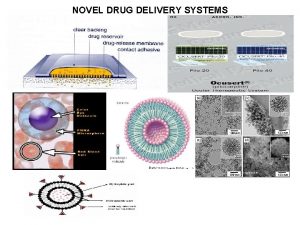
Drug Delivery Systems Content Introduction to Drug Delivery


















![Introduction Fig. 1. UNOS organ transplant statistics for 1990 to 1999 [6] documenting the Introduction Fig. 1. UNOS organ transplant statistics for 1990 to 1999 [6] documenting the](https://slidetodoc.com/presentation_image_h/05cc5bc4bd4dd72b0b1e6fa0ee9952e7/image-58.jpg)

















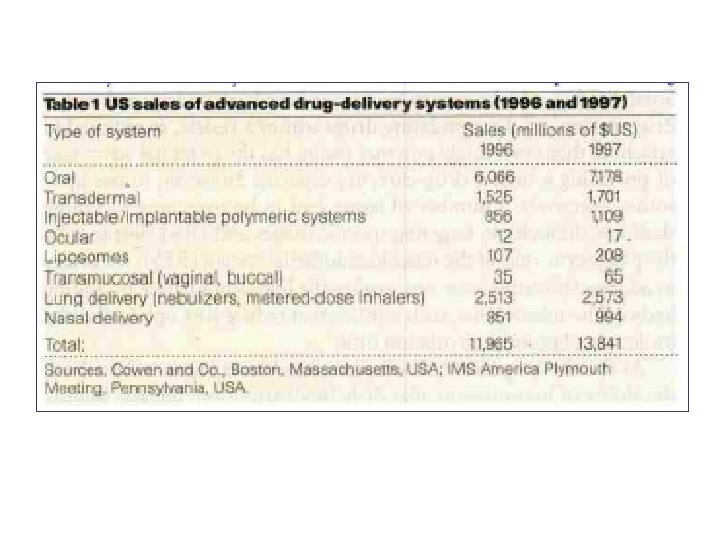
- Slides: 93

Drug Delivery Systems


Content • Introduction to Drug Delivery Systems (DDSs) • Mechanisms of Polymer-based DDSs • Progress in DDSs • Gene Delivery and gene therapy




Mechanisms of Polymer-based Drug Delivery Systems

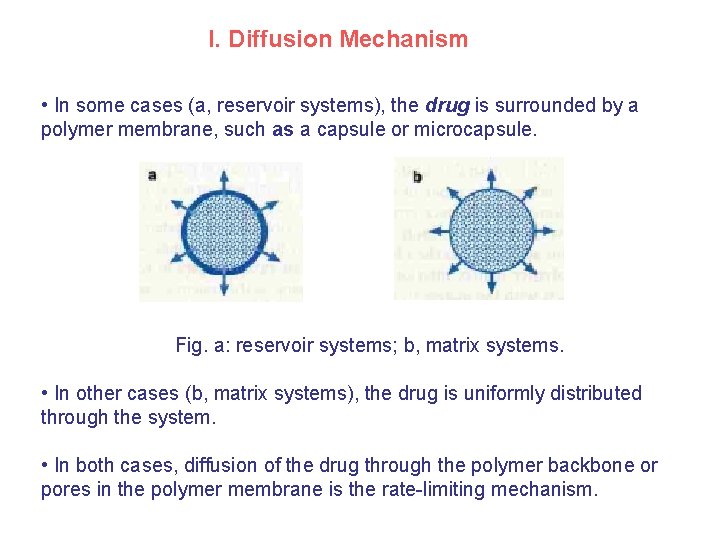
I. Diffusion Mechanism • In some cases (a, reservoir systems), the drug is surrounded by a polymer membrane, such as a capsule or microcapsule. Fig. a: reservoir systems; b, matrix systems. • In other cases (b, matrix systems), the drug is uniformly distributed through the system. • In both cases, diffusion of the drug through the polymer backbone or pores in the polymer membrane is the rate-limiting mechanism.

• Release rates from membranes are determined by the steady-state Fick’s Law diffusion equation: Here D is the concentration-independent drug diffusion coefficient in the membrane: 扩散系数(m 2/s) J* is the drug molar flux:J为扩散通量 atoms/(m 2·s)或kg/(m 2·s) dc/dx: is the drug concentration gradient within the membrane.

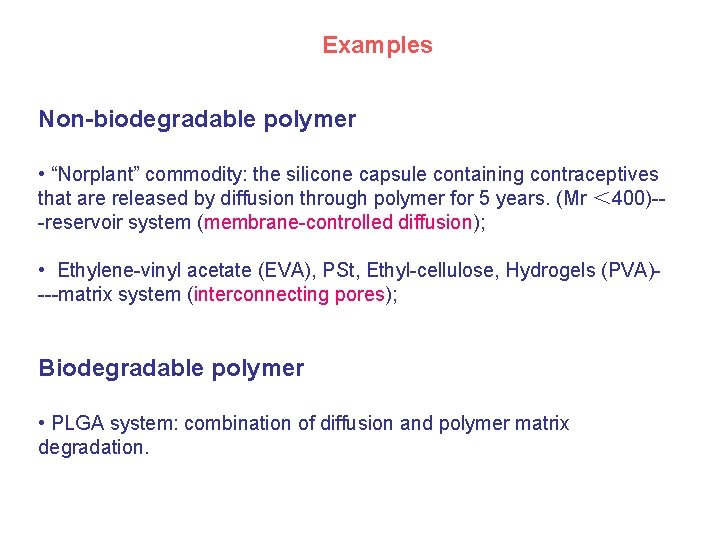
Examples Non-biodegradable polymer • “Norplant” commodity: the silicone capsule containing contraceptives that are released by diffusion through polymer for 5 years. (Mr < 400)--reservoir system (membrane-controlled diffusion); • Ethylene-vinyl acetate (EVA), PSt, Ethyl-cellulose, Hydrogels (PVA)---matrix system (interconnecting pores); Biodegradable polymer • PLGA system: combination of diffusion and polymer matrix degradation.

II. Solvent-Activated Mechanism • The osmotically controlled release system involves a tablet containing an osmotic agent surrounded by a semipermeable membrane (permeable to water but impermeable to salt or drug). The membrane contains a single laser-drilled hole. The external solvent, water, enters the tablet through the membrane at a constant rate and drives the drug out through the laserdrilled hole at a constant rate. Fig. c: osmotic system.

• An equation that describes release rates from these systems: where K is a constant equal to the product of the membrane’s hydraulic permeability and its reflection coefficient, II is the osmotic pressure of the osmotic agent of the core formulation, C is the drug concentration inside the osmotic tablet core, and l is the membrane thickness. • Examples: EVA, PMMA, PAA, Cellulose derivatives membranes.

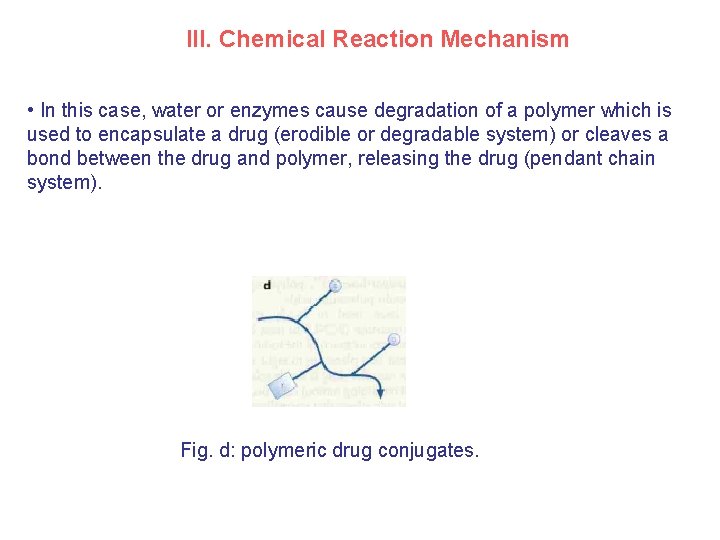
III. Chemical Reaction Mechanism • In this case, water or enzymes cause degradation of a polymer which is used to encapsulate a drug (erodible or degradable system) or cleaves a bond between the drug and polymer, releasing the drug (pendant chain system). Fig. d: polymeric drug conjugates.

Examples • From a chemical standpoint, bioerodible systems can be distinguished by three dissolution mechanisms: (1) water-soluble polymers insolubilized by degradable cross-links; (2) water-insoluble polymers solubilized by hydrolysis, ionization, or protonation of pendant side groups; and (3) water -insoluble polymers solubilized by backbone-chain cleavage to small water -soluble molecules. • The most commonly used biodegradable polymer is poly(lactic acid) or lactic/glycolic copolymers (type 3). • Others include poly(vinylpyrrolidine) (type l), copolymers of methyl vinyl ether (n-butyl half-ester) and maleic anhydride (type 2), poly(anhydrides) (type 3), poly(ortho esters) (type 3), poly(ecaprolactone) (type 3), and poly(amino acids) (type 3).

Progress in Drug Delivery Systems


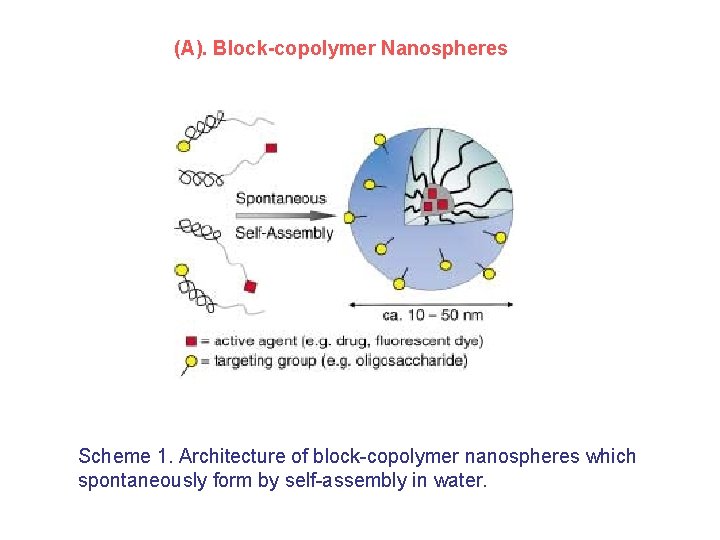
(A). Block-copolymer Nanospheres Scheme 1. Architecture of block-copolymer nanospheres which spontaneously form by self-assembly in water.

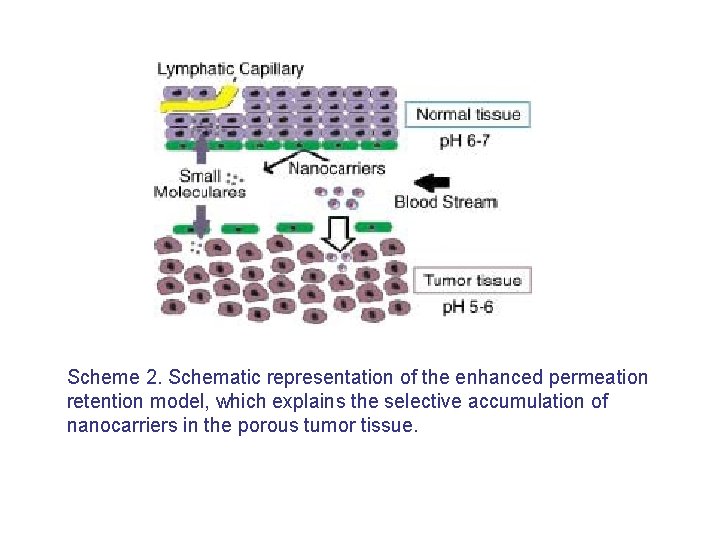
Scheme 2. Schematic representation of the enhanced permeation retention model, which explains the selective accumulation of nanocarriers in the porous tumor tissue.

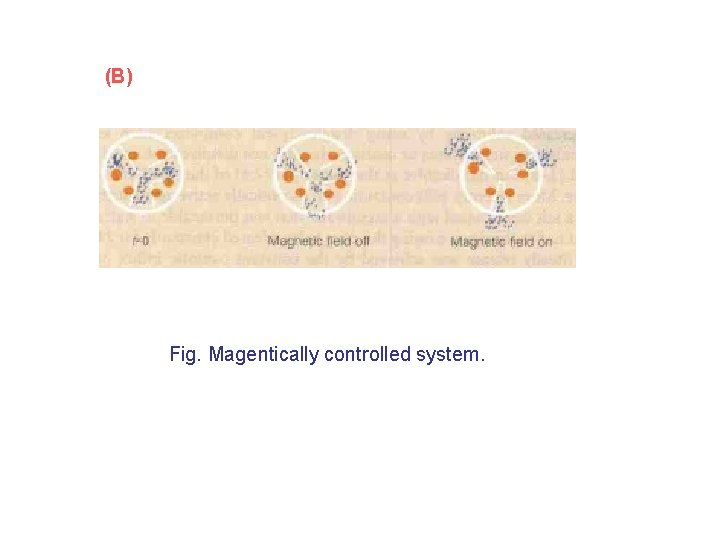
(B) Fig. Magentically controlled system.



Fig. p. H or temperature controlled system.

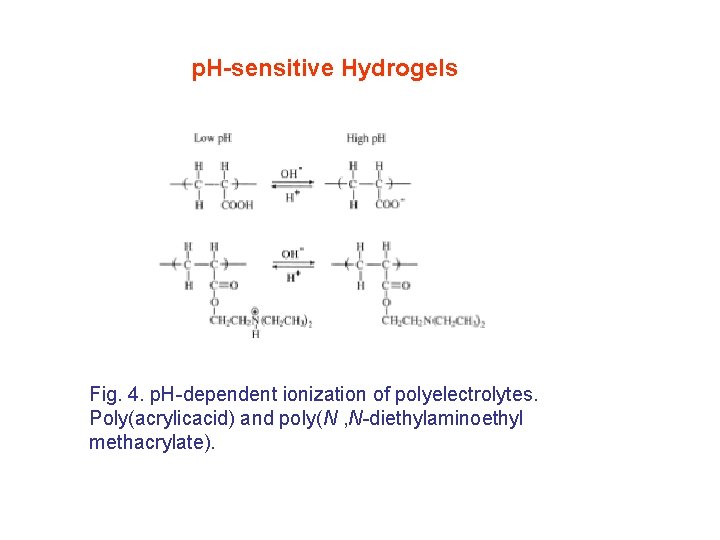
p. H-sensitive Hydrogels Fig. 4. p. H-dependent ionization of polyelectrolytes. Poly(acrylicacid) and poly(N , N-diethylaminoethyl methacrylate).

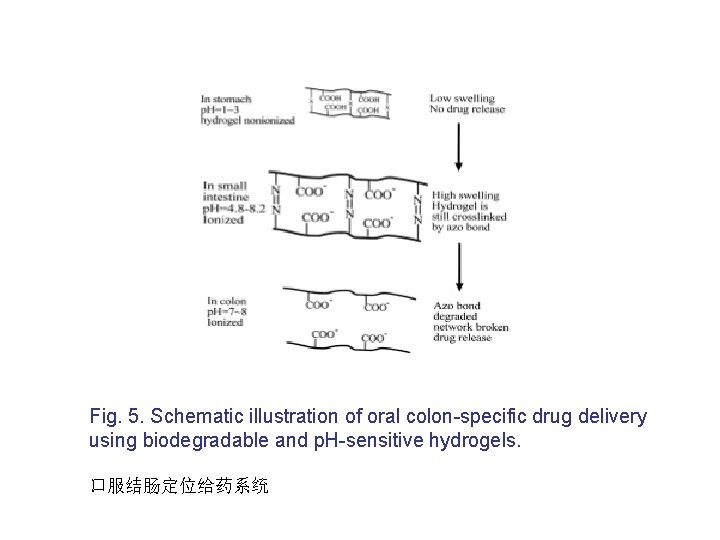
Fig. 5. Schematic illustration of oral colon-specific drug delivery using biodegradable and p. H-sensitive hydrogels. 口服结肠定位给药系统

Gene Delivery/Therapy • Introduction • Progress in non-viral gene delivery • Prospects in gene delivery



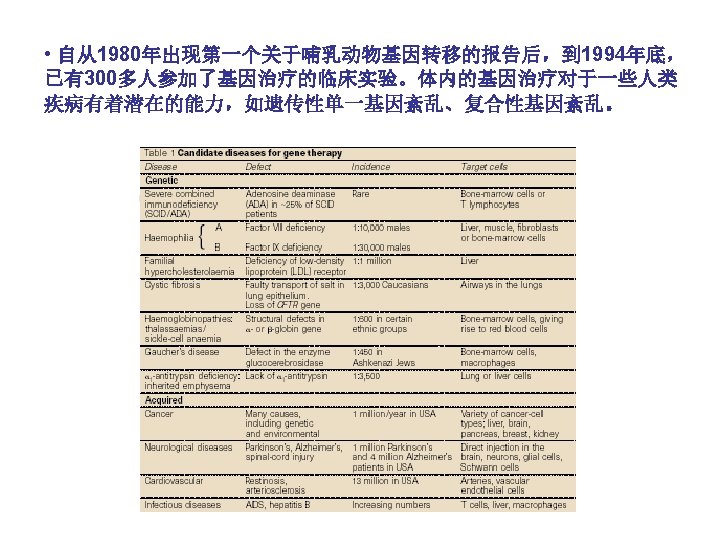
(B) Classification and Characteristics • The vectors available now: the non-viral and viral vectors. • These techniques are categorized into two general groups: naked DNA delivery by a physical method, such as electroporation and gene gun and delivery mediated by a chemical carrier such as cationic polymer and lipid. • Viral vectors suffer from several drawbacks: (1) a need for packaging cell lines (细胞系), (2) problems with safety, toxicity, (3) the elicitation of an immune response, (4) the lack of cell-specific targeting, (5) viral vector systems are rapidly cleared from the circulation, limiting transfection to ‘first-pass’ organs, such as the lungs, liver and spleen. • Viral vectors have been implicated in the death of at least one patient, leading the suspension of clinical trials.

Non-viral Vectors Advantages of non-viral vectors • they are easy to prepare and to scale-up, • they are more flexible with regard to the size of the DNA being transferred, • they are generally safer in vivo, • they do not elicit a specific immune response and can therefore be administered repeatedly, • they are better for delivering cytokine genes because they are less immunogenic than viral vectors. Disadvantages and Current Status • less efficient in delivering DNA and in initiating gene expression, particularly when used in vivo. • for this reason, few nonviral vectors have reached clinical trials, including naked DNA, DNA–cationic-LIPOSOME complexes (lipoplexes), DNA–polymer complexes and combinations of these.

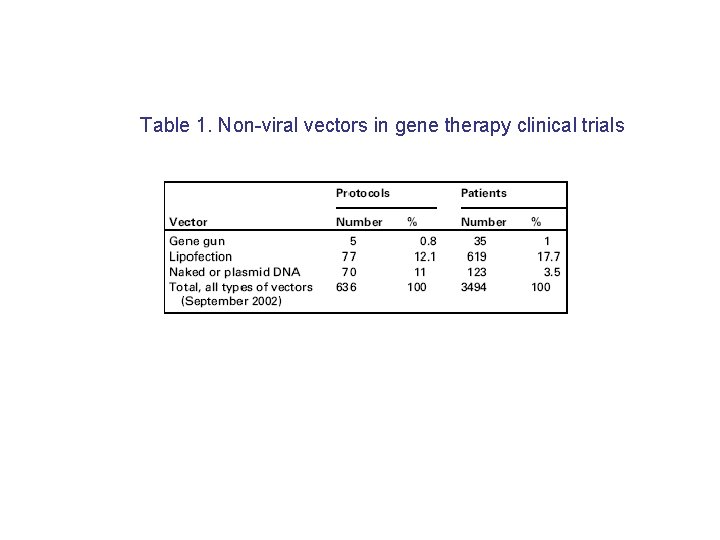
Table 1. Non-viral vectors in gene therapy clinical trials

(C) Properties of the ideal gene therapy vector Goals: the ideal gene delivery system should be specifically targeting, biodegradable, non-toxic, non-inflammatory, nonimmunogenic and stable for storage. It should also have a large capacity for genetic material, efficient transfection and the capacity to be produced in high concentrations at low cost. • Easy production The vector should be easy to produce at high titre on a commercial scale. (such as concentration technology for delivery in small volumes), and should have a reasonable shelf-life for transport and distribution. • Sustained Expression The vector, once delivered, should be able to express its genetic cargo over a sustained period or expression should be regulable in a precise way. Different disease states have different requirements (for example, regulated expression in diabetes and lifetime expression in haemophilia,血友病).

• Immunologically inert The vector components should not elicit an immune response after delivery. A humoral (体液) antibody response will make a second injection of the vector ineffective, whereas a cellular response will eliminate the transduced cells. • Tissue targeting Delivery to only certain cell types is highly desirable, especially where the target cells are dispersed throughout the body, or if the cells are part of a heterogeneous population (such as in the brain). • Size capacity The vector should have no size limit to the genetic material it can deliver. The coding sequence of a therapeutic gene varies from 350 base pairs for insulin, to over 12, 000 base pairs for dystrophin(营养不 良). • Replication, segregation or integration The vector should allow for site-specific integration of the gene into the chromosome of the target cell, or should reside in the nucleus as an episome (附加体, 游离基因); that will faithfully divide and segregate on cell division.

Progress in non-viral gene delivery

Progress in non-viral gene delivery • Naked DNA delivery by physical method: to overcome safety issue and to realize efficient gene expression in vivo; • Gene delivery using a chemical carrier: to establish functional gene delivery in vivo; • Nonviral vector modifications with peptides to increase intracellular gene delivery; • Reduction of immune responses by modifying the administration protocol or the composition of the DNA; • Design of tissue-specific, self-replicating and integrating plasmid expression systems to facilitate long-lasting gene expression.

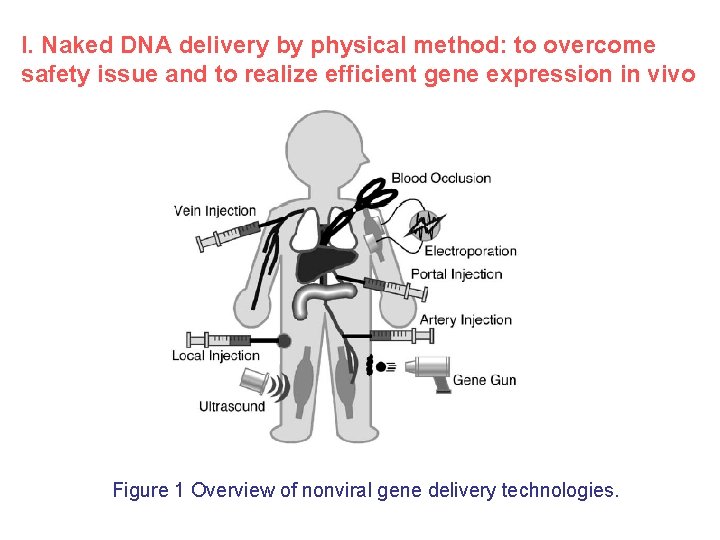
I. Naked DNA delivery by physical method: to overcome safety issue and to realize efficient gene expression in vivo Figure 1 Overview of nonviral gene delivery technologies.

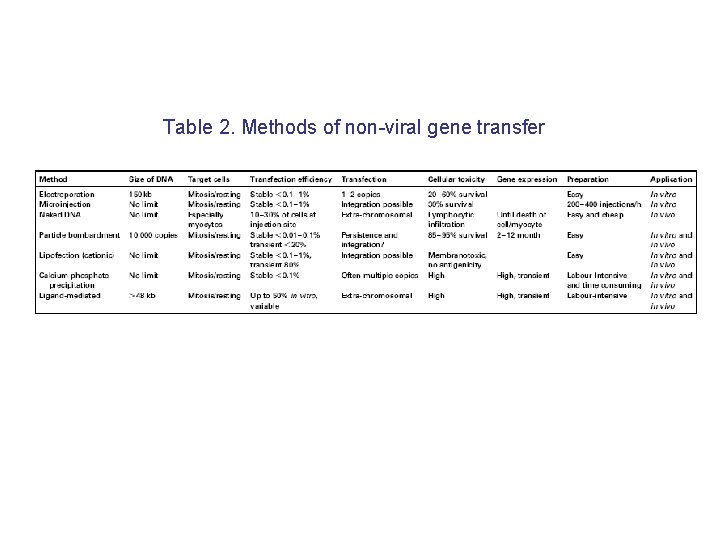
Table 2. Methods of non-viral gene transfer

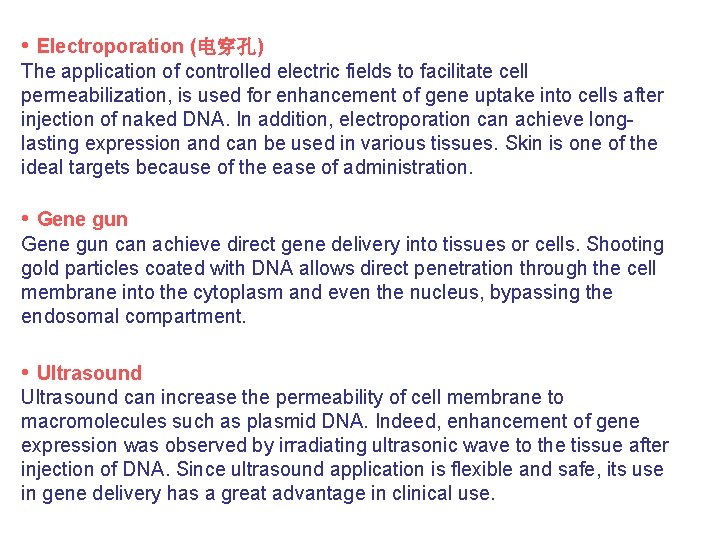
• Electroporation (电穿孔) The application of controlled electric fields to facilitate cell permeabilization, is used for enhancement of gene uptake into cells after injection of naked DNA. In addition, electroporation can achieve longlasting expression and can be used in various tissues. Skin is one of the ideal targets because of the ease of administration. • Gene gun can achieve direct gene delivery into tissues or cells. Shooting gold particles coated with DNA allows direct penetration through the cell membrane into the cytoplasm and even the nucleus, bypassing the endosomal compartment. • Ultrasound can increase the permeability of cell membrane to macromolecules such as plasmid DNA. Indeed, enhancement of gene expression was observed by irradiating ultrasonic wave to the tissue after injection of DNA. Since ultrasound application is flexible and safe, its use in gene delivery has a great advantage in clinical use.

• Hydrodynamic injection, a rapid injection of a large volume of naked DNA solution (eg 5 mg plasmid DNA injected in 5– 8 s in 1. 6 ml saline solution for a 20 g mouse) via the tail vein, can induce potent gene transfer in internal organs, especially the liver. • Blood Occlusion Significant gene expression can be achieved in the liver by transiently restricting blood flow through the liver immediately following peripheral intravenous injection of naked DNA. Occlusion of blood flow either at vena cava or at hepatic artery and portal vein increased the expression level in the liver. Presumably, the injected DNA is internalized into the hepatic cells by receptor-mediated mechanism as proposed by Budker et al or via a nonreceptor-mediated pathway.

II. Gene delivery using a chemical carrier: to establish functional gene delivery in vivo Novel carriers to achieve high-level gene expression and functional delivery have been designed. Gene carriers can be categorized into several groups: • those forming condensed complexes with the DNA to protect the DNA from nucleases and other blood components; • those designed to target delivery to specific cell types; • those designed to increase delivery of DNA to the cytosol or nucleus; • those designed to dissociate from DNA in the cytosol; • those designed to release DNA in the tissue to achieve a continuous or controlled expression. • Lipids and polymers are mainly used for gene delivery.

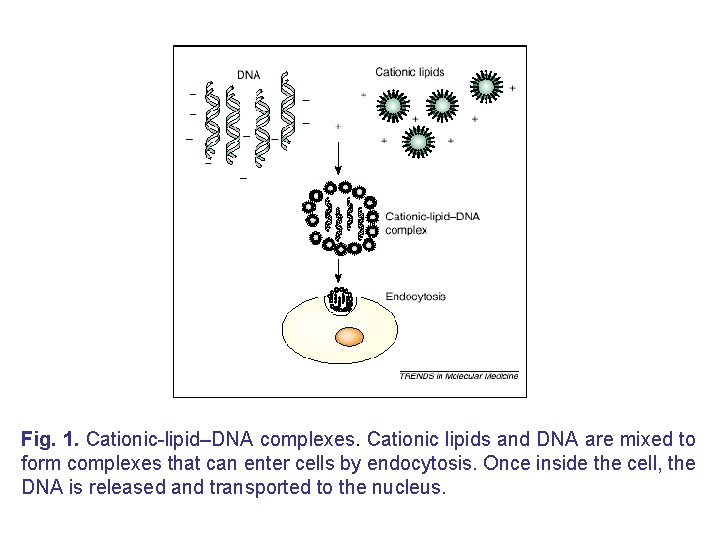
(A) Lipid-mediated gene delivery • Liposome-based gene delivery, first reported by Felgner in 1987, is still one of the major techniques for gene delivery into cells. In 1990 s, a large number of cationic lipids, such as quaternary ammonium detergents, cationic derivatives of cholesterol and diacylglycerol, and lipid derivatives of polyamines, were reported. • However, the development of novel types of lipid molecules appears to be saturated, and most of the efforts have shifted to improving efficacy by the modification listed above, as well as to specific in vivo applications.

Fig. 1. Cationic-lipid–DNA complexes. Cationic lipids and DNA are mixed to form complexes that can enter cells by endocytosis. Once inside the cell, the DNA is released and transported to the nucleus.

• Cationic-liposome-mediated gene transfer has, however, been successfully used in vitro and in vivo in gene therapy experimental models, and has also been evaluated in several clinical protocols. • In several phase-I human trials, direct in vivo injection of a p. DNA– lipid complex expressing the major-histocompatibility (组织相容性)complex-class-I gene, HLA-B 7, produced a clinical response in HLAB 7 -negative melanoma patients. • An interleukin [白(细胞)介素, 白细胞间素]-2 -expressing p. DNA–lipid complex was evaluated in a phase-I and -II trial of patients with melanoma (黑素瘤), sarcoma (肉瘤) or renal cell carcinoma.

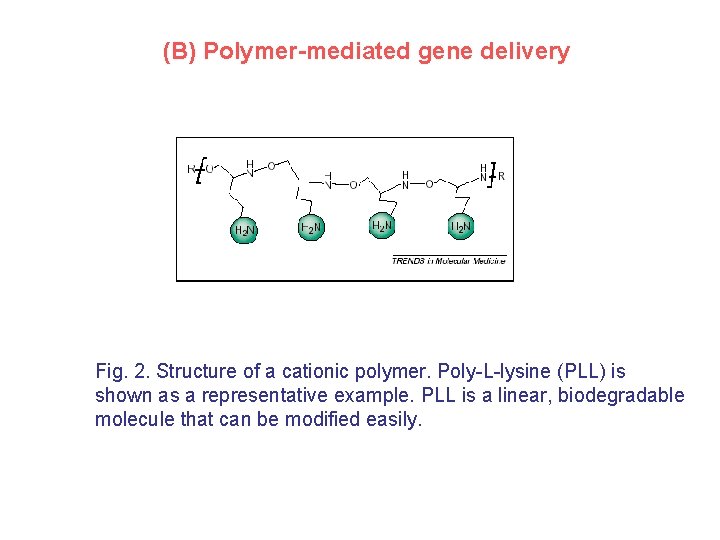
(B) Polymer-mediated gene delivery Fig. 2. Structure of a cationic polymer. Poly-L-lysine (PLL) is shown as a representative example. PLL is a linear, biodegradable molecule that can be modified easily.

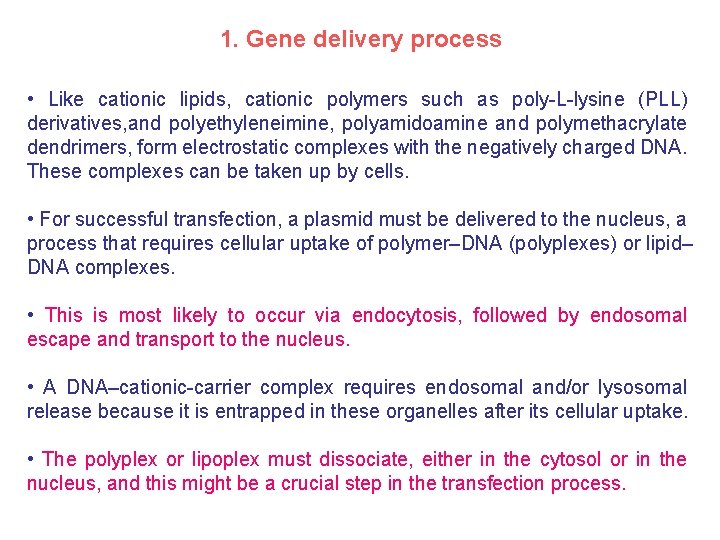
1. Gene delivery process • Like cationic lipids, cationic polymers such as poly-L-lysine (PLL) derivatives, and polyethyleneimine, polyamidoamine and polymethacrylate dendrimers, form electrostatic complexes with the negatively charged DNA. These complexes can be taken up by cells. • For successful transfection, a plasmid must be delivered to the nucleus, a process that requires cellular uptake of polymer–DNA (polyplexes) or lipid– DNA complexes. • This is most likely to occur via endocytosis, followed by endosomal escape and transport to the nucleus. • A DNA–cationic-carrier complex requires endosomal and/or lysosomal release because it is entrapped in these organelles after its cellular uptake. • The polyplex or lipoplex must dissociate, either in the cytosol or in the nucleus, and this might be a crucial step in the transfection process.

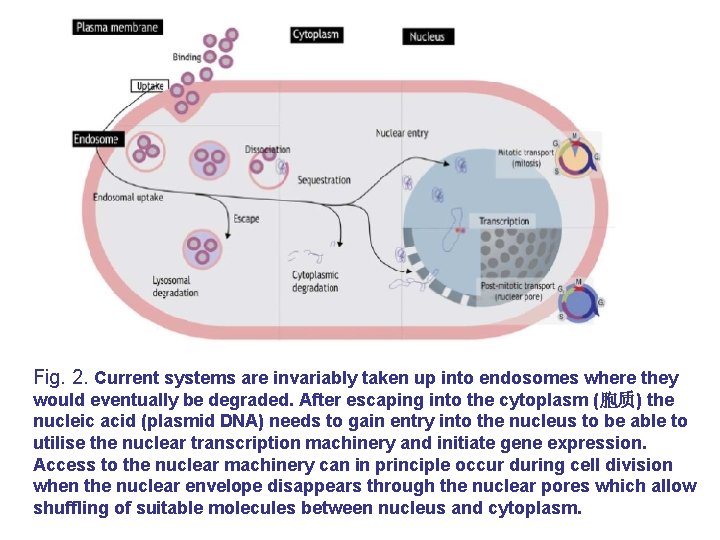
Fig. 2. Current systems are invariably taken up into endosomes where they would eventually be degraded. After escaping into the cytoplasm (胞质) the nucleic acid (plasmid DNA) needs to gain entry into the nucleus to be able to utilise the nuclear transcription machinery and initiate gene expression. Access to the nuclear machinery can in principle occur during cell division when the nuclear envelope disappears through the nuclear pores which allow shuffling of suitable molecules between nucleus and cytoplasm.

2. Other Polymer-based systems • Polymer-based systems (e. g. using collagen, lactic or glycolic acid, polyanhydride or polyethylene vinyl coacetate) provide several potential advantages for therapeutic delivery of DNA (or of drugs). • First, DNA encapsulation within the polymer can protect against degradation until release. • Second, injection or implantation of the polymer into the body can be used to target a particular cell type or tissue. • Third, drug release from the polymer and into the tissue can be designed to occur rapidly (a bolus delivery) or over an extended period of time; • Thus, the delivery system can be tailored to a particular application. The choice of polymer and its physical form determine the time-scale of release.

3. Control over DNA delivery • Control over DNA delivery can be achieved by the formation of both synthetic and natural polymers in a variety of geometries and configurations, such as reservoirs, matrices, and microspheres. • Microspheres (or pellets) can be delivered in a minimally invasive manner (e. g. by direct injection or by oral delivery), • Matrices can be implanted at the appropriate site, for example, for applications in tissue repair and wound healing.

4. Targeting of gene transfer has also been achieved by modification of gene carriers using cell targeting ligands, • such as asialoglycoproteins for hepatocytes (肝细胞), • anti-CD 3 and anti-CD 5 antibodies for T cells, • transferrin (转运蛋白) for some cancer cells, insulin, or galactose. • In addition, a targeted folate-expressing, cationic-liposome-based transfection complex has been shown to specifically transfect folatereceptor-expressing cells and tumours, suggesting that this is a potential therapy for intraperitoneal (腹膜内的) cancers.

5. Drawbacks and Modification • However, intrinsic drawbacks with cationic carriers, such as solubility, cytotoxicity and low transfection efficiency, have limited their use in vivo. These vectors sometimes attract serum proteins and blood cells when entering the circulation, resulting in dynamic changes in their physicochemical properties. • Polyamidoamine and polyethyleneimine dendrimers have a high transfection efficiency in vitro and in vivo, but are cytotoxic and have low solubility when complexed with DNA. • The attachment of polyethylene glycol to PLL provides a biocompatible protective coating for the DNA complex.

• More complex gene transfer systems use cationic amphiphiles, such as polymeric polyethylimines, polyamidoamine ‘starburst’ dendrimers, polylysine conjugates and cationic liposomes, which can be combined with naked DNA, m. RNA or larger DNA fragments to produce complex particles. Polycation addition leads to electrostatic neutralization of anionic charges, and condenses the polynucleotide structure thereby protecting it against nuclease digestion. • p. DNA and cationic amphiphiles can be formulated in different ratios to produce complexes of diverse size and surface-charge properties. • Additionally, complexes bearing a net positive charge display enhanced binding to negatively charged cell membranes, leading to increased cellular uptake.

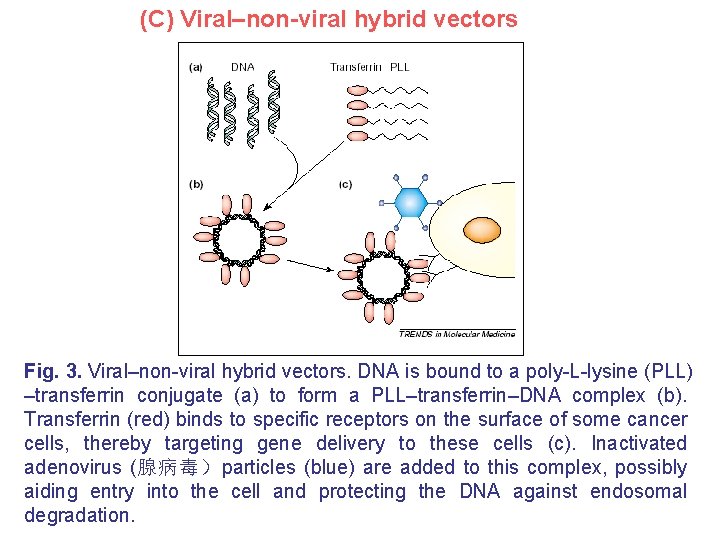
(C) Viral–non-viral hybrid vectors Fig. 3. Viral–non-viral hybrid vectors. DNA is bound to a poly-L-lysine (PLL) –transferrin conjugate (a) to form a PLL–transferrin–DNA complex (b). Transferrin (red) binds to specific receptors on the surface of some cancer cells, thereby targeting gene delivery to these cells (c). Inactivated adenovirus (腺病毒)particles (blue) are added to this complex, possibly aiding entry into the cell and protecting the DNA against endosomal degradation.

Progress in hybrid vector • Hybrid vectors incorporate viruses or viral peptides into traditional cationic-amphiphile-based vector systems. The efficiency of synthetic vectors can be improved using artificial nucleic-acid carriers incorporating functional elements that mimic viruses. • For example, the adenovirus hexon protein enhances the nuclear delivery and increases the transgene expression of polyethyleneimine– p. DNA vectors. • Non-viral vectors have also been designed to mimic the receptor mediated cell entry of adenoviruses; • Formulations that combine the merits of both viral and non-viral systems, such as a virus–cationic-liposome–DNA complex, ‘haemagglutinating virus of Japan’ liposomes, and cationic-lipid–DNA mixed with the G glycoprotein from the ‘vesicular stomatitis virus’ envelope, have been developed.

(D) Peptide-based gene delivery system • Amphiphilic a-helical peptides, containing cationic amino-acids, can be used as gene carriers into cells. • These peptides are readily available, owing to recent developments in production methods, allowing the design and synthesis of functional gene carrier molecules, such as carbohydrate-modified peptides, for targeted gene delivery. • Furthermore, the use of peptide-based gene carriers enables the construction of well-defined molecules, which cannot be achieved using polymer-based carriers.

(E) Nanoparticle-based gene delivery system • Novel polymeric delivery systems (e. g. nanospheres), which can be administered in novel ways (e. g. aerosols, 气溶胶), are being developed. • The smaller the size of the condensed DNA particles, the better the in vivo diffusion towards target cells and the trafficking within the cell. • Individual plasmid molecule can be collapsed to a nanoparticle using designed detergents. For example, in mice, nanoparticle-based gene delivery, targeted to the neovasculature using an integrin-targeting ligand, resulted in tumour regression.

(F) A physical and chemical combination: magnetofection • The association of non-viral gene vectors with supramagnetic nanoparticles, targeted by application of a magnetic field, increased efficacy by up to several-hundred-fold. • The high TRANSDUCTION efficiency observed in vitro was reproduced in vivo using magnetic-field-guided local transfection in the gastrointestinal (胃与肠的) tract and in blood vessels.

Prospects in gene delivery • Physical techniques for gene delivery into cells such as electroporation, with and without adjuvants (佐剂), will be significantly optimized; • Knowledge of the interaction of naked DNA with serum components and cell surface receptors will continue to accumulate. Immune responses originating from Cp. G motifs and nonviral gene carriers will diminish; • The structure of gene carriers will be further optimized and tailored for specific uses such as systemic administration, local injection or organspecific delivery; • Novel ligands for targeted delivery of DNA will be found; • Translocation mechanisms for plasmid DNA within the cell will be identified – these may provide novel strategies for efficient delivery; • More tissue-specific, site-specific integrating or self-replicating plasmid vectors are likely to appear.

Tissue Engineering • Introduction • Polymer scaffolds for tissue engineering • Progress in tissue engineering
![Introduction Fig 1 UNOS organ transplant statistics for 1990 to 1999 6 documenting the Introduction Fig. 1. UNOS organ transplant statistics for 1990 to 1999 [6] documenting the](https://slidetodoc.com/presentation_image_h/05cc5bc4bd4dd72b0b1e6fa0ee9952e7/image-58.jpg)
Introduction Fig. 1. UNOS organ transplant statistics for 1990 to 1999 [6] documenting the wait-listed patients (O) and transplants(●).




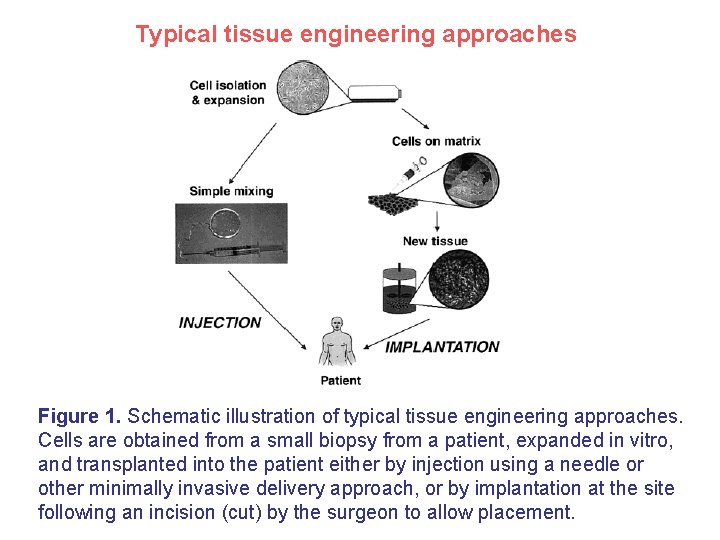
Typical tissue engineering approaches Figure 1. Schematic illustration of typical tissue engineering approaches. Cells are obtained from a small biopsy from a patient, expanded in vitro, and transplanted into the patient either by injection using a needle or other minimally invasive delivery approach, or by implantation at the site following an incision (cut) by the surgeon to allow placement.

Polymer scaffolds in tissue engineering

I. Polymer Scaffolds • Tissues or organs can be potentially engineered with a number of different strategies, but a particularly appealing approach utilizes a combination of a patient’s own cells combined with polymer scaffolds. • A variety of tissues are being engineered using this approach including fabricated artery, bladder, skin, cartilage, bone, ligament, and tendon. • Several of these tissues are now at or near clinical uses. II. Polymeric Hydrogels for Scaffolds • An exciting alternative approach to cell delivery for tissue engineering is the use of polymers (i. e. , hydrogels) that can be injected into the body. • This approach enables the clinician to transplant the cell and polymer combination in a minimally invasive manner.







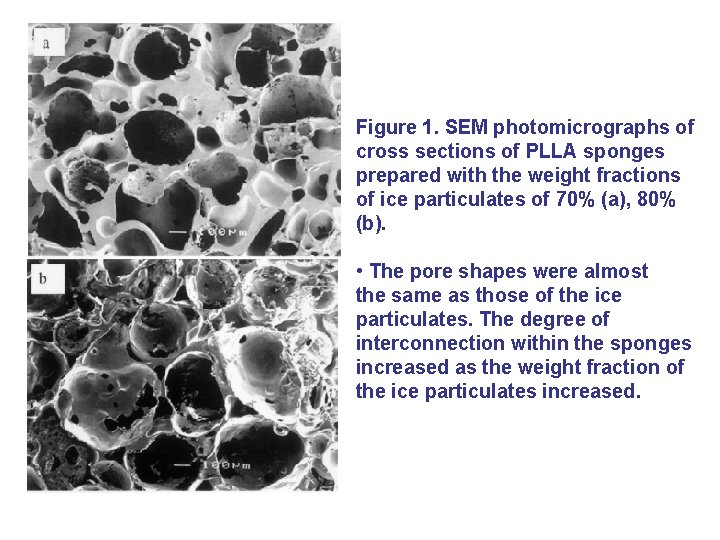
Figure 1. SEM photomicrographs of cross sections of PLLA sponges prepared with the weight fractions of ice particulates of 70% (a), 80% (b). • The pore shapes were almost the same as those of the ice particulates. The degree of interconnection within the sponges increased as the weight fraction of the ice particulates increased.

II. Polymeric hydrogels for Scaffolds

A. Design Parameters for Hydrogels in Tissue Engineering • Hydrogels in tissue engineering must meet a number of design criteria to function appropriately and promote new tissue formation. • These criteria include both classical physical parameters (e. g. , degradation and mechanics) as well as biological performance parameters (e. g. , cell adhesion). • The mechanical properties of hydrogels are important design parameters in tissue engineering, as the gel must create and maintain a space for tissue development. • The interactions of cells with hydrogels significantly affects their adhesion as well as migration and differentiation. • An absolutely critical parameter is the biocompatibility of hydrogels.

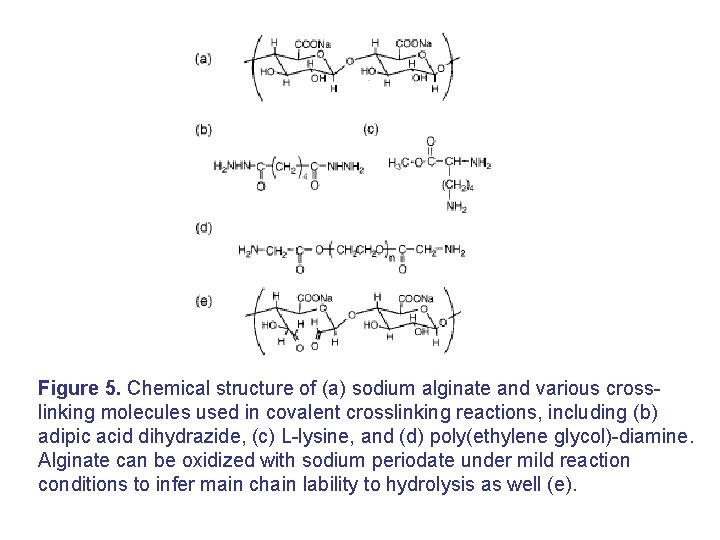
B. Example: Alginate • Alginate is a well-known biomaterial obtained from brown algae and is widely used for drug delivery and in tissue engineering due to its biocompatibility, low toxicity, relatively low cost, and simple gelation with divalent cations such as Ca 2+, Mg 2+, Ba 2+, and Sr 2+ (Figure 5 a). • Alginate has found uses to date as an injectable cell delivery vehicle as well as wound dressing, dental impression, and immobilization matrix. • Alginate gel beads have also been prepared and used for transplantation of chondrocytes, hepatocytes, and islets of Langerhans to treat diabetes. • Alginate itself may not be an ideal material because it degrades via a process involving loss of divalent ions into the surrounding medium, and subsequent dissolution. This process is generally uncontrollable and unpredictable. Therefore, covalent cross-linking with various types of molecules and different cross-linking densities has been attempted to precisely control the mechanical and/or swelling properties of alginate gels (Figure 5 b-d).

Figure 5. Chemical structure of (a) sodium alginate and various crosslinking molecules used in covalent crosslinking reactions, including (b) adipic acid dihydrazide, (c) L-lysine, and (d) poly(ethylene glycol)-diamine. Alginate can be oxidized with sodium periodate under mild reaction conditions to infer main chain lability to hydrolysis as well (e).

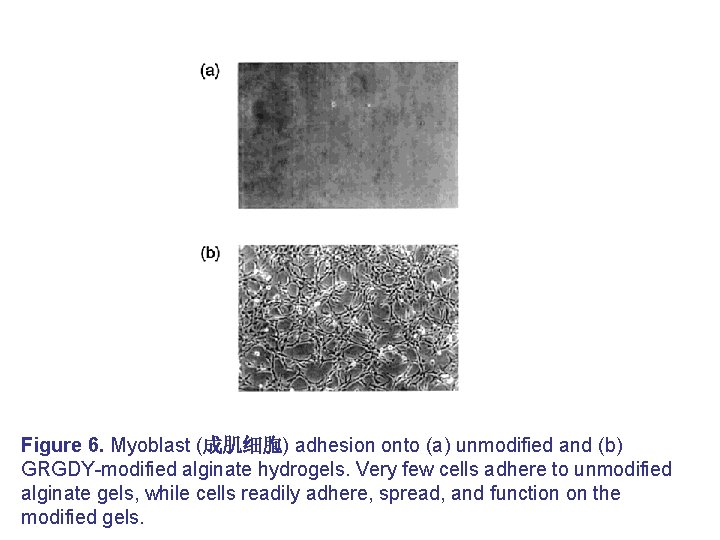
• Another potential limitation in using alginate gels in tissue engineering is the lack of cellular interaction. • Alginate is known to discourage protein adsorption due to its hydrophilic character, and it is unable to specifically interact with mammalian cells. • Therefore, alginate has been modified with lectin, a carbohydrate specific binding protein, to enhance ligand-specific binding properties. • An RGD-containing cell adhesion ligand has also been covalently coupled to alginate gels to enhance cell adhesion. • These modified alginate gels have been demonstrated to provide for the adhesion, proliferation, and expression of differentiated phenotype of skeletal muscle cells (Figure 6).

Figure 6. Myoblast (成肌细胞) adhesion onto (a) unmodified and (b) GRGDY-modified alginate hydrogels. Very few cells adhere to unmodified alginate gels, while cells readily adhere, spread, and function on the modified gels.

Progress in tissue engineering

I. Genetically Engineered Polypeptides Hydrogels • In brief, one may insert DNA templates of predetermined sequences into the genome of bacteria and produce polypeptides with predetermined structure and controlled properties. • This method enables one to design and engineer various sequences of polypeptides with known functions, including elasticity, stiffness, degradation, and cellular interactions. • Silk-like polypeptides have been prepared by this technique, and a Gly-Alarich sequence has been introduced into these artificial proteins to form reversible hydrogels in response to environmental changes of p. H or temperature.

• Elastin-mimetic polypeptides, comprised of a Gly-Val-Pro-Gly-any amino acid sequence, have also been studied and considered to have potential for artificial extracellular matrices in tissue engineering. • This technique is not appropriate to economically produce biomaterials in large scale at the current time, and one is also unable to easily modify the polymer product as any change requires re-engineering of the entire system.

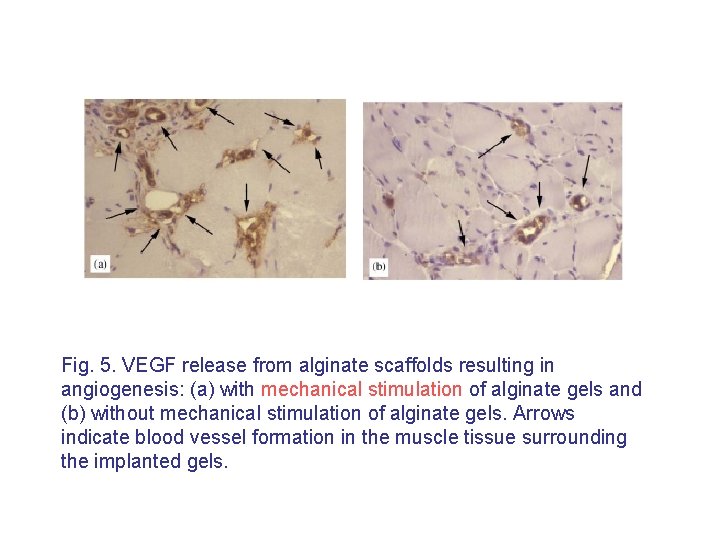
II. Appropriate mechanical properties of hydrogels • Another critical issue in the design of hydrogels for tissue engineering is that many tissues (e. g. , bone, muscle, and blood vessels) exist in a mechanically dynamic environment. • Many current hydrogels do not possess appropriate mechanical properties for these mechanically dynamic environments. • It has been previously demonstrated that mechanical signals result in alterations of cellular structure, metabolism, and transcription and/or translation of various genes. • The gels must appropriately convey the mechanical signals to these incorporated cells. It have recently reported that mechanical signals may be exploited to control growth factor release from hydrogels, and this could provide a novel approach to guide tissue formation in mechanically stressed environments.

Fig. 5. VEGF release from alginate scaffolds resulting in angiogenesis: (a) with mechanical stimulation of alginate gels and (b) without mechanical stimulation of alginate gels. Arrows indicate blood vessel formation in the muscle tissue surrounding the implanted gels.

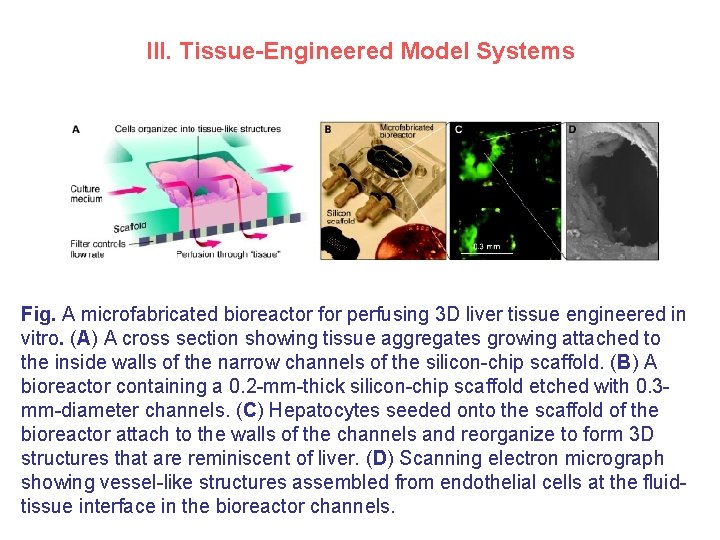
III. Tissue-Engineered Model Systems Fig. A microfabricated bioreactor for perfusing 3 D liver tissue engineered in vitro. (A) A cross section showing tissue aggregates growing attached to the inside walls of the narrow channels of the silicon-chip scaffold. (B) A bioreactor containing a 0. 2 -mm-thick silicon-chip scaffold etched with 0. 3 mm-diameter channels. (C) Hepatocytes seeded onto the scaffold of the bioreactor attach to the walls of the channels and reorganize to form 3 D structures that are reminiscent of liver. (D) Scanning electron micrograph showing vessel-like structures assembled from endothelial cells at the fluidtissue interface in the bioreactor channels.

• Tissue engineering can be applied to the development of drugs to treat many diseases that could be prevented or even cured if such drugs were available today. • The greatest impact of tissue engineering in the coming decade will be for designing in vitro physiological models to study disease pathogenesis and for developing molecular therapeutics. • For example, the creation of tissues containing hierarchical cell interactions under appropriate mechanical stresses (including perfusion shear as found in the microcirculation) will take in vitro systems even closer to living tissues.


• In 1981, a skin equivalent consisting of a silicone cover a sponge of porous collagen cross-linked with chondroitin was used successfully to treat severe burns. • In this decade, several products reached the market. • In 1996, Integra’s Artificial Skin was approved for as an in vivo, nonbiological tissue regeneration product. • In 1997, Apligraf, produced by Organogenesis, is the first manufactured living human organ, specifically multilayered skin, to be recommended for approval by an advisory panel to the FDA. Apligraf was approved for the treatment of venous leg ulcers in Canada, and was launched there in August 1997 by Novartis. Pharmaceuticals Canada (Dorval, Canada). • In 1998, the General and Plastic Surgery Devices Advisory Panel to the US Food and Drug Administration recommended unconditional approval of Apligraf (Graftskin) Human Skin Equivalent for the treatment of venous leg ulcers.

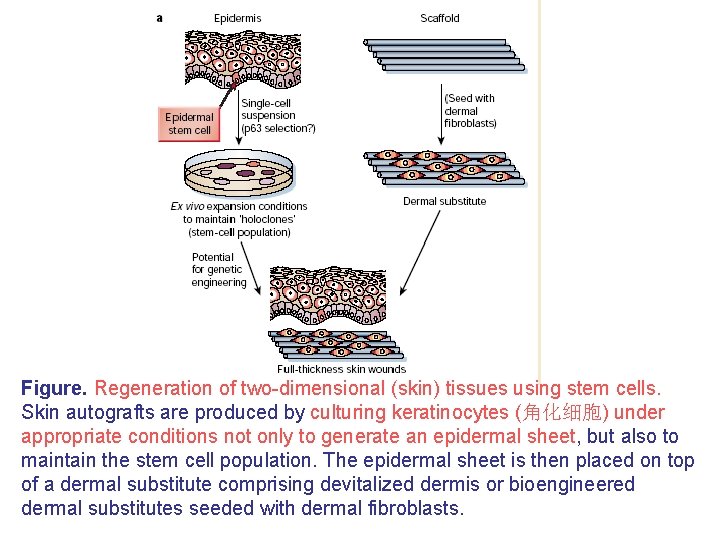
Figure. Regeneration of two-dimensional (skin) tissues using stem cells. Skin autografts are produced by culturing keratinocytes (角化细胞) under appropriate conditions not only to generate an epidermal sheet, but also to maintain the stem cell population. The epidermal sheet is then placed on top of a dermal substitute comprising devitalized dermis or bioengineered dermal substitutes seeded with dermal fibroblasts.


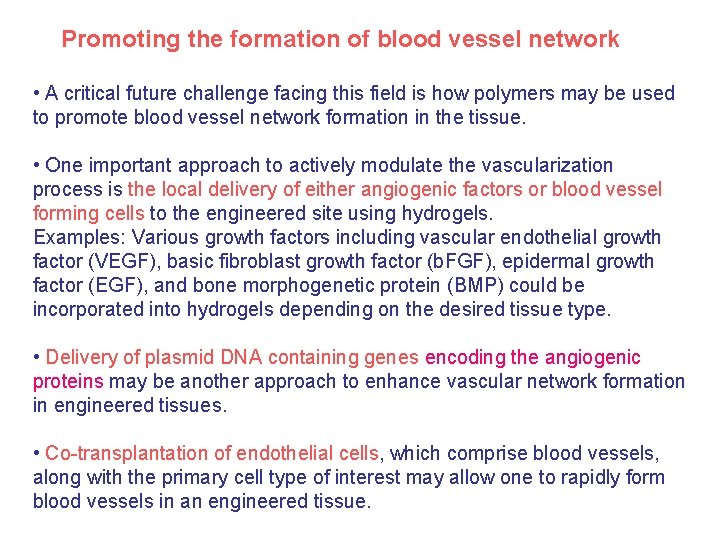
Promoting the formation of blood vessel network • A critical future challenge facing this field is how polymers may be used to promote blood vessel network formation in the tissue. • One important approach to actively modulate the vascularization process is the local delivery of either angiogenic factors or blood vessel forming cells to the engineered site using hydrogels. Examples: Various growth factors including vascular endothelial growth factor (VEGF), basic fibroblast growth factor (b. FGF), epidermal growth factor (EGF), and bone morphogenetic protein (BMP) could be incorporated into hydrogels depending on the desired tissue type. • Delivery of plasmid DNA containing genes encoding the angiogenic proteins may be another approach to enhance vascular network formation in engineered tissues. • Co-transplantation of endothelial cells, which comprise blood vessels, along with the primary cell type of interest may allow one to rapidly form blood vessels in an engineered tissue.

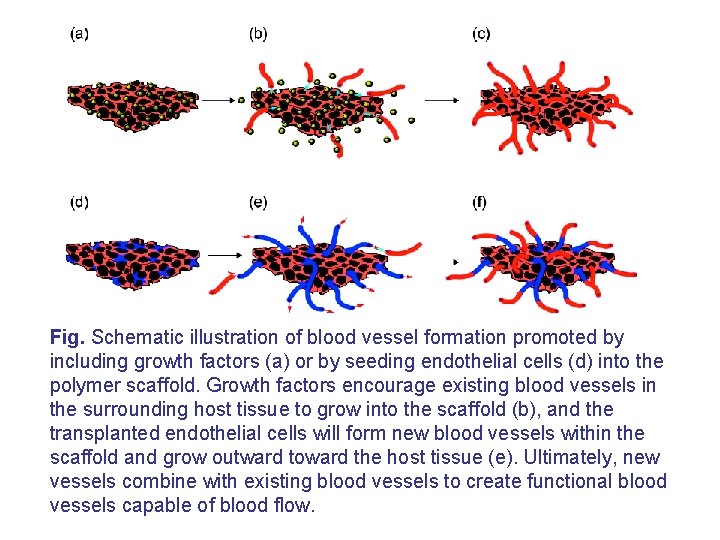
Fig. Schematic illustration of blood vessel formation promoted by including growth factors (a) or by seeding endothelial cells (d) into the polymer scaffold. Growth factors encourage existing blood vessels in the surrounding host tissue to grow into the scaffold (b), and the transplanted endothelial cells will form new blood vessels within the scaffold and grow outward toward the host tissue (e). Ultimately, new vessels combine with existing blood vessels to create functional blood vessels capable of blood flow.


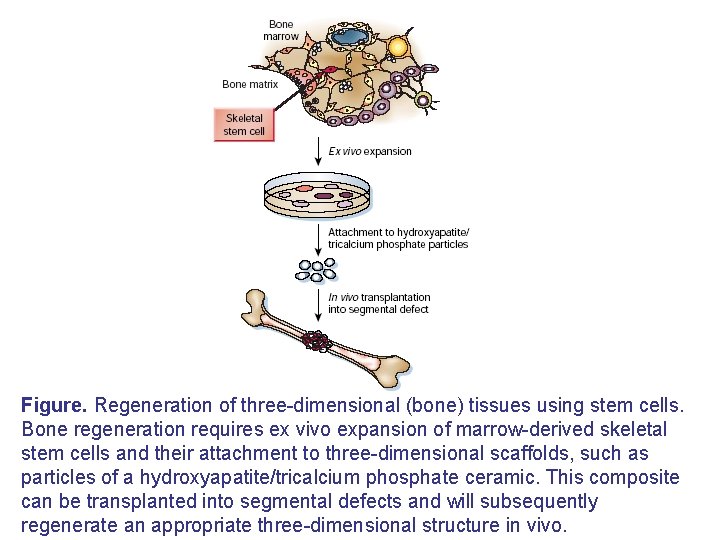
Figure. Regeneration of three-dimensional (bone) tissues using stem cells. Bone regeneration requires ex vivo expansion of marrow-derived skeletal stem cells and their attachment to three-dimensional scaffolds, such as particles of a hydroxyapatite/tricalcium phosphate ceramic. This composite can be transplanted into segmental defects and will subsequently regenerate an appropriate three-dimensional structure in vivo.

 Punch method pharmacy definition
Punch method pharmacy definition Shelf life of drug
Shelf life of drug Dosage forms and drug delivery systems
Dosage forms and drug delivery systems Dosage forms and drug delivery systems
Dosage forms and drug delivery systems Content management system database
Content management system database Introduction to healthcare delivery systems
Introduction to healthcare delivery systems Carrier content and real content in esp
Carrier content and real content in esp Static content vs dynamic content
Static content vs dynamic content Methods of adulteration of crude drugs
Methods of adulteration of crude drugs Algorithmic nuggets in content delivery
Algorithmic nuggets in content delivery Microsoft ajax content delivery network
Microsoft ajax content delivery network New drug delivery system
New drug delivery system Transdermal drug delivery system
Transdermal drug delivery system Ocular inserts
Ocular inserts Advantages of controlled drug delivery system
Advantages of controlled drug delivery system Introduction to dynamic web content
Introduction to dynamic web content Introduction to dynamic web content
Introduction to dynamic web content Content introduction
Content introduction Delivery approaches accenture recommends
Delivery approaches accenture recommends Social service delivery systems
Social service delivery systems Decision support systems and intelligent systems
Decision support systems and intelligent systems Dicapine
Dicapine Embedded systems vs cyber physical systems
Embedded systems vs cyber physical systems Engineering elegant systems: theory of systems engineering
Engineering elegant systems: theory of systems engineering Introduction to system analysis and design
Introduction to system analysis and design Introduction to recommender systems
Introduction to recommender systems Recommender systems an introduction
Recommender systems an introduction I/o device management in operating system
I/o device management in operating system Chapter 3 lesson 1 introduction to global systems
Chapter 3 lesson 1 introduction to global systems Sap systems introduction
Sap systems introduction Introduction to radar systems
Introduction to radar systems Introduction to information systems 6th edition
Introduction to information systems 6th edition Erp system overview
Erp system overview Principles of information systems
Principles of information systems Multimedia system definition
Multimedia system definition Introduction to information systems 3rd edition
Introduction to information systems 3rd edition Introduction to information systems 5th edition
Introduction to information systems 5th edition Introduction to artificial intelligence and expert systems
Introduction to artificial intelligence and expert systems Introduction to signals and systems
Introduction to signals and systems Introduction to ems
Introduction to ems User productivity system
User productivity system Introduction to accounting information system
Introduction to accounting information system An introduction to database systems
An introduction to database systems Introduction to global systems
Introduction to global systems Air conditioning basics ppt
Air conditioning basics ppt Introduction to manufacturing systems
Introduction to manufacturing systems Introduction to embedded systems lee seshia solution manual
Introduction to embedded systems lee seshia solution manual Introduction to electrical power systems
Introduction to electrical power systems Introduction to erp systems
Introduction to erp systems Operating system
Operating system System software: an introduction to systems programming
System software: an introduction to systems programming Introduction to digital control
Introduction to digital control 101101-100111
101101-100111 Mcit 593
Mcit 593 Introduction to low voltage systems
Introduction to low voltage systems Introduction to information systems 3rd edition
Introduction to information systems 3rd edition Digital control
Digital control 15-213 introduction to computer systems
15-213 introduction to computer systems Systems engineering consulting firms
Systems engineering consulting firms Introduction to information systems 3rd edition
Introduction to information systems 3rd edition 15-213 introduction to computer systems
15-213 introduction to computer systems Essay structure
Essay structure Examples of language objectives
Examples of language objectives Content words and function words
Content words and function words Open class words examples
Open class words examples What are content buckets
What are content buckets Content specific vocabulary
Content specific vocabulary Content validity
Content validity Conclusion validity definition
Conclusion validity definition Forms of validity
Forms of validity Mobile user-generated content
Mobile user-generated content Tributaries of pulmonary vein
Tributaries of pulmonary vein Web content managment
Web content managment Poor insight and judgement examples
Poor insight and judgement examples Muscles of scapula region
Muscles of scapula region Content pillars for instagram
Content pillars for instagram Ternary cam
Ternary cam How to write content points
How to write content points Declarative content clause
Declarative content clause Disclaimer for sensitive content
Disclaimer for sensitive content Gauginf
Gauginf Security content automation protocol (scap)
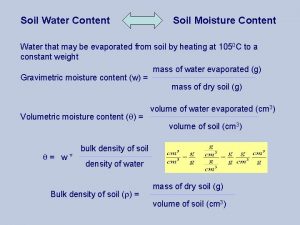
Security content automation protocol (scap) Water content of soil
Water content of soil 55x2080
55x2080 Stress sentence
Stress sentence Semantic content enrichment
Semantic content enrichment Prehn sign pronunciation
Prehn sign pronunciation Sharable content object
Sharable content object What is communicative achievement
What is communicative achievement Reviewing content strategies
Reviewing content strategies Which of the following is an example of quantitative
Which of the following is an example of quantitative Unified content strategy
Unified content strategy Content analysis interviews
Content analysis interviews Contoh content validity
Contoh content validity