Transdermal drug delivery systems Patch REFERENCES Novel drug
























- Slides: 52

Transdermal drug delivery systems Patch REFERENCES Ø Novel drug delivery systems, 2 nd edition, by Y. W. Chein page no. : 338 – 380. 1

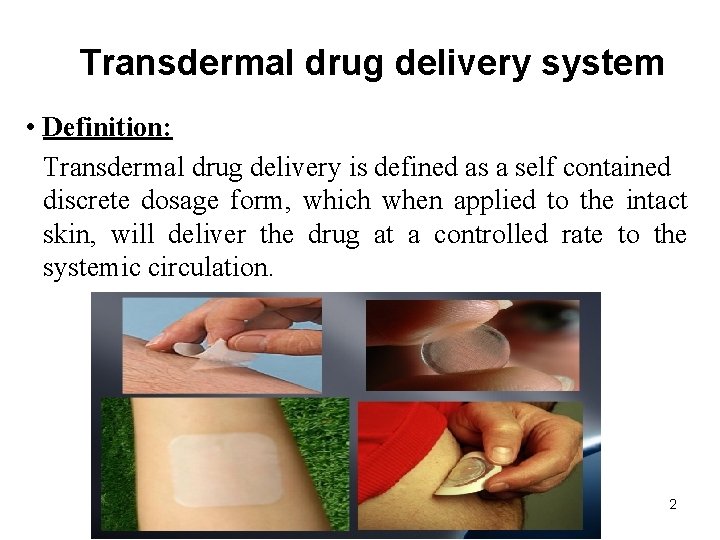
Transdermal drug delivery system • Definition: Transdermal drug delivery is defined as a self contained discrete dosage form, which when applied to the intact skin, will deliver the drug at a controlled rate to the systemic circulation. 2

POTENTIAL BENEFITS OF TRANSDERMAL DRUG DELIVERY (ADVANTAGES) • • • Easy to use. Avoid GIT absorption problems for drugs. Avoids First Pass hepatic metabolism of drugs. More improved and convenient patient compliance. Rapid termination in case of toxicity is possible. Self medication is possible. Reduces frequency of dosing. Maintains therapeutic level for 1 to 7 days. Controlled delivery resulting in more reliable and predictable blood levels. 3

DISADVANTAGES • • Daily dose of more than 10 mg is not possible. Local irritation is a major problem. Drug requiring high blood levels are unsuitable. Drug with long half life can not be formulated in TDDS. Uncomfortable to wear. May not be economical. Barrier function changes from person to person and within the same person. • Heat, cold, sweating (perspiring) and showering prevent the patch from sticking to the surface of the skin for more than one day. A new patch has to be applied daily. 4

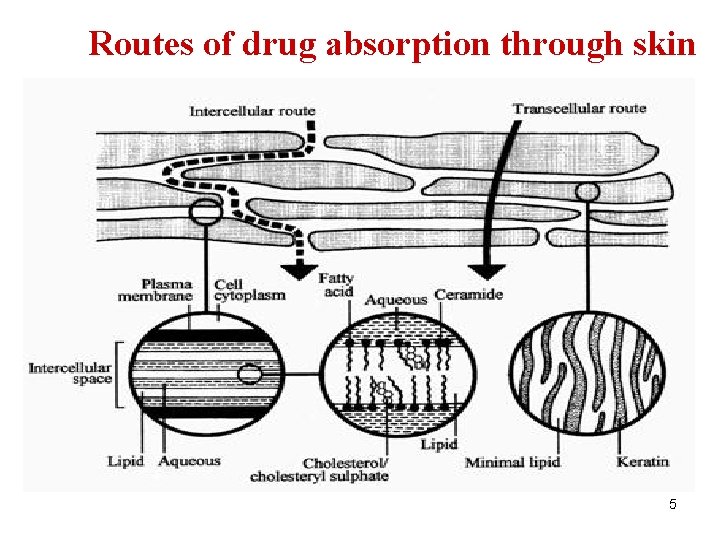
Routes of drug absorption through skin 5

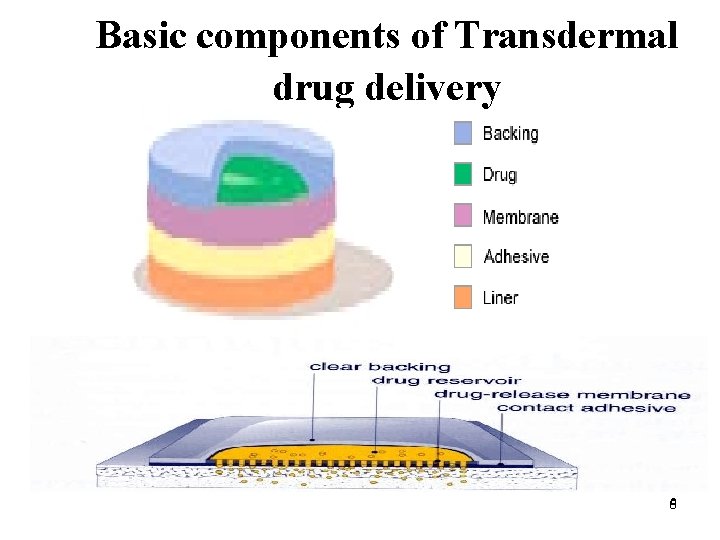
Basic components of Transdermal drug delivery 66

BASIC COMPONENTS OF TRANSDERMAL DRUG DELIVERY SYSTEM COMPONENT OF TRANSDERMAL DEVICE INCLUDE: 1) POLYMER MATRIX 2) THE DRUG 3) PERMEATION ENHANCER 4) OTHER EXCEPIENTS 7

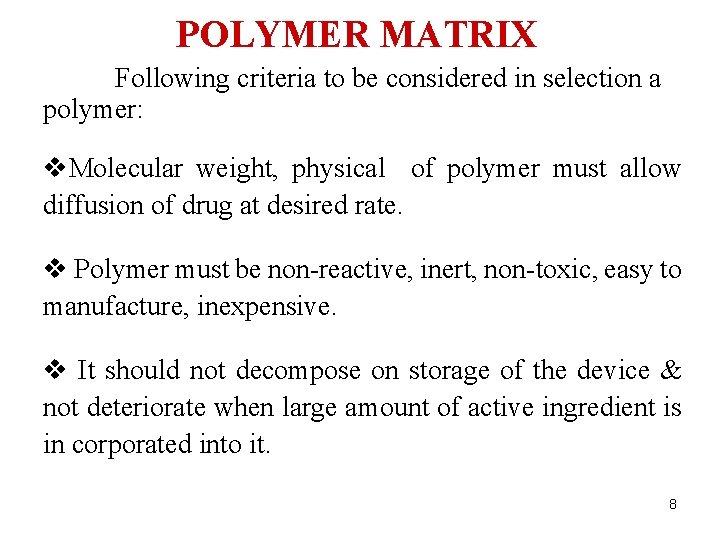
POLYMER MATRIX Following criteria to be considered in selection a polymer: v. Molecular weight, physical of polymer must allow diffusion of drug at desired rate. v Polymer must be non-reactive, inert, non-toxic, easy to manufacture, inexpensive. v It should not decompose on storage of the device & not deteriorate when large amount of active ingredient is in corporated into it. 8

LIST OF POLYMERS USED NATURAL POLYMERS: Cellulose derivatives, Zein, Gelatin, Shellac, Waxes, Gums & Natural rubber SYNTHETIC ELASTOMER POLYBUTADIENE: Polysiloxane, Silicon rubber, Nitrile, Acrylonitryle, Butyl rubber, Styrene butadiene rubber. SYNTHETIC POLYMER Poly vinyl alcohol, Poly vinyl chloride, Polyethylene, Poly propylene, Poly urea, PVP, Polymethacrylate 9

DRUG For successful developing transdermal delivery, drug should be chosen with great care physicochemical properties 1. Mol. wt. less than 1000 Daltons 2. Affinity for both lipophilic & hydrophilic phase 3. Drug should have low melting point 10

Ideal molecular properties for drug penetration - A low molecular weight (generally less than 500 Daltons) An adequate solubility in oil and water A balanced partition coefficient A low melting point Potent drug (maximum 10 mg/day) Half life of drug should be short. Non irritant to skin. Drug prone to ‘first pass effect’ and which degrade in GIT are ideal candidate.

DRUG Hormones • Estradiol and progesterone • Avoid hepatic metabolism Cardiovascular drugs • Hypertension and angina • Betablockers : timolol, propranolol • Hepatic metabolism of propranolol Analgesics • Control of chronic pain by transdermal therapy Antihistamines • Treatment of allergy E. X Chlorpheniramine • Maintain histamine-receptor antagonism while reducing CNS side effects such as drowsiness Central nervous system drugs • Physostigmine : cholinesterase inhibitors • To inhibit breakdown of acetylcholine by 30 to 40% over 4 days

PERMEATION ENHANCERS Optimization of Percutaneous Absorption: • Vehicle or device to maximize drug partition into the skin • Incorporate penetration enhancer into formulation ª penetration enhancer are the agents which promote the skin permeability by altering the skin as a barrier to the flux of desired penetrant. ª Flux J across the skin can be given by J= D. dc/dx D= diffusion coefficient C= concentration x=Spatial coordinate ª D is function of size, shape, flexibility of diffusing drug molecule 13

IDEAL CHARACTERISTIC OF PENETRATION ENHANCERS 1) IT SHOULD BE INERT 2) NON-TOXIC, NON- IRRITATING 3) ACTION SHOULD BE IMMEDIATE& PREDICTABLE 4) SHOULD NOT CAUSE REMOVAL OF BODY FLUID 5) SHOLD BE COMPATIBLE WITH DRUG& EXIPIENTS 6) A SUITABLE SOLVENT FOR DRUG 7) SPREAD WELL ON THE SKIN 8) COSMETICALLY ACCEPTABLE 9) ODORLESS, TASTELESS, COLORLESS & CHEAP 14

SOLVENTS The compounds increase penetration possibly by swelling the polar pathway and fluidizing the lipid e. g. . Methanol, pyrolidiones, propylene glycol, glycerol etc. . SURFACTANTS They enhance polar pathway transport of hydrophillic drugs • ANIONIC SURFACTANTS : Dioctyl sulpho succinate, SLS, decodemethyl sulphoxide • NON -IONIC SURFACTANTS : Pluronic F 127, pluronic F 58 15

The Theory for Activity of penetration enhancers Ä Interaction with the polar head groups of lipid via hydrogen and ionic bonding Ä Change in hydration sphere of lipids and affect the head region the packing at Ä Increase volume of the aqueous layer swelling and hydration Ä Protein modification- open up the dense keratin structure and make it more permeable 16

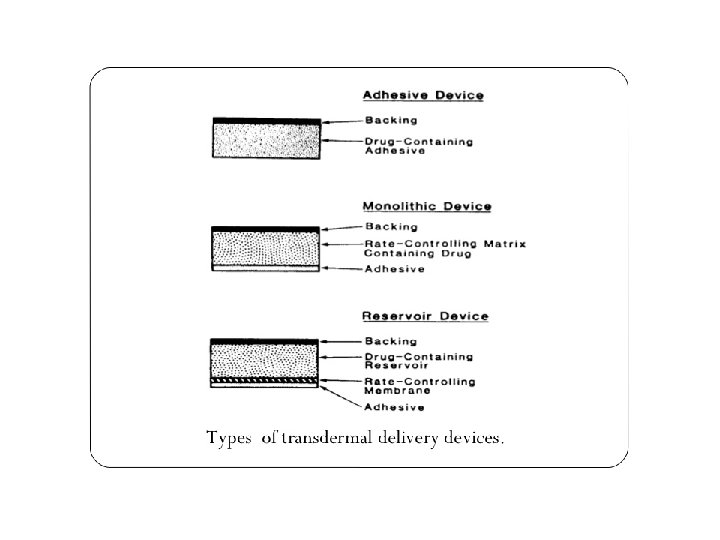
Backing membrane FThey are flexible and provide a good bond to the drug reservoir, prevent the drug from leaving the dosage form through top. FIt is an impermeable membrane that protects the product during the use on the skin. a Contains formulation throughout shelf-life and during wear period a Must be compatible with formulation (non adsorptive) a Printable FE. g. : Metallic plastic laminate , plastic backing with adsorbent pad adhesive foam pad. 17 17

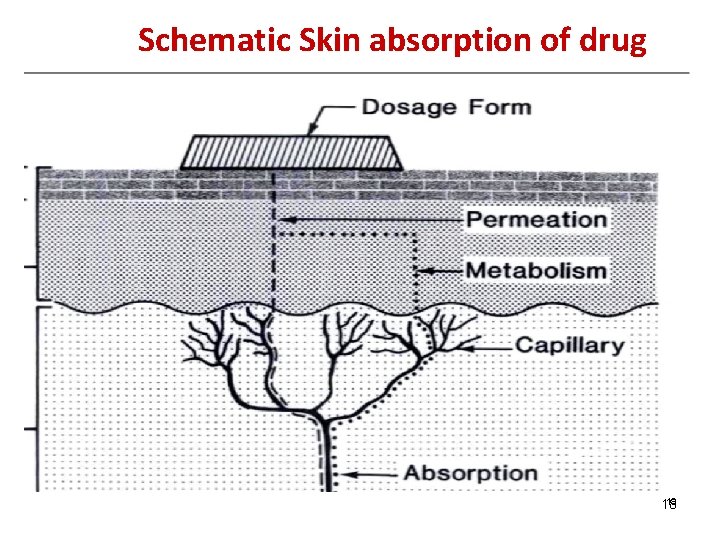
Schematic Skin absorption of drug 18 18

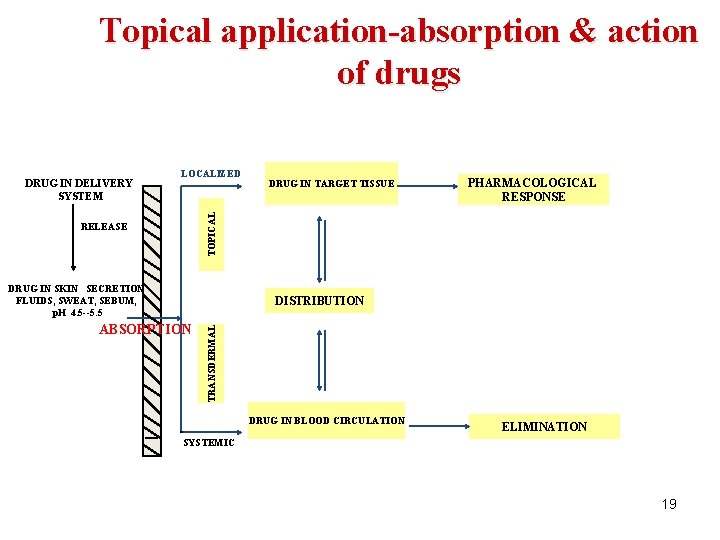
Topical application-absorption & action of drugs LOCALIZED DRUG IN TARGET TISSUE PHARMACOLOGICAL RESPONSE TOPICAL DRUG IN DELIVERY SYSTEM RELEASE DRUG IN SKIN SECRETION FLUIDS, SWEAT, SEBUM, p. H 4. 5 --5. 5 ABSORPTION TRANSDERMAL DISTRIBUTION DRUG IN BLOOD CIRCULATION ELIMINATION SYSTEMIC 19

FORMULATION APPROACHES FOR DEVELOPMENT OF TRANSDERMAL DRUG DELIVERY SYSTEM 20

TYPES OF FORMULATION Ø PLATFORM FOR THE DRUG: • Liquids • Semisolids : ointments and gels • Non flowing material That is … ÊPolymeric film or rubbery gels and Ê Solid-state platform 21

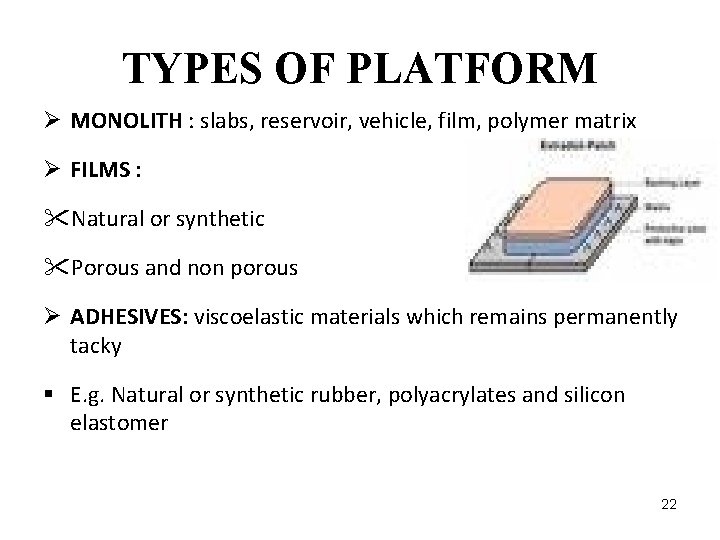
TYPES OF PLATFORM Ø MONOLITH : slabs, reservoir, vehicle, film, polymer matrix Ø FILMS : "Natural or synthetic "Porous and non porous Ø ADHESIVES: viscoelastic materials which remains permanently tacky § E. g. Natural or synthetic rubber, polyacrylates and silicon elastomer 22


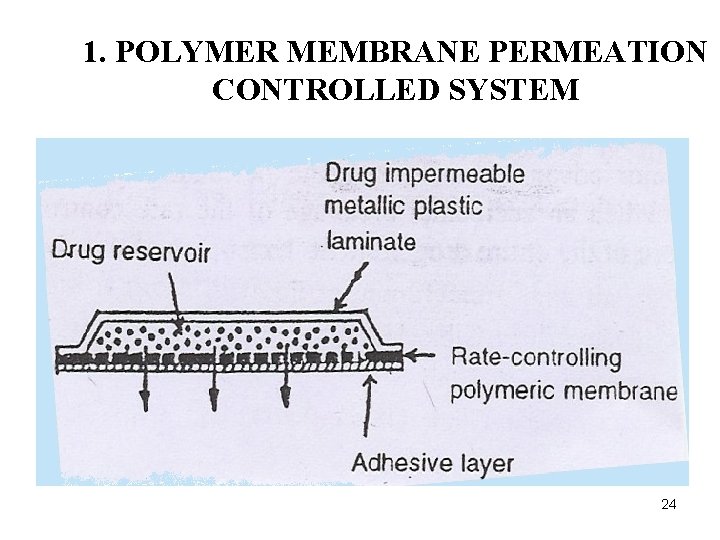
1. POLYMER MEMBRANE PERMEATION CONTROLLED SYSTEM 24

1. POLYMER MEMBRANE PERMEATION CONTROLLED SYSTEM Ø The drug reservoir is sandwiched between a drug impermeable backing laminate and a rate controlling polymeric membrane. Ø The drug molecules are permitted to release only through the membrane. Ø The drug reservoir compartment: The drug is: 1. Dispersed in solid polymer matrix (polyisobutylene) 2. Suspended in unleachable viscous liquid (silicone fluid) to form paste like suspension. 3. Dissolved in a releasable solvent (alkyl alcohol) to form a 25 clear drug solution

Ø The membrane: Ø Microporous or non porous ploymeric (ethylene vinyl acetate copolymer). Ø A thin layer of drug compatible, hypoallergenic adhesive polymer e. g. Silicon or polyacrylet adhesive may be applied to the external surface. Ø Rate of drug release affect by varying the polymer composition, permeability coefficient and thickness of rate limiting membrane and adhesive.

Ø Accidental breakage of the rate controlling membrane can result in dose dumping or a rapid release of the entire drug content. E. g. § Nitroglycerine releasing trans dermal system for once a day medication for angina 27

§ Scopolamine-releasing transdermal system for 72 hr. prophylaxis of motion sickness. § Clonidine releasing transdermal system for 7 day therapy of hypertension. § Estradiol-releasing transdermal system for treatment of menopausal syndrome for 3 -4 days. 28

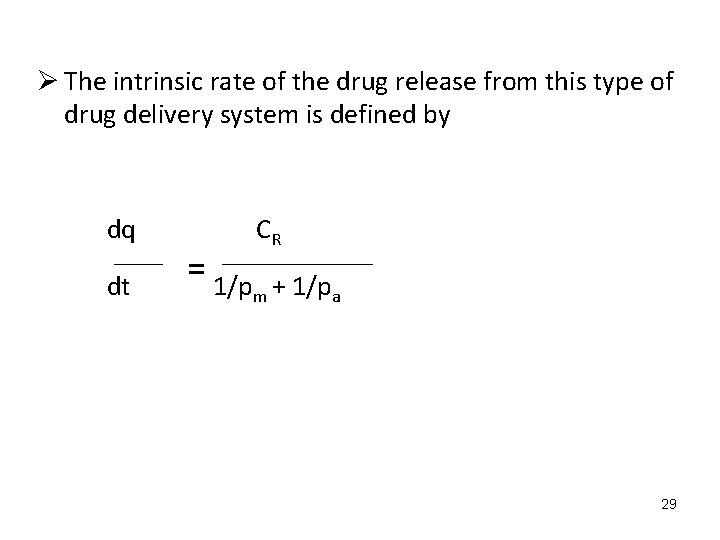
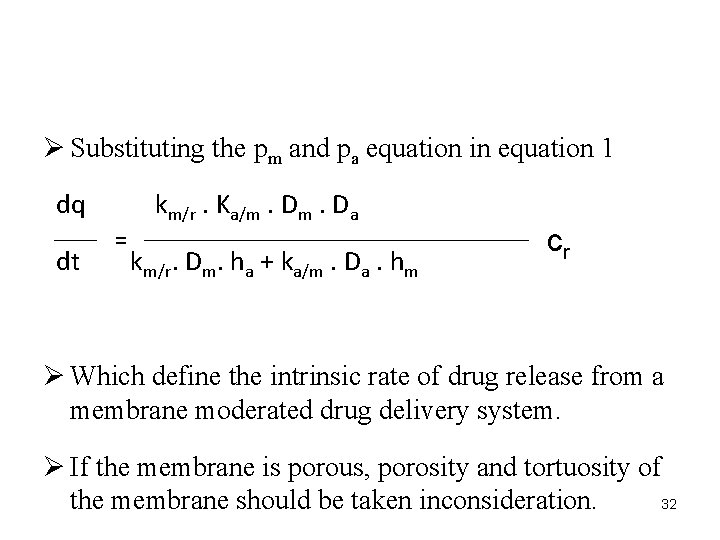
Ø The intrinsic rate of the drug release from this type of drug delivery system is defined by dq CR dt = 1/p + 1/p m a 29

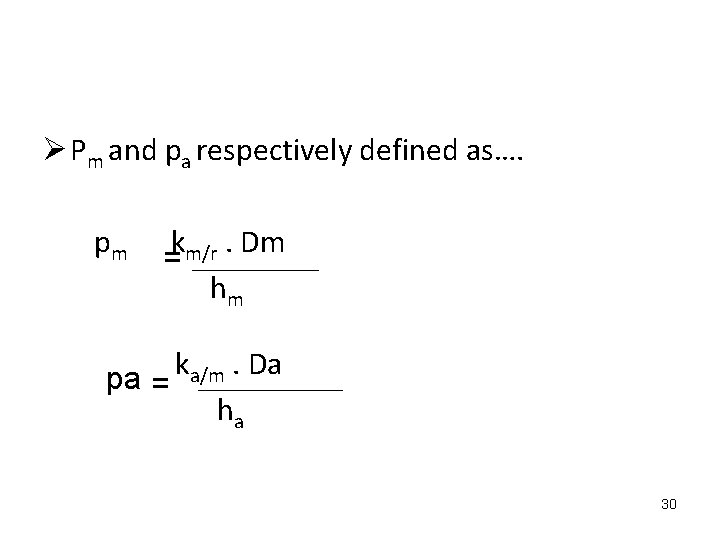
Ø Pm and pa respectively defined as…. pm km/r. Dm = hm k. Da a/m pa = ha 30

Ø Where, " Km/r and ka/m are the partition coefficient for the interfacial partitioning of the drug from reservoir to the membrane and from the membrane to adhesive layer respectively. " Dm and Da are diffusion coefficient and " hm and ha are thickness 31

Ø Substituting the pm and pa equation in equation 1 dq dt km/r. Ka/m. Da = km/r. Dm. ha + ka/m. Da. hm cr Ø Which define the intrinsic rate of drug release from a membrane moderated drug delivery system. Ø If the membrane is porous, porosity and tortuosity of 32 the membrane should be taken inconsideration.

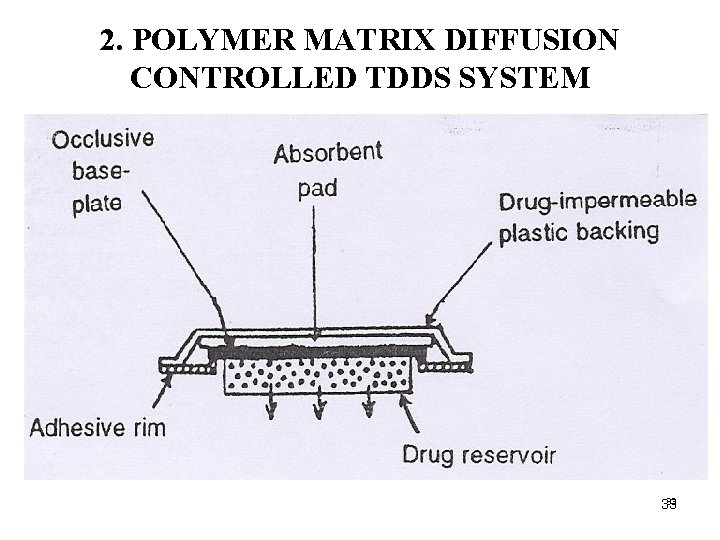
2. POLYMER MATRIX DIFFUSION CONTROLLED TDDS SYSTEM 33 33

Ø The drug reservoir: Ø Homogeneous dispersion of the drug solids in hydrophilic or lipophilic polymer matrix, and the medicated polymer is then molded into medicated discs with defined surface area and thickness. Ø Then mounted onto impermeable plastic backing. Ø The adhesive polymer is applied to the circumference of the patch.

Ø E. g. of this type of system is nitro-dur I and nitrodur II. for continuous transdermal fusion of nitroglycerine at a daily dose of 0. 5 mg/cm 2 for therapy of angina pectoris. Ø Nitro dur II is modified version of I in which the drug is dispersed in acrylic based polymer adhesive with a resinous cross linking agent which result in much thinner and more elegant patch. 35

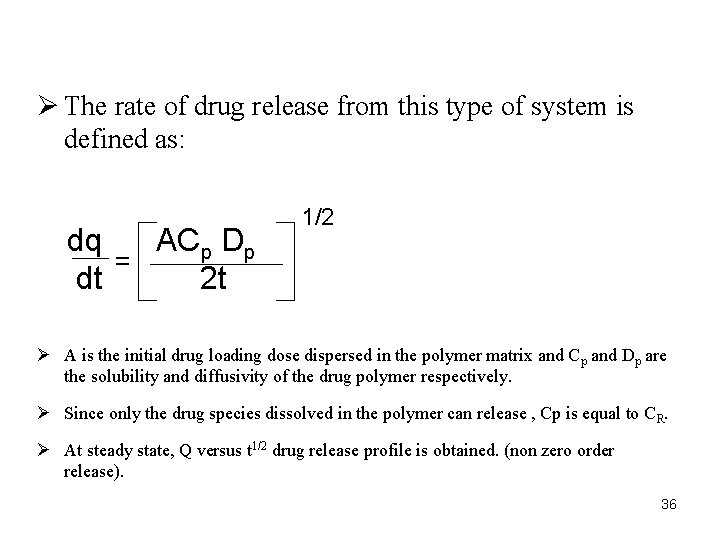
Ø The rate of drug release from this type of system is defined as: dq ACp Dp = dt 2 t 1/2 Ø A is the initial drug loading dose dispersed in the polymer matrix and Cp and Dp are the solubility and diffusivity of the drug polymer respectively. Ø Since only the drug species dissolved in the polymer can release , Cp is equal to CR. Ø At steady state, Q versus t 1/2 drug release profile is obtained. (non zero order release). 36

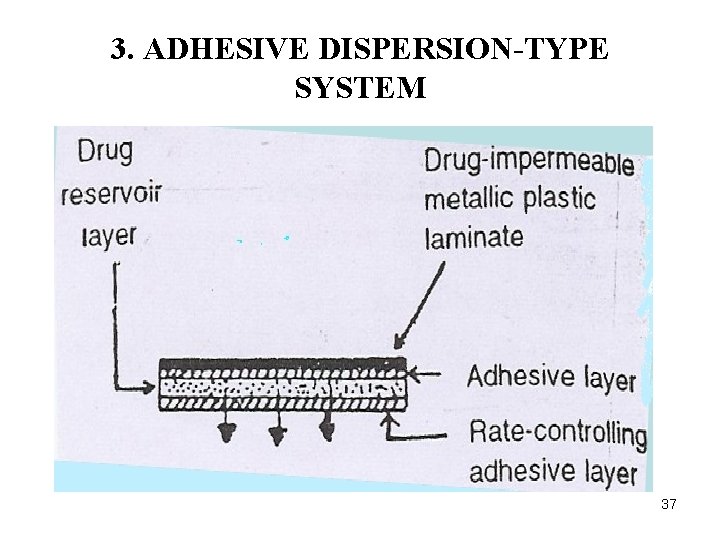
3. ADHESIVE DISPERSION-TYPE SYSTEM 37

§ e. g. of adhesive polymer is poly(isobutylene) or poly(Acrylet) adhesive § E. g. of this type of system is isosorbide dinitrate releasing transdermal therapeutic system for once a day medication of angina pectoris. Ø It is used for the administration of verapamil. 38

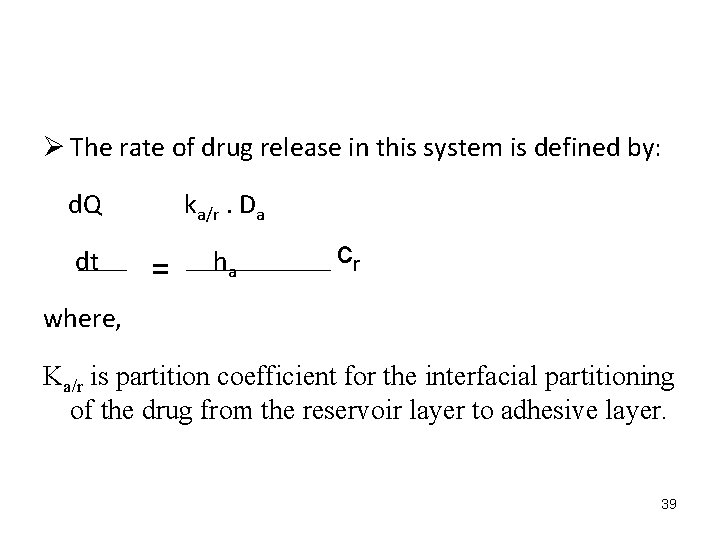
Ø The rate of drug release in this system is defined by: d. Q ka/r. Da dt ha = cr where, Ka/r is partition coefficient for the interfacial partitioning of the drug from the reservoir layer to adhesive layer. 39

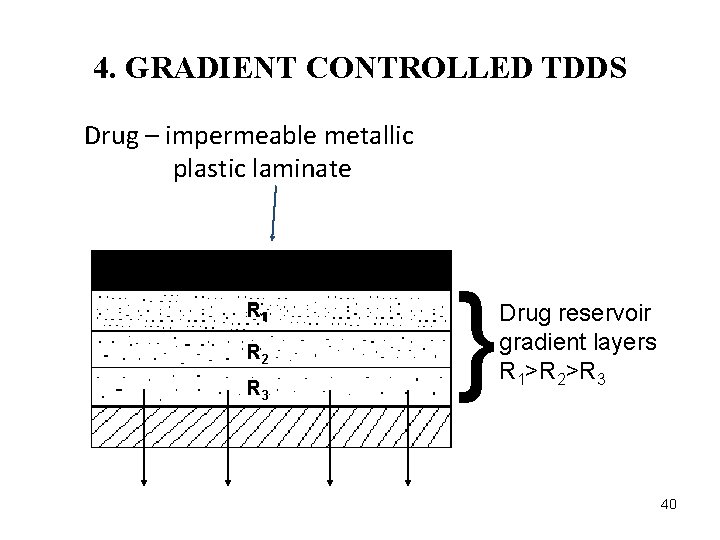
4. GRADIENT CONTROLLED TDDS Drug – impermeable metallic plastic laminate R 11 R 2 R 3 } Drug reservoir gradient layers R 1>R 2>R 3 40

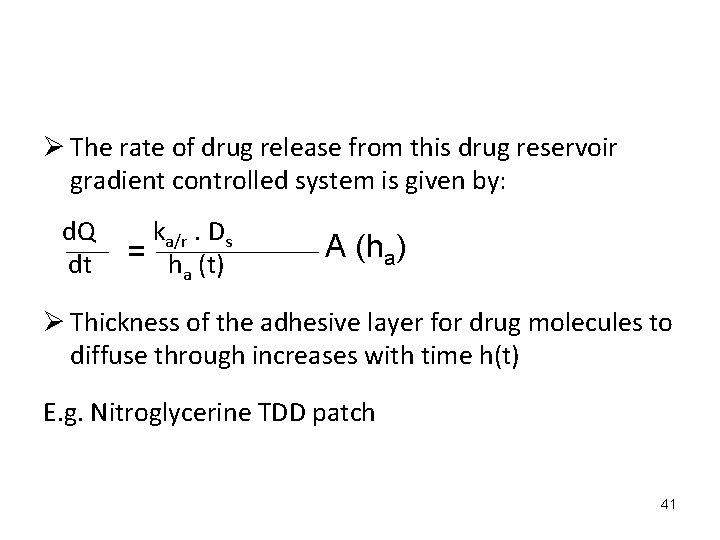
Ø The rate of drug release from this drug reservoir gradient controlled system is given by: d. Q dt ka/r. Ds = h (t) a A (ha) Ø Thickness of the adhesive layer for drug molecules to diffuse through increases with time h(t) E. g. Nitroglycerine TDD patch 41

Ø To overcome the non zero order release, the drug loading level in the reservoir is varied incrementally. Ø The thickness of the diffusional path increases with time, to compensate for this, the drug loading level in the multilaminate adhesive layer is increased Ø Deponit system: nitroglycerin releasing TDDS.

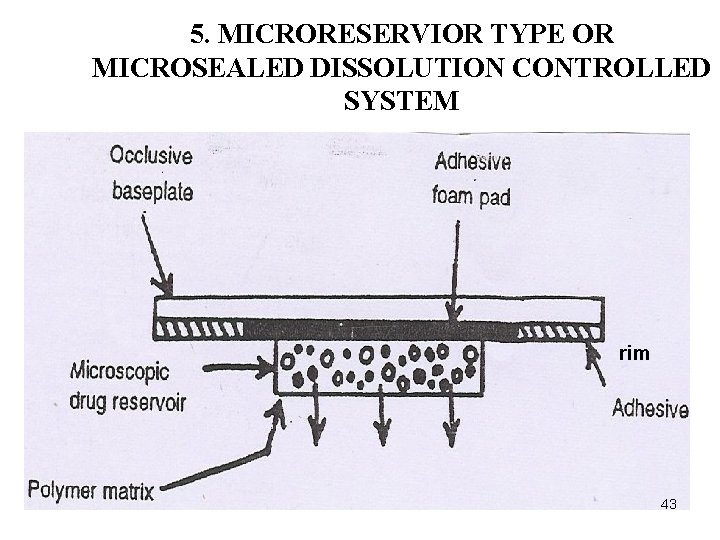
5. MICRORESERVIOR TYPE OR MICROSEALED DISSOLUTION CONTROLLED SYSTEM rim 43

Ø Hybrid of reservoir and matrix dispersion type. Ø The drug is suspended in aqueous solution of water miscible drug solubilizer, then dispersing the drug suspension in a lipophilic polymer by shear mechanical force to form thousands of microscopic drug reservoirs. Ø The medicated disc is then mounted at the center of an adhesive pad.

Ø It is successfully utilized in the preparation of nitrodisc, a nitroglycerine releasing trans dermal therapeutic system used in angina pectoris. Ø This system followed zero order release of drug without the danger of dose dumping. 45

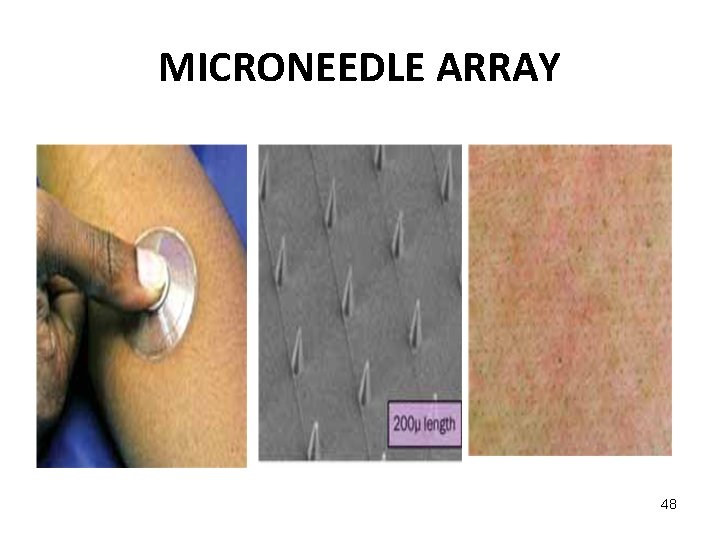
ADVANCED RESEARCHES MICROARRAY NEEDLE Ø Advanced micro-needle Patch transdermal system allowing continuous delivery through the skin of proteins and water-soluble drugs. 46

ADVANCED RESEARCHES • The device create painlessly micropores in the S. C. known as microstructered arrays or microneedles. • These devices have about 400 microneedles. • The solid silicone needles (coated with drug) or hollow metal needles (filled with drug solution) penetrate the horny layer without breaking it or stimulating nerves in deeper tissues. • Flux increase up to 1, 000 fold are reported. 47

MICRONEEDLE ARRAY 48

MICRO TRANS Applications : Ø Delivery of large proteins, fragile antibodies, and hormones. Ø Delivery of small molecules, particularly those with difficulty diffusing through skin layers. Ø Delivery of vaccines, both conventional and DNAbased. Ø Fluid sensing of glucose, hormones, blood gases, and therapeutic drug levels. 49

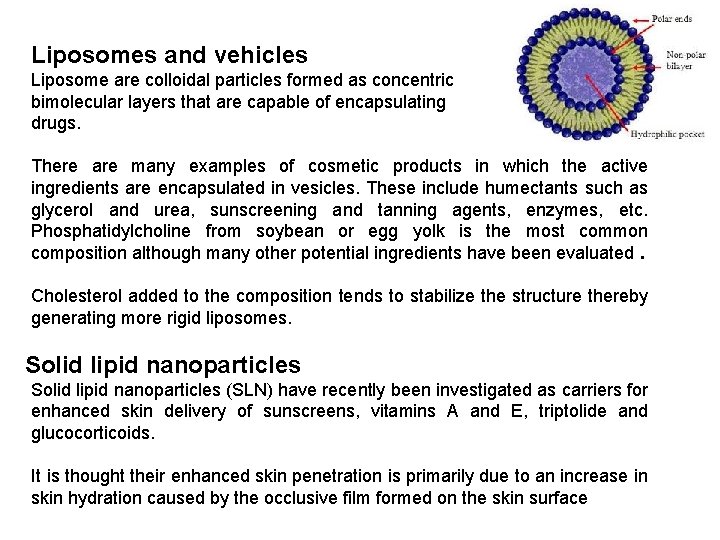
Liposomes and vehicles Liposome are colloidal particles formed as concentric bimolecular layers that are capable of encapsulating drugs. There are many examples of cosmetic products in which the active ingredients are encapsulated in vesicles. These include humectants such as glycerol and urea, sunscreening and tanning agents, enzymes, etc. Phosphatidylcholine from soybean or egg yolk is the most common composition although many other potential ingredients have been evaluated. Cholesterol added to the composition tends to stabilize the structure thereby generating more rigid liposomes. Solid lipid nanoparticles (SLN) have recently been investigated as carriers for enhanced skin delivery of sunscreens, vitamins A and E, triptolide and glucocorticoids. It is thought their enhanced skin penetration is primarily due to an increase in skin hydration caused by the occlusive film formed on the skin surface

Find an appropriate place to put the patch Ø Choose a dry, unbroken, non-hairy part of your skin. The buttocks, lower abdomen, lower back, and upper arm (outer part) are good choices. If the area you choose has body hair, clip (do not shave) the hair close to the skin with scissors. Ø the area is clean. If there is any oil or powder (from bath products, for example), the patch may not stick properly. Ø Attach the adhesive side of the patch to skin in the chosen area. 51

Find an appropriate place to put the patch 52