promise as a means of peptide and protein administration.

A number of drugs have been the subject of iontophoretic studies, they include n lidocaine n dexamethasone n amino acids, n peptides n insulin n Verapamil (维拉帕米) n Propranolol (普萘洛尔)

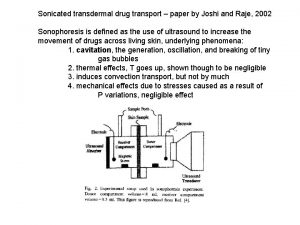
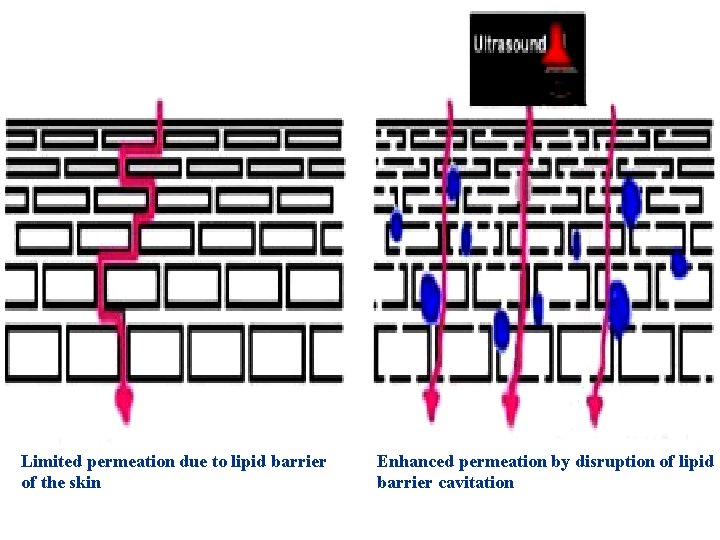
Sonophoresis, is a process that exponentially increases the absorption of topical compounds (transdermal delivery) with high-frequency ultrasound. n Sonophoresis occurs because ultrasound waves stimulate micro-vibrations within the skin epidermis and increase the overall kinetic energy of molecules making up topical agents. n

Limited permeation due to lipid barrier of the skin Enhanced permeation by disruption of lipid barrier cavitation

It is thought that high-frequency ultrasound can influence the integrity of the stratum corneum and thus affect its penetrability. Among the agents examined are n hydrocortisone, n lidocaine, n salicylic acid in such formulations as gels, creams, and lotions. n

III. Design features of transdermal drug delivery systems TDDSs are designed to support the passage of drug substances from the surface of the skin through its various layers and into the systemic circulation. (将药物设计成经皮给药系统能使药物透过 皮肤表面、穿过皮肤的不同层进入体循环)。

Transdermal drug delivery systems may be constructed of a number of layers, including 1) an occlusive backing membrane to protect the system from environmental entry and from loss of drug from the system or moisture from the skin; 2) the drug at the skin-site; 3) a release liner, which is removed before application and enables drug release; 4) an adhesive layer to maintain contact with the skin after application.

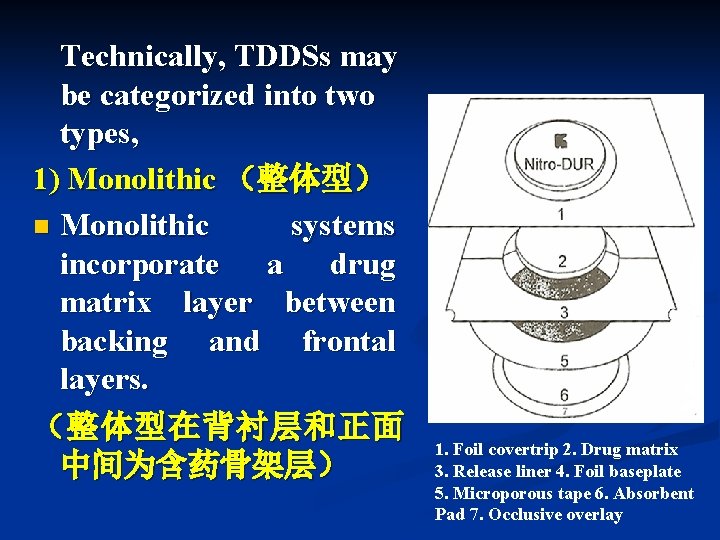
Technically, TDDSs may be categorized into two types, 1) Monolithic (整体型) n Monolithic systems incorporate a drug matrix layer between backing and frontal layers. (整体型在背衬层和正面 中间为含药骨架层) 1. Foil covertrip 2. Drug matrix 3. Release liner 4. Foil baseplate 5. Microporous tape 6. Absorbent Pad 7. Occlusive overlay

The drug-matrix layer is composed of a polymeric material in which the drug is dispersed. (药物-骨架层由分散有药物的聚合物组成) n The polymer matrix controls the rate at which the drug is released for percutaneous absorption. (聚合物骨架控制经皮吸收药物的释放速率) n

In the preparation of monolithic systems, the drug and the polymer are dissolved or blended together, cast as the matrix, and dried. (在制备整体型时,药物和聚合物一起溶解或 混合,作为骨架并干燥)。 n

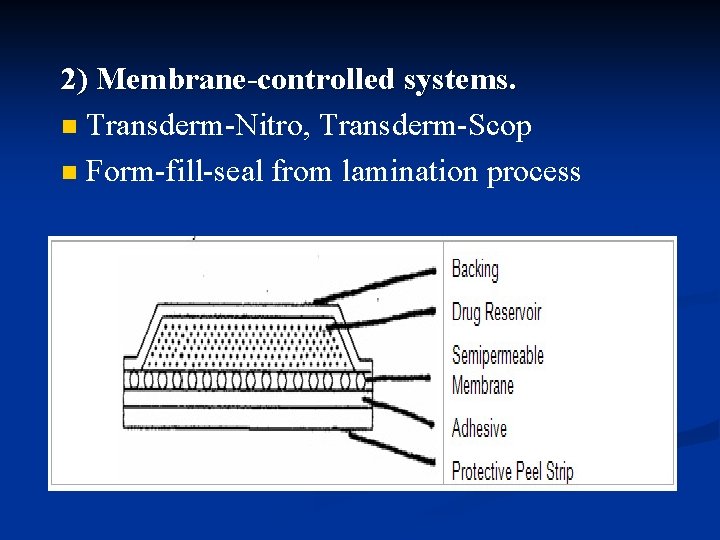
2) Membrane-controlled systems. n Transderm-Nitro, Transderm-Scop n Form-fill-seal from lamination process

Membrane-controlled transdermal systems are designed to contain a drug reservoir, or pouch, usually in liquid or gel form, a ratecontrolling membrane, and backing, adhesive, and protecting layers. (膜控型经皮给药系统设计为有药物储库或 药囊,通常以液态或粘胶态存在,含有控 释膜、背衬层、粘胶层和保护层。)

n Membrane-controlled systems have the advantage over monolithic systems in that as long as the drug solution in the reservoir remains saturated, the release rate of drug through the controlling membrane remains constant. n 膜控型比整体型的优点在于,只要药物贮 库中溶液保持饱和,药物通过控释膜的速 率保持恒定。


Delivery Modifiers Proprietary Drug Delivery Systems & Designs Polymer Technologies Patch System Designs Micro-Delivery Systems



IV. Percutaneous absorption models 1. 1) 2) In vivo studies In vivo skin penetration studies may be undertaken for one or more of the following purposes: To verify and quantify the cutaneous bioavailability of a topically applied drug. To verify and quantify the systemic bioavailability of a transdermal drug.

3) To establish bioequivalence of different topical formulations of the same drug substance. 4) To determine the incidence and degree of systemic toxicologic risk following topical application of a specific drug or drug product. 5) To relate resultant blood levels of drug in human to systemic therapeutic effects.

n The most relevant studies are performed in humans, however, animal models may be used insofar as they may be effective as predictors of human response. n Biologic samples used in drug penetration and drug absorption studies include skin sections, venous blood from the application site, blood from the systemic circulation, and excreta (urine, feces, and expired air).

2. In vitro studies n Skin permeation may be tested in vitro using various skin tissues (human or animal whole skin, dermis or epidermis) in a diffusion cell. n In vitro penetration studies using human skin are limited because of difficulties of procurement, storage, expense, and variation in permeation. n Animal skins are much more permeable than human skin. One alternative that has been shown to be effective is shed snake skin (蛇蜕).

The material may be used in cell culture studies or in standard diffusion cells. n Diffusion cell systems are employed in vitro to quantify the release rates of drugs from topical preparations. n In these systems, skin membranes or synthetic membranes may be employed as barriers to the flow of drug and vehicle to simulate the biologic system. n


V. Advantages and disadvantages of TDDSs The advantages of TDDSs are: 1. They can avoid gastrointestinal drug absorption difficulties caused by gastrointestinal p. H, enzymatic activity and drug interactions with food, drink, or other orally administered drugs. 2. They can substitute for oral administration of medication when that route is unsuitable, as in instances of vomiting and/or diarrhea.

3. They avoid the first-pass effect, that is, the initial pass of a drug substance through the systemic and portal circulation following gastrointestinal absorption, theraby possibly avoiding the drug’s deactivation by digestive and liver enzymes. 4. The systems are noninvasive, avoiding the inconvenience of parenteral therapy.

5. They provide extended therapy with a single application, thereby improving patient compliance over other dosage forms requiring more frequent dose administration. 6. The activity of drugs having short half-lives is extended through the reservoir of drug present in therapeutic delivery system and its controlled release characteristics.

7. Drug therapy may be terminated rapidly by removal of the application from the surface of the skin. 8. Ease of rapid identification of the medication in emergencies (e. g. , nonresponsive, unconscious, or comatose patient) due to the physical presence, features and identifying-markings on the TDDS.

The disadvantages of TDDSs are: 1. Only relatively potent drugs are suitable candidates for transdermal delivery due to the natural limits of drug entry imposed by the skin’s impermeability. (由于皮肤的不透过性,仅有部分活性大的药物适 合制成经皮给药。) 2. Some patients may develop contact dermatitis at the site of application due to one or more of the system components, necessitating discontinuation. (一些病人在用药部位可产生接触性皮炎,无法继 续用药。)

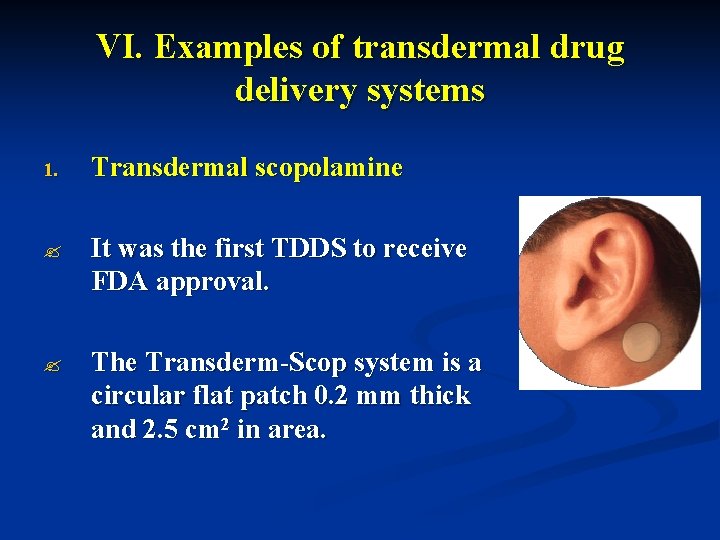
VI. Examples of transdermal drug delivery systems 1. Transdermal scopolamine ? It was the first TDDS to receive FDA approval. ? The Transderm-Scop system is a circular flat patch 0. 2 mm thick and 2. 5 cm 2 in area.

? The TDDS contains 1. 5 mg of scopolamine and is designed to deliver approximately 1 mg of scopolamine at an approximately constant rate to the systemic circulation over the 3 day life-time of the system. ? The patch is worn in a hairless area behind the ear. Because of the small size of the patch, the system is unobtrusive, convenient, and well accepted by the patient.

2. Transdermal Nitroglycerin A number of nitroglycerin-containing TDDSs have been developed, including ? Deponit (Schwarz) ? Minitran (3 M Pharmaceuticals) ? Nitro-Dur (Key) ? Transderm-Nitro (Novartis) Each of these products maintains nitroglycerin drug delivery for 24 hours after application.

? Nitroglycerin is used widely in the prophylactic (预防) treatment of angina. ? It has a relatively low dose, short plasma half-life, high peak plasma levels, and inherent (固有的) side effects when taken sublingually, a popular route. ? It is rapidly metabolized by the liver when taken orally, this first-pass effect is bypassed by the transdermal route.

? The various nitroglycerin TDDSs control the rate of drug delivery through a membrane and/or controlled release from the matrix or reservoir. ? The rate of drug release depends on the system. In the Transderm-Nitro system, nitroglycerin 0. 02 mg is delivered per hour for every square centimeter of patch, whereas in the Deponit system, each square centimeter delivers approximately 0. 013 mg of nitroglycerin per hour.

? Not all nitroglycerin systems have the same construction. For example, the Transderm. Nitro TDDS is a four-layer drug pouch system, whereas the Deponit TDDS is a thin two-layer matrix system resembling. ? Patients should be given explicit instructions regarding the use of nitroglycerin transdermal systems. Generally, these TDDSs are placed on the chest, back, upper arms, or shoulders.

? The patient should understand that physical exercise and elevated ambient temperatures, such as in a sauna, may increase the absorption of nitroglycerin.

3. Transdermal clonidine ? The first transdermal system for hypertension, Catapres TTS (clonidine transdermal therapeutic system, Boehringer Ingelheim), was marketed in 1985. ? Clonidine lends itself to transdermal delivery because of its lipid solubility, high volume of distribution, and therapeutic effectiveness in low plasma concentrations. ? The TDDS provides controlled release of clonidine.

? Catapres TTS is available in several sizes, with the amount of drug released proportional to the patch size. ? To ensure constant release over the 7 -day use period, the drug content is greater than the total amount of drug delivered. ? Catapres TTS is available in several sizes, with the amount of drug released proportional to the patch size.

C Clonidine flows in the direction of the lower concentration at a constant rate limited by a rate-controlling membrane. C The system is applied to the hairless area of intact skin on the upper outer arm or chest.

4. Transdermal Nicotine C Nicotine TDDSs are used as adjuncts in smoking cessation programs. C In a blinded study, users of nicotine TDDSs are more than twice as likely to quit smoking than individuals wearing a placebo patch. C The nicotine TDDSs provide sustained blood levels of nicotine as “nicotine-replacementtherapy” to help the patient establish and sustain remission from smoking.

C C C The commercially available patches contain from 7 to 22 mg of nicotine for daily application during the course of treatment ranging from about 6 to 12 weeks. A nicotine TDDS usually is applied to the arm or upper front torso, with patients advised not to smoke when wearing the system. Some of the nicotine replacement programs provide a gradual reduction in nicotine dosage form during the treatment program.

5. Transdermal Estradiol C The Estraderm TDDS delivers 17 -estradiol through a rate-limiting membrane continuously upon application to intact skin. C Two systems provide delivery of 0. 05 or 0. 1 mg estradiol per day.

C Estradiol is indicated for the treatment of moderate to severe vasomotor symptoms associated with menopause, female hypogonadism ( 性 腺 功 能 减 退 ) , female castration ( 女 性 卵 巢 切 除 术 ) , primary ovarian failure ( 原 发 性 卵 巢 功 能 衰 竭 ) , and atrophic (萎 缩 ) conditions caused by deficient endogenous estrogen production, such as atrophic vaginitis and kraurosis value.

Transdermal administration produces therapeutic serum levels of estradiol with lower circulating levels of estrone and estrone conjugates than does oral therapy and requires a smaller total dose. C The systemic side effects from oral estrogens can be reduced by using the transdermal dosage forms.

6. Transdermal testosterone C Testosterone transdermal systems, Testoderm (Alza) and Androderm (Smith. Kline Beecham), are available with various delivery rates as hormone replacement therapy in men who have an absence or deficiency of testosterone. C The TDDS is applied daily, usually in the morning to mimic endogenous testosterone release. C Optimum serum levels are reached within 2 to 4 hours after application. The patch is worn 22 to 24 hours daily for 6 to 8 weeks.

7. Other transdermal therapeutic systems C C C Diltiazem 地尔硫卓 Isosorbide dinitrate 硝酸异山梨醇 Propranolol 普奈洛尔 Nifedipine 硝苯地平 Mepindolol 甲吲洛尔 Verapamil 维拉帕米

C C C C Cardiovascular agents, levonorgestrel/estradiol for hormonal contraception, Physostigmine(毒扁豆碱), xanomeline (占诺美林 )for Alzheimer’s disease therapy, Naltrexone(纳曲酶) and methadone for substance addiction, Buspirone(丁螺环酮)for anxiety, Bupropion (安非他酮) for smoking cessation, Papaverine(罂粟碱) for male impotence,

VII. General clinical considerations in the use of TDDSs The patient should be advised of the following general guidelines along with product-specific instructions in the use of TDDSs. 1. Percutaneous absorption may vary with the site of application. 2. TDDSs should be applied to clean, dry skin that is relatively free of hair and not oily, irritated, inflamed, broken, or callused.

3. Use of skin lotion should be avoided at the application site, because lotions affect skin hydration and can alter the partition coefficient between the drug and the skin. 4. TDDSs should not be physically altered by cutting, since this destroys the integrity of the system. 5. A TDDS should be removed from its protective package, with care not to tear or cut into the unit.

6. A TDDS should be placed at a site that will not subject it to being rubbed off by clothing or movement. 7. A TDDS should be worn for the full period stated in the product’s instructions. Following that period, it should be removed and replaced with a fresh system as directed. 8. The patient or caregiver should be instructed to cleanse the hands thoroughly before and after applying a TDDS.

9. if the patient exhibits sensitivity or intolerance to a TDDS or if undue skin irritation results, the patient should seek reevaluation. 10. Upon removal, a used TDDS should be folded in half with the adhesive layer together so that it cannot be reused.

Questions 1. Explain shortly - transdermal drug delivery systems - iontophoresis - sonophoresis - percutaneous absorption enhancers 2. What factors could affect percutaneous drug absorption? 3. How to increase percutaneous absorption of drug by physical and chemical methods? 4. What types of drugs could be designed as TDDSs and how?

5. How to design transdermal drug delivery systems? 6. How to study the percutaneous absorption of drug using in vitro and in vivo models? 6. What are the characteristics of transdermal drug delivery systems? 7. How many different types of transdermal nitroglycerin are in the market? Explain their characteristics respectively. 8. What are the clinical considerations in the use of TDDSs?
 Transdermal drug delivery system
Transdermal drug delivery system Transdermal patch placement chart
Transdermal patch placement chart Transdermal kontraseptif patch
Transdermal kontraseptif patch Transdermal buprenorphine patch
Transdermal buprenorphine patch Transdermal
Transdermal Punch method of capsule filling
Punch method of capsule filling Rate limiting steps in drug absorption
Rate limiting steps in drug absorption Dosage forms and drug delivery systems
Dosage forms and drug delivery systems Parenteral dosage form example
Parenteral dosage form example Example of crude drug adulterated with exhausted drug
Example of crude drug adulterated with exhausted drug Hamlet act iii scene iii
Hamlet act iii scene iii Newer drug delivery system
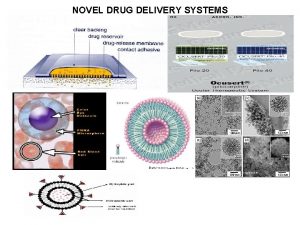
Newer drug delivery system Ocular drug delivery system
Ocular drug delivery system Diffusion controlled modified release system consists of
Diffusion controlled modified release system consists of Accenture delivery suite website link
Accenture delivery suite website link Introduction to healthcare delivery systems
Introduction to healthcare delivery systems Social service delivery systems
Social service delivery systems Decision support systems and intelligent systems
Decision support systems and intelligent systems Dicapine
Dicapine Embedded systems vs cyber physical systems
Embedded systems vs cyber physical systems Engineering elegant systems: theory of systems engineering
Engineering elegant systems: theory of systems engineering Work portfolio examples
Work portfolio examples Deep perineal pouch contents
Deep perineal pouch contents Trali symptoms
Trali symptoms Ffp
Ffp Middle mediastinum: contents mnemonic
Middle mediastinum: contents mnemonic Content of popliteal fossa
Content of popliteal fossa Superior mediastinum
Superior mediastinum The immortal life of henrietta lacks table of contents
The immortal life of henrietta lacks table of contents Contents of internal capsule
Contents of internal capsule Rhomboideus minor
Rhomboideus minor Ark of the covenant lampstand
Ark of the covenant lampstand So we meet again my dear doctor
So we meet again my dear doctor Porta hepatis
Porta hepatis Mla table of contents
Mla table of contents Stylistic synonyms lexicology
Stylistic synonyms lexicology Cover page for school magazine
Cover page for school magazine Continuous variable
Continuous variable Appendices in report
Appendices in report Platelets transfusion indication
Platelets transfusion indication Anterior mediastinum contents
Anterior mediastinum contents Mediastinum and pericardium
Mediastinum and pericardium Lecture abstract example
Lecture abstract example Persepolis table of contents
Persepolis table of contents Persepolis table
Persepolis table Interactive notebook table of contents
Interactive notebook table of contents Cryoprecipitate contains
Cryoprecipitate contains Brainstem
Brainstem Coverings of the spermatic cord
Coverings of the spermatic cord Adductor magnus nerve supply
Adductor magnus nerve supply Swot event management
Swot event management Contents of the middle mediastinum
Contents of the middle mediastinum Posterior mediastinum contents
Posterior mediastinum contents Carotid sheath contents
Carotid sheath contents Triangular space contents
Triangular space contents Contents of curriculum vitae
Contents of curriculum vitae Ctd module 3
Ctd module 3 Cost audit report
Cost audit report Civics interactive notebook
Civics interactive notebook The city of ember summary
The city of ember summary Fresh frozen plasma contents
Fresh frozen plasma contents Contents of air pollution
Contents of air pollution Content page magazine
Content page magazine Cubital fossa anatomy
Cubital fossa anatomy Olecrannon fossa
Olecrannon fossa Triangle
Triangle Thigh muscles medial
Thigh muscles medial Medial aspect of antecubital fossa
Medial aspect of antecubital fossa Anterior
Anterior Transverse humeral ligament attachments
Transverse humeral ligament attachments Hamlet table of contents
Hamlet table of contents Contents of optab
Contents of optab Ctd module 5 table of contents
Ctd module 5 table of contents Urogenital triangle
Urogenital triangle