Lactate production and lactic acidosis Glucose enter muscle




































- Slides: 48

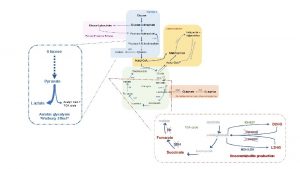
Lactate production and lactic acidosis : Glucose enter muscle post- prandially under the influence of insulin and is stored as glycogen. Because of the absence of (G 6 Pase) this glycogen can not be converted to glucose and can only supply local needs. Muscular contraction (activity), the glycogenolysis is stimulated by adrenalin and the resultant (G 6 P) is drawn upon by rapid glycolysis and by oxidation in TCA cycle to supply necessary energy

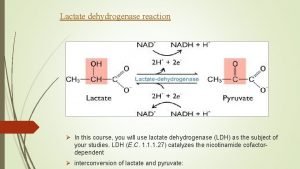
these conditions the rate of glycolysis may out strip the availability of oxygen and glycolytic products then exceed the immediate aerobic capacity to oxidize them The overall reaction for anaerobic glycolysis is : glucose→ 2 lactate + 2 H + 2 ATP the lactate is transported in the blood stream to the liver where it can be used for gluconeogensis providing further glucose for muscle ( Cori cycle ). During gluconeogensis hydrogen ion (H+) is also reutilized. Under aerobic conditions the liver consumes much more lactate than it produces. This physiological accumulation of lactic acid during muscle contraction is temporary phenomena, and rapidly disappears at rest when slowing glycolysis allows aerobic processes to "catch up ".

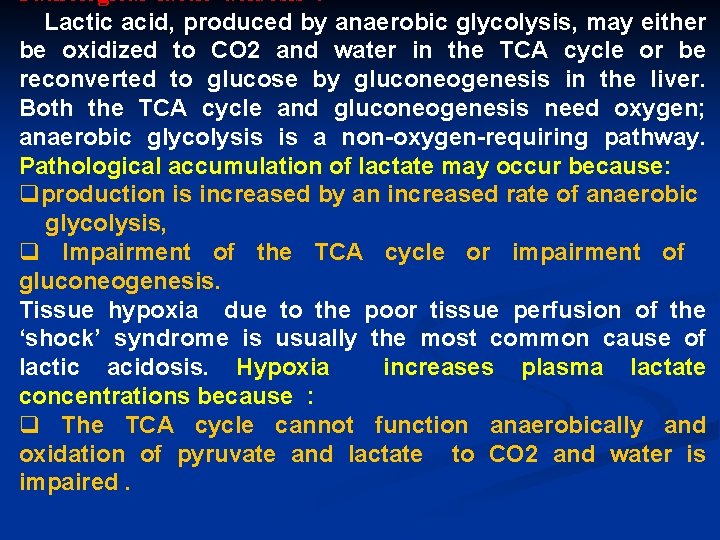
Pathological lactic acidosis : Lactic acid, produced by anaerobic glycolysis, may either be oxidized to CO 2 and water in the TCA cycle or be reconverted to glucose by gluconeogenesis in the liver. Both the TCA cycle and gluconeogenesis need oxygen; anaerobic glycolysis is a non-oxygen-requiring pathway. Pathological accumulation of lactate may occur because: qproduction is increased by an increased rate of anaerobic glycolysis, q Impairment of the TCA cycle or impairment of gluconeogenesis. Tissue hypoxia due to the poor tissue perfusion of the ‘shock’ syndrome is usually the most common cause of lactic acidosis. Hypoxia increases plasma lactate concentrations because : q The TCA cycle cannot function anaerobically and oxidation of pyruvate and lactate to CO 2 and water is impaired.

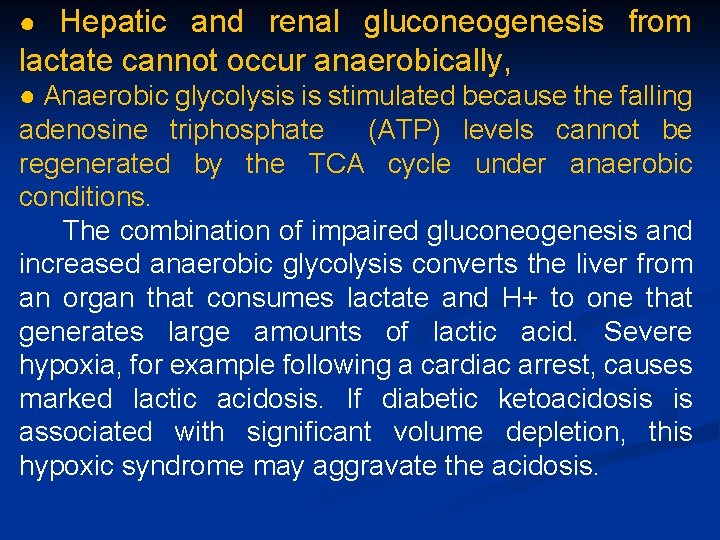
● Hepatic and renal gluconeogenesis from lactate cannot occur anaerobically, ● Anaerobic glycolysis is stimulated because the falling adenosine triphosphate (ATP) levels cannot be regenerated by the TCA cycle under anaerobic conditions. The combination of impaired gluconeogenesis and increased anaerobic glycolysis converts the liver from an organ that consumes lactate and H+ to one that generates large amounts of lactic acid. Severe hypoxia, for example following a cardiac arrest, causes marked lactic acidosis. If diabetic ketoacidosis is associated with significant volume depletion, this hypoxic syndrome may aggravate the acidosis.

Metabolic adaptations in prolong starvation : Atypical well-nourished 70 -Kg man has a fuel reserves of some 1600 Kcal in glycogen , 2400 Kcal in mobilizable protein and 135000 Kcal in triacylglycerol. The energy need for 24 hrs period range from about 1600 Kcal in the basal state to 6000 Kcal depending on the extent of the activity. Thus, stored fuels suffice to meet caloric needs in starvation for one to three months. However , the CHO reserves are exhausted in only a day. Even under these conditions the blood glucose level is maintained above 50 mg/dl.

The brain can not tolerate appreciably lower glucose level for even short periods. Hence, the first priority of metabolism in starvation is to provide sufficient glucose to the brain and other tissue (such as red blood cells) that are absolutely dependant on this fuel. However , precursor of glucose are not abundant. Most of the energy is stored in the fatty acyl moieties of triacylglycerol. Recall that fatty acid can not be convered into glucose because the acetyl Co-A cannot be transformed into pyruvate. The glycerol moiety of TG can be converted into glucose, but only limited amount is available.

The only other potential source of glucose is amino acid derived from breakdown of proteins. Muscle is the largest potential source of amino acid during starvation, however survival for most animals depend on being able to move rapidly, which requires a large muscle mass. Thus, the second priority of metabolism in starvation is to preserve protein , this accomplished by shifting the fuel being used from glucose to fatty acid and ketone bodies.

The metabolic change during the first day of starvation are like those after an overnight fast. The low blood glucose levels lead to decreased secretion of insulin and increase secretion of glucagon. The dominant metabolic processes are the mobilization of triacylglycerol in adipose tissue and gluconeogensis by the liver. The liver obtain energy for its own needs by oxidizing fatty acid released from adipose tissue , the concentration of acetyl-Co. A and citrate consequently increase which switches off glycolysis. the uptake of glucose by muscle is markedly diminished because of low insulin level , where as fatty acid enter freely.

F. A. for fuel. The β-oxidation of F. A by muscle halts the conversion of pyruvate into acetyl-Co. A. Hence, pyruvate , lactate and alanine are exported to the liver for conversion into glucose. proteolysis of muscle protein provide some of these 3 -carbon precursors of glucose. Glycerol derived from cleavage of triacylglycerol is another raw material for systhesis of glucose by the liver. The most important change after about 3 -days of starvation is that large amounts of acetoacetate and Bhydroxybutyrate (keton bodies) are formed by the liver , their synthesis from acetyl-Co. A increase markedly because the TCA-cycle is enable to oxidize all of the acyl units generated by the degradation of F. A.

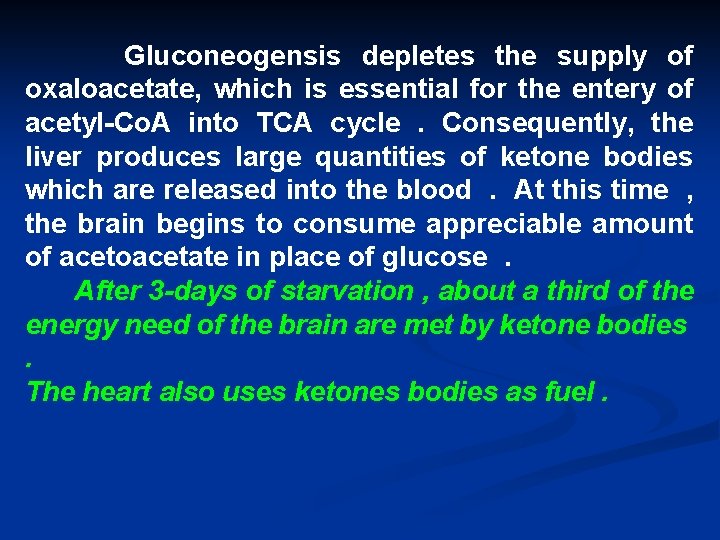
Gluconeogensis depletes the supply of oxaloacetate, which is essential for the entery of acetyl-Co. A into TCA cycle. Consequently, the liver produces large quantities of ketone bodies which are released into the blood. At this time , the brain begins to consume appreciable amount of acetoacetate in place of glucose. After 3 -days of starvation , about a third of the energy need of the brain are met by ketone bodies. The heart also uses ketones bodies as fuel.

These changes in fuel usage are accompanied by a rise in the level of ketone bodies in the plasma. After several weeks of starvation , ketone bodies become the major fuel of the brain. only 40 gm of glucose is needed per day for the brain , compared with about 120 gm in the first day of starvation. The effective conversion of F. A. into ketone bodies by the liver and their use by the brain markedly diminishes the need for glucose. hence , less muscle is degraded than in the first day of starvation. The breakdown of 20 gm of muscle compared with 75 gm early in starvation is most important for survival. the duration of starvation compatible with life is mainly determined by the size of tiacylglycerol depot.

Diabetes mellitus: Is a clinical syndrome characterized by hyperglycemia due to absolute or relative deficiency of insulin. It has be defined by W. H. O on the basis of laboratory finding as fasting plasma venous glucose (126 mg/dl) or more , or concentration of (200 mg/dl) or more at two hours after CHO meals or two hrs after ingestion of equivalent of 75 gm of glucose , even if the fasting concentration is normal. Sever cases have persistent hyperglycemia , it is divided into two types : A - Primary : 1 - ( type I ) : Previously called insulin-dependent diabetes mellitus, this is the term used to describe the condition in patients for whom insulin therapy is essential because they are prone to develop ketoacidosis. It usually presents during childhood or adolescence.

Individuals most at risk are those with human leucocyte antigen (HLA) types DR 3 and DR 4. it has been suggested that many cases follow a viral infection which has destroyed the β-cells of pancreatic islets. Autoantibodies to islet cells. Glutamic decarboxylase (GAD) are found in about 90 per cent of cases. There is a form of type 1 diabetes called idiopathic diabetes mellitus that is not autoimmune mediated but is strongly inherited and more common in black and Asian people. There is also LADA (latent autoimmune diabetes of adults), sometimes called slow-onset type 1 diabetes.

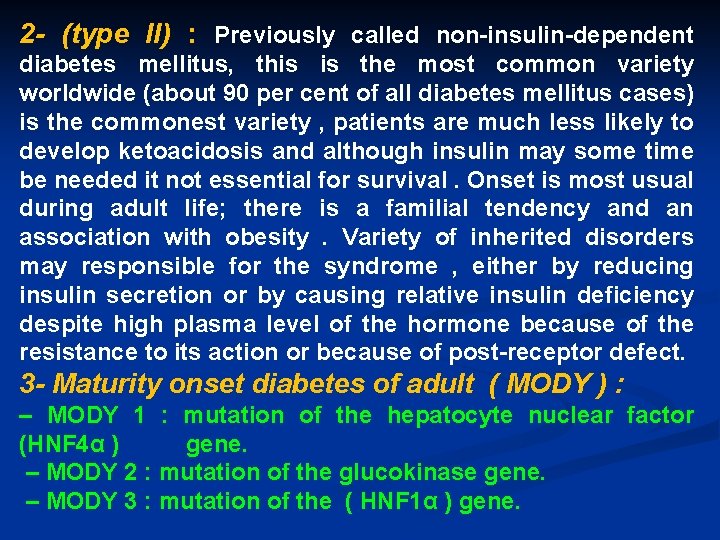
2 - (type II) : Previously called non-insulin-dependent diabetes mellitus, this is the most common variety worldwide (about 90 per cent of all diabetes mellitus cases) is the commonest variety , patients are much less likely to develop ketoacidosis and although insulin may some time be needed it not essential for survival. Onset is most usual during adult life; there is a familial tendency and an association with obesity. Variety of inherited disorders may responsible for the syndrome , either by reducing insulin secretion or by causing relative insulin deficiency despite high plasma level of the hormone because of the resistance to its action or because of post-receptor defect. 3 - Maturity onset diabetes of adult ( MODY ) : – MODY 1 : mutation of the hepatocyte nuclear factor (HNF 4α ) gene. – MODY 2 : mutation of the glucokinase gene. – MODY 3 : mutation of the ( HNF 1α ) gene.

4 - gestational diabetes mellitus. In the UK, about (4– 5 %) of pregnancies are complicated by gestational diabetes mellitus (GDM). It is associated with increased fetal abnormalities, for example high birth weight, cardiac defects and polyhydramnios. In addition, birth complications, maternal hypertension and the need for caesarean section may occur. If maternal diet/lifestyle factors fail to restore glucose levels, insulin is usually required to try to reduce the risk of these complications. Women at high risk for GDM include those who have had GDM before, have previously given birth to a high-birth weight baby, are obese, have a family history of diabetes mellitus.

B- secondary : Diabetes associated with other conditions include : Absolute insuline deficiency : due to pancreatic diseases (chronic pancreatitis , haemochromatosis , cystic fibrosis). Relative insulin deficiency : due to excessive growth hormone (acromegaly) or glucocorticoid secretion (cushing syndrome) or increase glucocorticoid level due to administration of steroid , drugs such as thiazide diuretics .

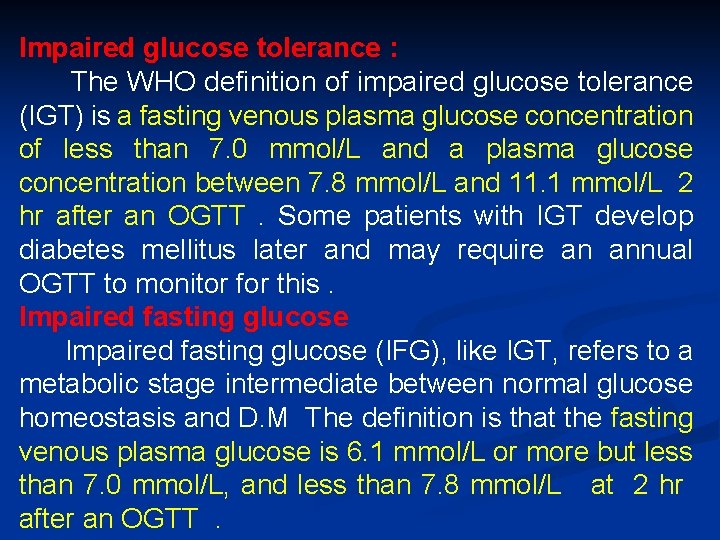
Impaired glucose tolerance : The WHO definition of impaired glucose tolerance (IGT) is a fasting venous plasma glucose concentration of less than 7. 0 mmol/L and a plasma glucose concentration between 7. 8 mmol/L and 11. 1 mmol/L 2 hr after an OGTT . Some patients with IGT develop diabetes mellitus later and may require an annual OGTT to monitor for this. Impaired fasting glucose (IFG), like IGT, refers to a metabolic stage intermediate between normal glucose homeostasis and D. M The definition is that the fasting venous plasma glucose is 6. 1 mmol/L or more but less than 7. 0 mmol/L, and less than 7. 8 mmol/L at 2 hr after an OGTT .

Subjects at risk of developing diabetes mellitus A strong family history of diabetes mellitus may suggest that an individual is at risk of developing diabetes mellitus (particularly type 2), as may a family history of GDM, IGT or IFG. Those with predisposing HLA types and autoimmune disease may be susceptible to developing type 1 diabetes. Type 2 diabetes is more common in certain racial groups, such as Afro Caribbean's, South Asians and Pacific Islanders. One of the reasons why type 2 diabetes is on the increase is the increasing tendency to obesity and central adiposity in urbanized and more sedentary

Insulin resistance syndrome or metabolic syndrome: There is an aggregation of lipid and non-lipid risk factors of metabolic origin. A particular cluster is known as the metabolic syndrome, syndrome X or Reaven’s syndrome and is closely linked to insulin resistance. One definition is the presence of three or more of the following features: q Abdominal obesity (waist circumference) - male more than 102 cm (40 in), - female more than 88 cm (35 in) q Fasting plasma triglycerides more than 1. 7 mmol/L. q Fasting plasma high-density lipoprotein (HDL) cholesterol : male less than 1. 0 mmol/L female less than 1. 3 mmol/L q Blood pressure more than or equal to 130/85 mm. Hg.

Plasma levels of insulin would be expected to be raised, that is, hyperinsulinaemia. Other associated features may include polycystic ovary syndrome, fatty liver, raised fibrinogen and plasminogen activator inhibitor 1 concentrations, renal sodium retention, hyperuricaemia and dense low-density lipoprotein (LDL) particles.

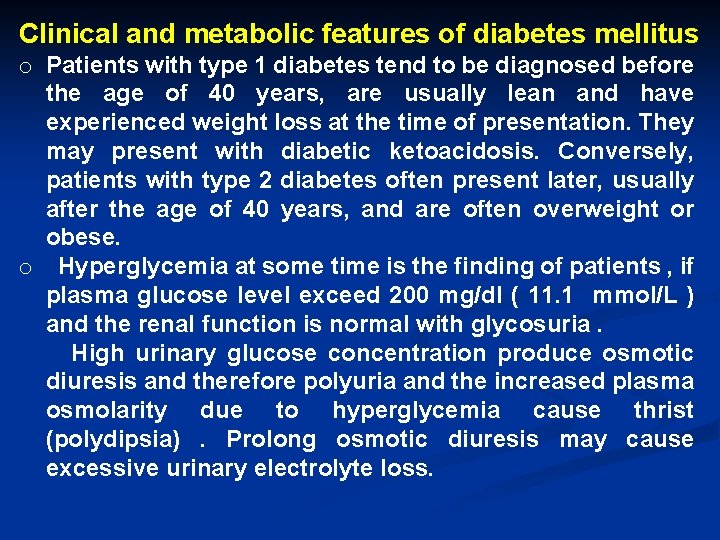
Clinical and metabolic features of diabetes mellitus o Patients with type 1 diabetes tend to be diagnosed before the age of 40 years, are usually lean and have experienced weight loss at the time of presentation. They may present with diabetic ketoacidosis. Conversely, patients with type 2 diabetes often present later, usually after the age of 40 years, and are often overweight or obese. o Hyperglycemia at some time is the finding of patients , if plasma glucose level exceed 200 mg/dl ( 11. 1 mmol/L ) and the renal function is normal with glycosuria. High urinary glucose concentration produce osmotic diuresis and therefore polyuria and the increased plasma osmolarity due to hyperglycemia cause thrist (polydipsia). Prolong osmotic diuresis may cause excessive urinary electrolyte loss.

The classical symptoms of D. M. are only present in advanced cases. Abnormalities in lipid metabolism may be secondary to insulin deficiency , lipolysis is enhanced and plasma FAA level raise. In the liver FAA are converted to acetyl Co. A and ketones , or are reesterified to form endogenous triglyceride and incorporated to VLDL , if insulin deficiency is very sever chylomicron may be accumulate in the blood. The rate of cholesterol synthesis is also increase with associated increase in LDL. Increase breakdown of protein may cause muscle wasting.

Long-term effects of diabetes mellitus Vascular disease is a common complication of diabetes mellitus. Macrovascular disease due to abnormalities of large vessels may present as coronary artery, cerebrovascular or peripheral vascular insufficiency. The condition is probably related to alterations in lipid metabolism and associated hypertension. The most common cause of death is cardiovascular disease, including myocardial infarction. Microvascular disease due to abnormalities of small blood vessels particularly affects the retina (diabetic retinopathy) and the kidney

q Kidney disease is associated with several abnormalities, including proteinuria and progressive renal failure , nephrotic syndrome. The presence of small amounts of albumin in the urine (microalbuminuria) is associated with an increased risk of developing progressive renal disease, which may sometimes be prevented by more stringent plasma glucose and blood pressure control. q The renal complications may be partly due to the increased glycation of structural proteins in the arterial walls supplying the glomerular basement membrane; similar vascular changes in the retina may account for the high incidence of diabetic retinopathy. Glycation of protein in the lens may cause cataracts. Infections are also more common in diabetic patients, for example urinary tract or chest infections, cellulitis and candida.

- Diabetic neuropathy can occur, which can be peripheral or autonomic. It has been suggested that sorbitol is implicated in the aetiology of diabetic neuropathy through the action of aldolase reductase. - Diabetic ulcers, for example of the feet, can lead to gangrene and amputation. - The ulcers can be ischaemic , neuropathic or infective.

Acute metabolic mellitus: complications of diabetes 1 -Hypoglycaemia : This is the most common cause of coma seen in diabetic patients. Hypoglycaemia is most commonly caused by accidental over administration of insulin or (OHD) sulphonylureas or meglitinides. Precipitating causes include too high a dose of insulin or hypoglycaemic drug; conversely, the patient may have missed a meal or taken excessive exercise after the usual dose of insulin or ( OHD ). Hypoglycaemia is very dangerous, and some patients lack awareness of this; i, e they lose warning signs such as sweating, dizziness, palpitation and headaches. Driving is a major hazard under such circumstances. Patients should monitor their own blood glucose closely, carry glucose preparations to abort severe hypoglycaemia and avoid high-risk activities during which

2 - Diabetic ketoacidosis : Diabetic ketoacidosis may be precipitated by infection, acute myocardial infarction or vomiting. In the absence of insulin, there is increased lipid and protein breakdown, enhanced hepatic gluconeogenesis and impaired glucose entry into cells. The clinical consequences of diabetic ketoacidosis are due to: ●hyperglycaemia causing plasma hyperosmolality, ● metabolic acidosis, ●glycosuria. Plasma glucose concentrations are usually in the range (20– 40 mmol/L) , hyperglycaemia causes glycosuria and hence an osmotic diuresis. Water and electrolyte loss due to vomiting, which is common in this syndrome, increases fluid depletion.

There may be haemoconcentration and reduction of the glomerular filtration rate enough to cause uraemia due to renal circulatory insufficiency. The extracellular hyperosmolality causes a shift of water out of the cellular compartment and severe cellular dehydration occurs. Loss of water from cerebral cells is probably the reason for the confusion and coma. Thus there is both cellular and extracellular volume depletion. The rate of lipolysis is increased because of decreased insulin activity; more free fatty acids are produced than can be metabolized by peripheral tissues. The free fatty acids are either converted to ketones by the liver or, of less immediate clinical importance, incorporated as endogenous TG into VLDL, sometimes causing severe hypertriglyceridaemia.

q H+ ions, produced with ketones other than acetone, are buffered by plasma bicarbonate. q. When the rate of production exceeds the rate of bicarbonate generation, the plasma bicarbonate falls. q Hydrogen ion secretion causes a fall in urinary p. H. The deep, sighing respiration (Kussmaul’s respiration) and the odour of acetone on the breath are classic features of diabetic ketoacidosis.

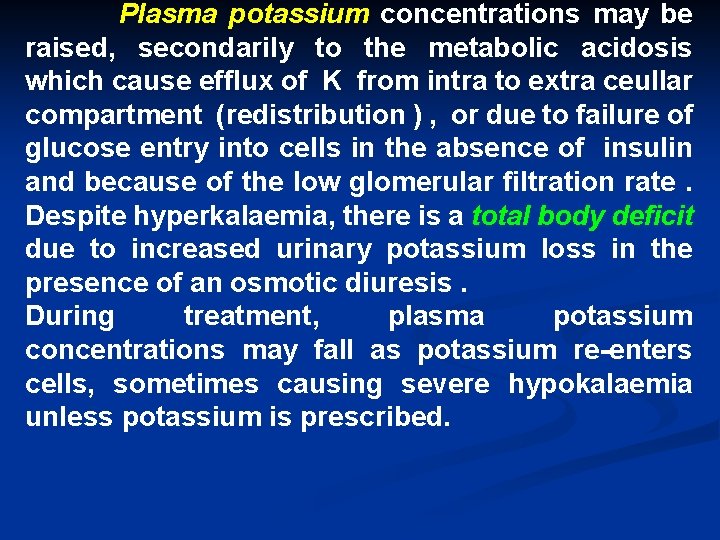
Plasma potassium concentrations may be raised, secondarily to the metabolic acidosis which cause efflux of K from intra to extra ceullar compartment (redistribution ) , or due to failure of glucose entry into cells in the absence of insulin and because of the low glomerular filtration rate. Despite hyperkalaemia, there is a total body deficit due to increased urinary potassium loss in the presence of an osmotic diuresis. During treatment, plasma potassium concentrations may fall as potassium re-enters cells, sometimes causing severe hypokalaemia unless potassium is prescribed.

Plasma sodium concentrations may be low (hyponatraemia) partly because of the osmotic effect of the high extracellular glucose concentration , which draws water from the cells and dilutes the sodium. In the presence of a very high plasma glucose concentration, a normal or raised plasma sodium concentration is suggestive of significant water depletion (dehydration). If there is severe hyperlipidaemia, the possibility of pseudohyponatraemia must be considered. During treatment if plasma sodium concentrations rise rapidly this occur if isosmolar or stronger saline solutions are given inappropriately , the patient may remain confused or even comatose as long as the plasma osmolality remains significantly raised, despite a satisfactory fall in plasma glucose concentration.

Hyperphosphataemia followed by hypophosphataemia as plasma phosphate concentrations parallel those of potassium may persist for several days after recovery from diabetic coma. Similarly, hypermagnesaemia can result, partly because of the acidosis. Plasma and urinary amylase activities may be markedly elevated. Sometimes severe hypertriglyceridaemia and chylomicronaemia result, due to reduced lipoprotein lipase activity in the face of insulin deficiency.

3 -Hyperosmolal non-ketotic coma: In diabetic ketoacidosis there is always plasma hyperosmolality due to the hyperglycaemia, and many of the symptoms, including those of confusion and coma, are related to it. The term ‘hyperosmolal’ coma or is usually confined to a condition in which there is marked hyperglycaemia but no detectable ketoacidosis. The reason for these different presentations is not clear. It has been suggested that insulin activity is sufficient to suppress lipolysis but insufficient to suppress hepatic gluconeogenesis or to facilitate glucose transport into cells.

q Hyperosmolal non-ketotic (HONK) coma now may be referred to as hyperosmolar hyperglycaemic state (HHS) and may be of sudden onset. It is more common in older patients. q Plasma glucose concentrations may exceed 900 mg /dl (50 mmol/L). The effects of glycosuria are as described above, but hypernatraemia due to predominant water loss is more commonly found than in ketoacidosis and aggravates the plasma hyperosmolality. q Cerebral cellular dehydration, which contributes to the coma, may also cause hyperventilation, and a respiratory alkalosis. There may also be an increased risk of thrombosis.

Investigation of patients with diabetes mellitus : • Urine glucose testing : By using sensitive glucose specific dipstick method normally there is no sugar in the urine, usually most filtered glucose is reabsorbed by proximal convoluted tubules. Glycosuria is defined as when the plasma , and therefore glomerular filtrate levels greatly exceed the tubular reabsorptive capacity , this may because : A- The plasma glomerular filtrate concentration are (more than 180 mg/dl) and therefore the normal tubular reabsorptive capacity is significantly exceeded. B- Renal threshold is reduced ( renal glycosuria) this usually harmless condition, like in pregnancy in which there is decrese in renal threshold secondly to an increase in GFR so glycosuria is common in normal pregnancy.

2 - blood testing : Ø when symptoms suggest D. M. the diagnosis may be confirm by random blood glucose concentration greater than 200 mg/dl , when R. B. glucose elevated but are not diagnostic of D. M. , glucose tolerance test is usually assessed either by fasting blood glucose estimation or by oral glucose tolerance test. Ø Diabetes is defined by a fasting plasma glucose of 126 mg/dl or above or a random plasma glucose of 200 mg/dl or above.

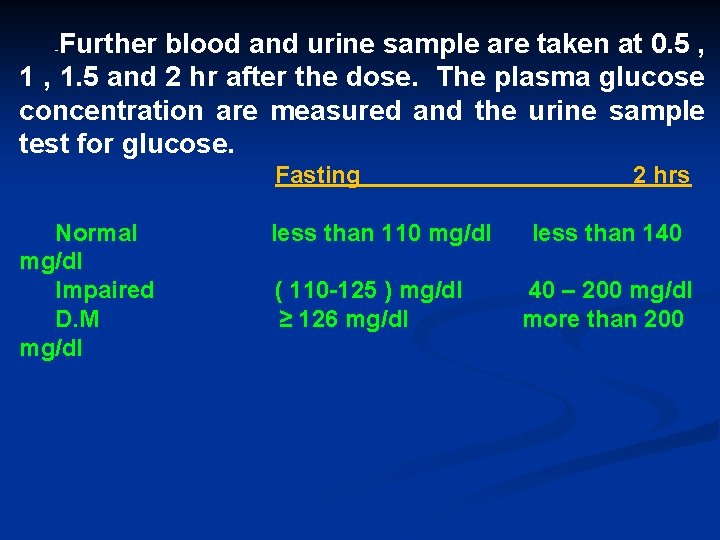
Oral glucose tolerance test Before starting this test , the patient should be resting and not smoke during the test- the patient fast overnight (for at least 10 hrs but not more than 16 hrs ) water only allowed. -A venous sample is withdrawn for plasma glucose estimation and urine sample is collected. - The equivalent of 75 gm of glucose ( for children 1. 75 gm/kg body weight up to a maximum 75 gm ) is given by dissolve it in 300 ml water and give it to patient.

Further blood and urine sample are taken at 0. 5 , 1. 5 and 2 hr after the dose. The plasma glucose concentration are measured and the urine sample test for glucose. - Fasting Normal mg/dl Impaired D. M mg/dl less than 110 mg/dl ( 110 -125 ) mg/dl ≥ 126 mg/dl 2 hrs less than 140 40 – 200 mg/dl more than 200

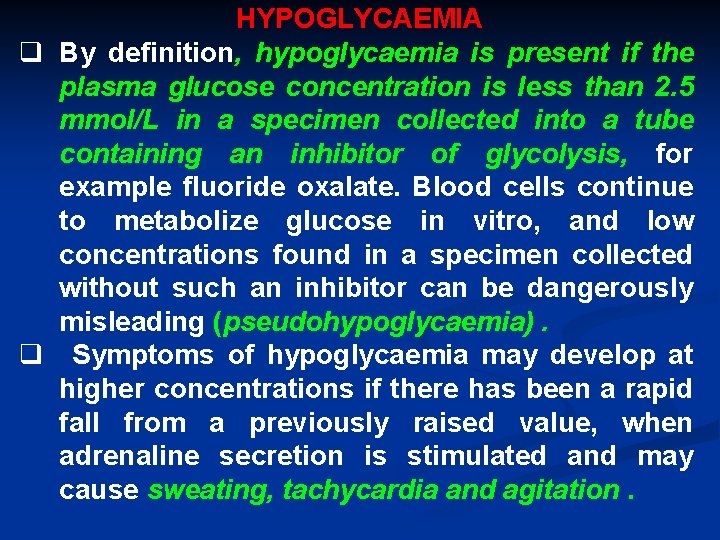
HYPOGLYCAEMIA q By definition, hypoglycaemia is present if the plasma glucose concentration is less than 2. 5 mmol/L in a specimen collected into a tube containing an inhibitor of glycolysis, for example fluoride oxalate. Blood cells continue to metabolize glucose in vitro, and low concentrations found in a specimen collected without such an inhibitor can be dangerously misleading (pseudohypoglycaemia). q Symptoms of hypoglycaemia may develop at higher concentrations if there has been a rapid fall from a previously raised value, when adrenaline secretion is stimulated and may cause sweating, tachycardia and agitation.

q Cerebral metabolism depends on an adequate supply of glucose from ECF, and the symptoms of hypoglycaemia may resemble those of cerebral hypoxia (neuroglycopenia). q Faintness, dizziness or lethargy may progress rapidly to coma and, if untreated, permanent cerebral damage or death may occur. q Existing cerebral or cerebrovascular disease may aggravate the clinical picture. hypoglycaemic, symptoms are relieved of on rapid raising the blood glucose.

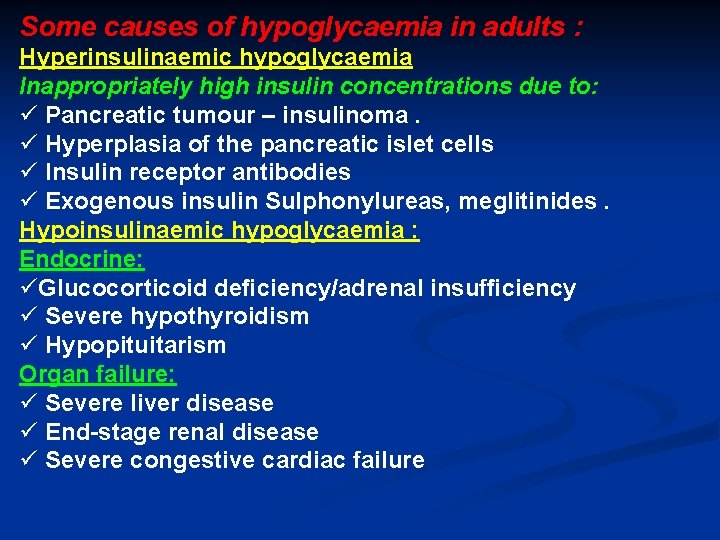
Some causes of hypoglycaemia in adults : Hyperinsulinaemic hypoglycaemia Inappropriately high insulin concentrations due to: ü Pancreatic tumour – insulinoma. ü Hyperplasia of the pancreatic islet cells ü Insulin receptor antibodies ü Exogenous insulin Sulphonylureas, meglitinides. Hypoinsulinaemic hypoglycaemia : Endocrine: üGlucocorticoid deficiency/adrenal insufficiency ü Severe hypothyroidism ü Hypopituitarism Organ failure: ü Severe liver disease ü End-stage renal disease ü Severe congestive cardiac failure

Some non-pancreatic islet cell tumours : Insulin-like growth factor (IGF)-2 -secreting tumours, e. g. liver, adrenal, breast. Leukaemias, lymphomas, myeloma Widespread metastases Reactive hypoglycaemia : Idiopathic , Post-gastric surgery and alcohol induced Miscellaneous causes : Von Gierke’s disease (type 1 glycogen storage disease)

Hyperinsulinaemic hypoglycaemia q Insulin or other drugs are probably the most common causes. It is most important to take a careful drug history. q Hypoglycaemia in a diabetic patient may be caused by accidental insulin overdosage, by changing insulin requirements, or by failure to eat after insulin has been given. q Self-administration for suicidal purposes, sulphonylureas or meglitinides may also induce hypoglycaemia, especially in the elderly. q Measurement of plasma C-peptide concentrations may help to differentiate exogenous insulin administration, when C-peptide secretion is inhibited, from endogenous insulin secretion, when plasma C-peptide is raised, whether it is from an insulinoma or following pancreatic stimulation by sulphonylurea drugs.

q An insulinoma is usually a small, histologically benign primary tumour of the islet cells of the pancreas, C-peptide and proinsulin are released in parallel with insulin, and plasma concentrations are therefore inappropriately high in the presence of hypoglycaemia. q Attacks of hypoglycaemia occur typically at night and before breakfast, associated with hunger, and may be precipitated by strenuous exercise. Insulin antibodies can form in response to exogenous insulin. q Sometimes insulin antibodies form despite the patient never having been exposed to exogenous insulin – autoimmune insulin syndrome (AIS). Insulin receptor antibodies may cause

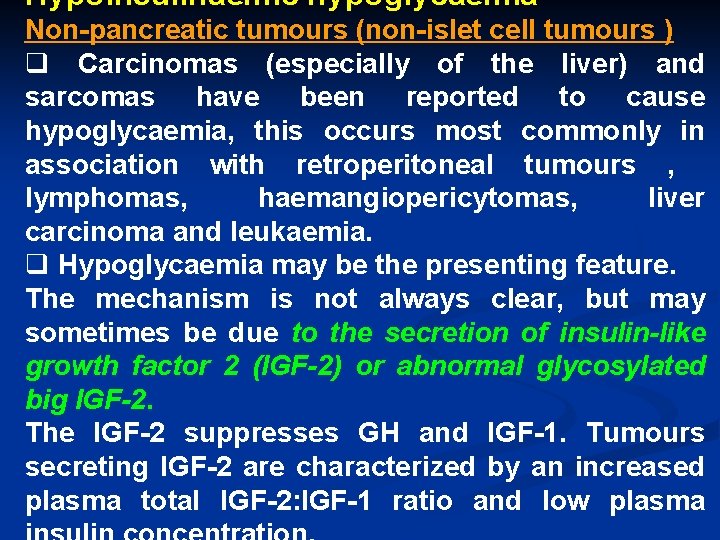
Hypoinsulinaemic hypoglycaemia Non-pancreatic tumours (non-islet cell tumours ) q Carcinomas (especially of the liver) and sarcomas have been reported to cause hypoglycaemia, this occurs most commonly in association with retroperitoneal tumours , lymphomas, haemangiopericytomas, liver carcinoma and leukaemia. q Hypoglycaemia may be the presenting feature. The mechanism is not always clear, but may sometimes be due to the secretion of insulin-like growth factor 2 (IGF-2) or abnormal glycosylated big IGF-2. The IGF-2 suppresses GH and IGF-1. Tumours secreting IGF-2 are characterized by an increased plasma total IGF-2: IGF-1 ratio and low plasma

Endocrine causes Hypoglycaemia may occur in hypothyroidism, pituitary or adrenal insufficiency. Impaired liver function The functional reserve of the liver is so great that, despite its central role in the maintenance of plasma glucose concentrations, hypoglycaemia is a rare complication of liver disease. It may complicate very severe hepatitis, or liver necrosis. Renal failure can result in hypoglycaemia as the kidney, like the liver, is a gluconeogenic organ. Reactive (functional) hypoglycaemia Some people develop symptomatic hypoglycaemia between 2 and 4 h after a meal or a glucose load. Loss of consciousness is very rare. Similar symptoms may follow a gastrectomy , when rapid passage of glucose into the intestine, and rapid absorption, may stimulate excessive insulin secretion (‘late dumping syndrome’). Reactive hypoglycaemia is

Alcohol-induced hypoglycaemia q Hypoglycaemia may develop between 2 and 10 h after the ingestion of large amounts of alcohol. It is found most often in undernourished subjects and chronic alcoholics but may occur in young subjects when they first drink alcohol. q Hypoglycaemia is probably caused by the suppression of gluconeogenesis during the metabolism of alcohol.

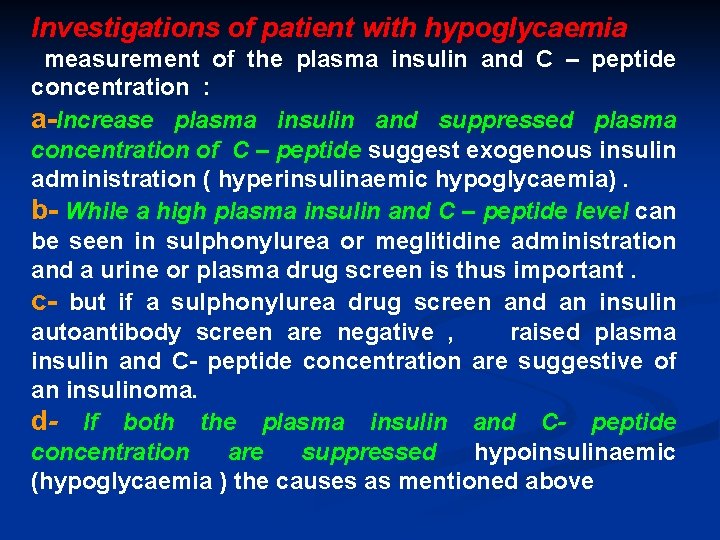
Investigations of patient with hypoglycaemia measurement of the plasma insulin and C – peptide concentration : a-Increase plasma insulin and suppressed plasma concentration of C – peptide suggest exogenous insulin administration ( hyperinsulinaemic hypoglycaemia). b- While a high plasma insulin and C – peptide level can be seen in sulphonylurea or meglitidine administration and a urine or plasma drug screen is thus important. c- but if a sulphonylurea drug screen and an insulin autoantibody screen are negative , raised plasma insulin and C- peptide concentration are suggestive of an insulinoma. d- If both the plasma insulin and C- peptide concentration are suppressed hypoinsulinaemic (hypoglycaemia ) the causes as mentioned above
 Metformin lactic acidosis symptoms
Metformin lactic acidosis symptoms Metformin lactic acidosis symptoms
Metformin lactic acidosis symptoms Lactic acidosis causes
Lactic acidosis causes Alcalosis metabolica
Alcalosis metabolica Beta glucose vs alpha glucose
Beta glucose vs alpha glucose Dihydroxyacetone fischer projection
Dihydroxyacetone fischer projection Pre production adalah
Pre production adalah Role of csf
Role of csf Glucose estimation methods
Glucose estimation methods Potato starch agar
Potato starch agar Synthesis of glucose from non-carbohydrate sources
Synthesis of glucose from non-carbohydrate sources Anaerobic respiration benefits
Anaerobic respiration benefits Gap junctions in smooth muscle
Gap junctions in smooth muscle What muscle fibers run in circles around your eye
What muscle fibers run in circles around your eye Respiratory acidosis
Respiratory acidosis Winters formula
Winters formula Objectives of fermentation
Objectives of fermentation Fermentation in microorganisms
Fermentation in microorganisms Lactic acid
Lactic acid Acid lactic structura
Acid lactic structura Formula for lactic acid fermentation
Formula for lactic acid fermentation Lactic acid vasodilation
Lactic acid vasodilation Percentage to empirical formula
Percentage to empirical formula Lactic acid fermentation
Lactic acid fermentation Lactic acid
Lactic acid The lactic acid energy system
The lactic acid energy system Blood flow control
Blood flow control Fermentation process
Fermentation process Hyporolemia
Hyporolemia C12h22014ca.h2o
C12h22014ca.h2o How to increase lactate inflection point
How to increase lactate inflection point Lactate dehydrogenase
Lactate dehydrogenase Gluconeogenesis importance
Gluconeogenesis importance Gluconeogenesis from lactate
Gluconeogenesis from lactate Clasificarea produselor lactate
Clasificarea produselor lactate Gluconeogenesis from lactate
Gluconeogenesis from lactate Pyruvate to lactate
Pyruvate to lactate Bisphoglycerate
Bisphoglycerate Lactag pe gustate
Lactag pe gustate Kaon jarabe
Kaon jarabe Respiratory acidosis
Respiratory acidosis Arritmias ventriculares
Arritmias ventriculares Timur graham
Timur graham What cause metabolic acidosis
What cause metabolic acidosis Metabolic acidosis definition
Metabolic acidosis definition Causas de acidosis lactica
Causas de acidosis lactica Maintenance fluid formula 4-2-1
Maintenance fluid formula 4-2-1 Compensated versus uncompensated
Compensated versus uncompensated Po2 esperada para la edad
Po2 esperada para la edad