VARIOUS METHOD OF GLUCOSE ESTIMATION GTT AND PRINCIPAL







































- Slides: 67

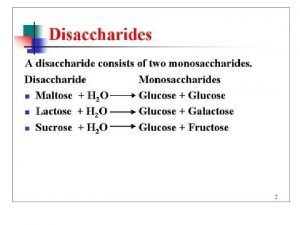
VARIOUS METHOD OF GLUCOSE ESTIMATION , GTT AND PRINCIPAL OF CARBOHYDRATES CHEMISTRY TEST Subject : Biochemistry Student name : 1) Jignasha Surti. 2) Mitali Rana. 3) Arpita Ktaria. 4) Priya Patel. 5) Vipra Patel.

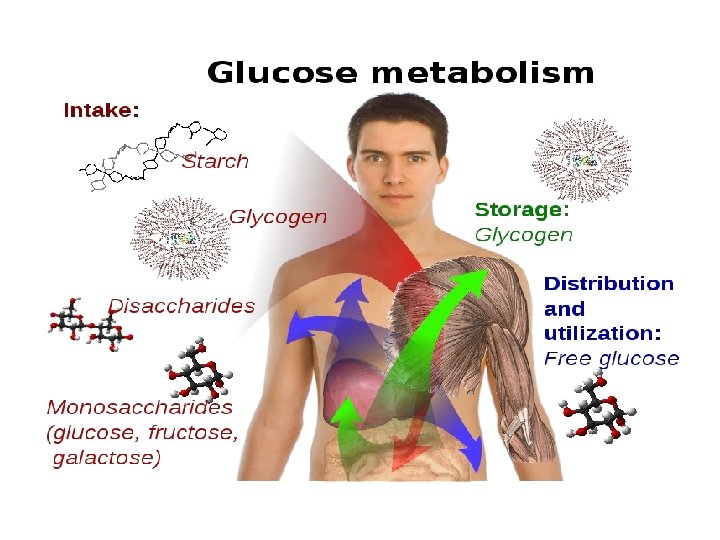
INTRODUCTION: • Glucose is a monosaccharide. • It is central molecule in carbohydrate metabolism. • Stored as glycogen in liver and skeletal muscle.


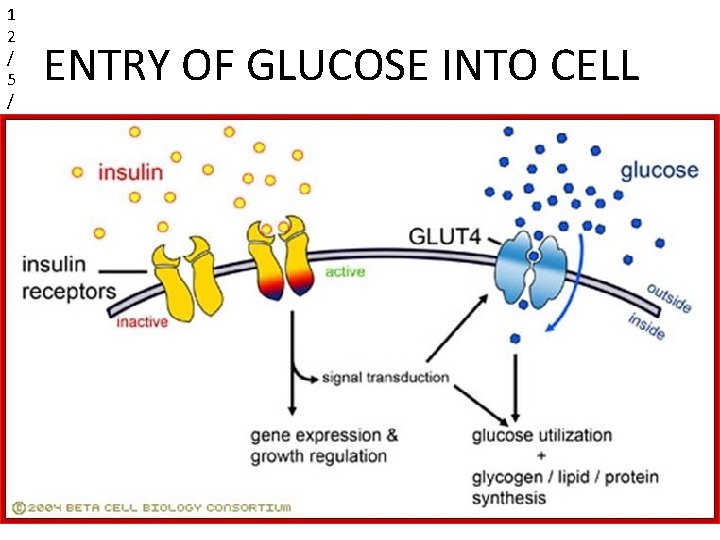
Entry of glucose into the cell Two specific transport system are used : 1. Insulin –independent transport system: • Carrier mediated uptake of glucose • Not dependent on insulin. • Present in hepatocytes, erythosytes & brain. 2. Insulin dependent transport system : • Present in Skeletal muscle.

1 2 / 5 / 2 0 ENTRY OF GLUCOSE INTO CELL

Blood collection for glucose estimation : • Flouride containing vials are used. • Fluoride inhibit glycolysis by inhibiting enolase enzyme. • In CSF, bacteria & other cells are also present so analyzed immediately. • For glucose estimation from urine, add 5 ml glacial acetic acid as preservative to inhibit bacterial growth.

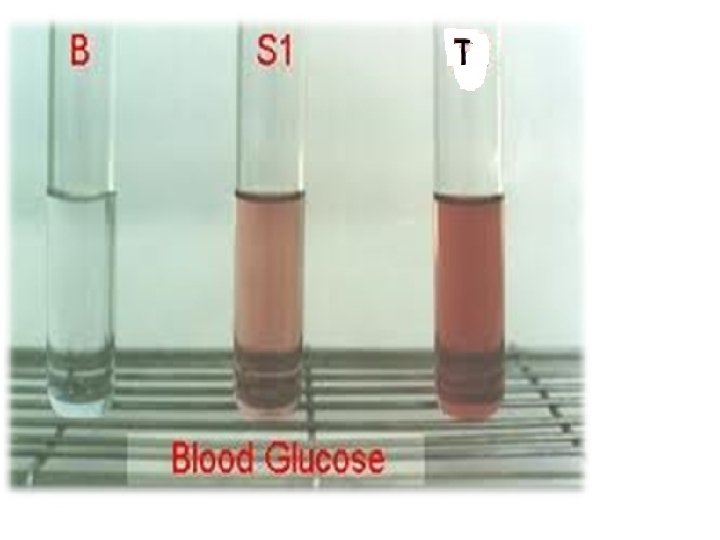
ENZYMETIC DETERMINATION: GOD POD METHOD PRINCIPLE: Glucose + H₂O + O₂ GOD Gluconic acid + H₂O₂ 4 Amino Phenazone + Phenol + H₂O₂ POD Quinonimine – Pink colour compound Intensity is determined at on 505 nm filter.

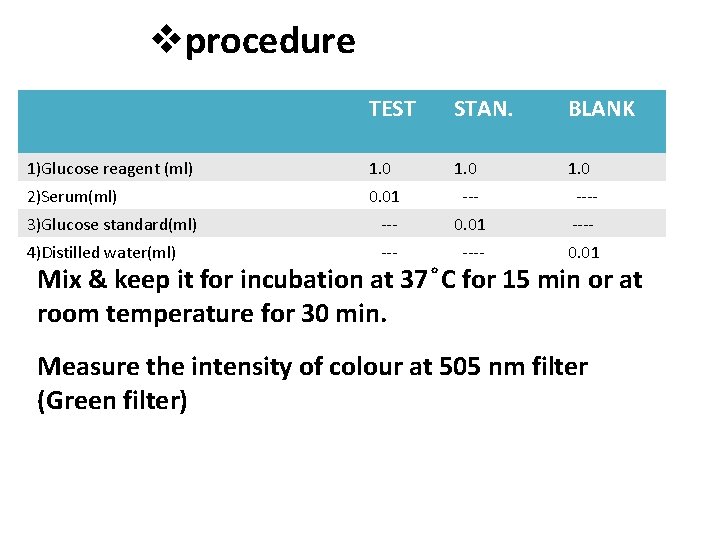
procedure TEST STAN. BLANK 1)Glucose reagent (ml) 1. 0 2)Serum(ml) 0. 01 ---- 3)Glucose standard(ml) --- 0. 01 ---- 4)Distilled water(ml) ---- 0. 01 Mix & keep it for incubation at 37 C for 15 min or at room temperature for 30 min. Measure the intensity of colour at 505 nm filter (Green filter)


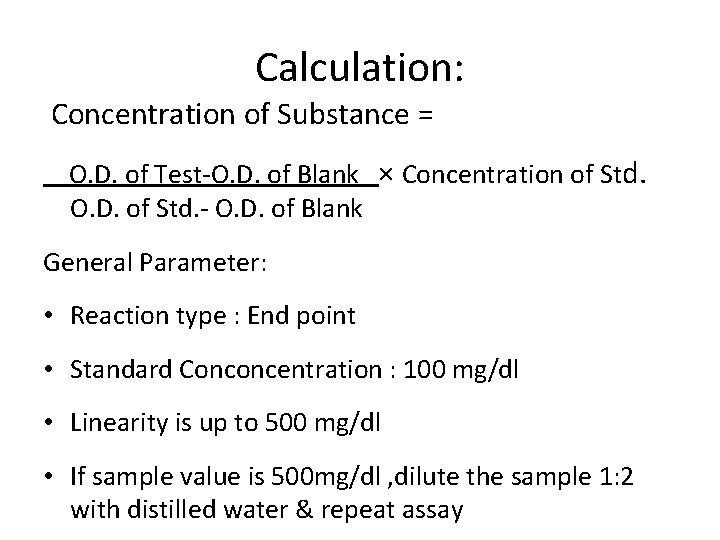
Calculation: Concentration of Substance = O. D. of Test-O. D. of Blank × Concentration of Std. O. D. of Std. - O. D. of Blank General Parameter: • Reaction type : End point • Standard Conconcentration : 100 mg/dl • Linearity is up to 500 mg/dl • If sample value is 500 mg/dl , dilute the sample 1: 2 with distilled water & repeat assay

ADVANTAGE AND DISADVANTAGES :

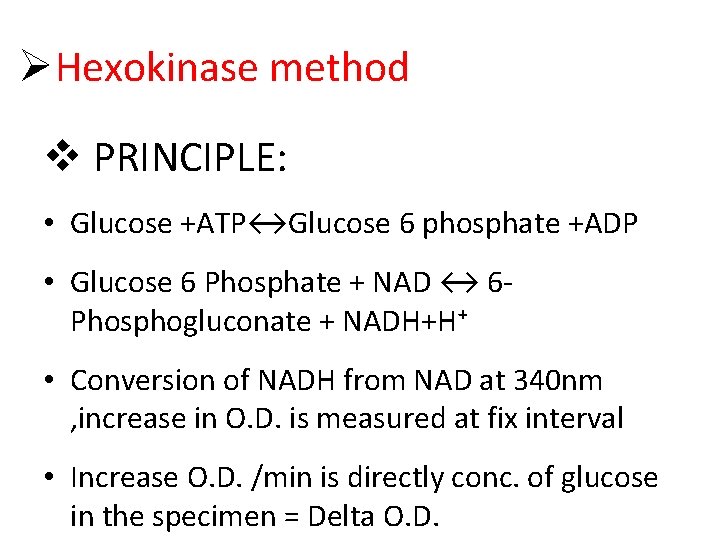
Hexokinase method PRINCIPLE: • Glucose +ATP↔Glucose 6 phosphate +ADP • Glucose 6 Phosphate + NAD ↔ 6 Phosphogluconate + NADH+H⁺ • Conversion of NADH from NAD at 340 nm , increase in O. D. is measured at fix interval • Increase O. D. /min is directly conc. of glucose in the specimen = Delta O. D.

PROCEDURE: • Pipette 1. 0 ml Of Glucose Reagent in Cuvette & Keep It In a Water-bath at 37 c For 1 min(for incubation) • Add 10 μl of sample mix well & read change in O. D /minute , up to 3 minute • Repeat steps 1, 2 & 3 by using Standard. CALCULATION: • Plasma glucose = Delta O. D. /min(test) × 100 Delta O. D. /min(Std. )

ADVANTAGES AND DISADVANTAGES

3. GLUCOSE DEHYDROGENASE METHOD • GLUCOSE ↔ D-GLUCONO-δ-LACTONE NAD⁺↔NADH + H⁺

PRINCIPLE PROCEDURE CALCULATION MERITS AND DEMERITS

DISADVANTAGES It is non-selective for glucose because it also detect maltose, xylose, galactose. Several drugs give high false positive result.

4. Orthotoluidine method PRINCIPLE: • Glucose react with orthotoluidin in hot acidic medium to form a Green color complex • Color intensity α Conc. Of Glucose • Measured in photometer at 620 nm to 660 nm. • It can measured other monosaccharide also. • It is Non-Specific Method.

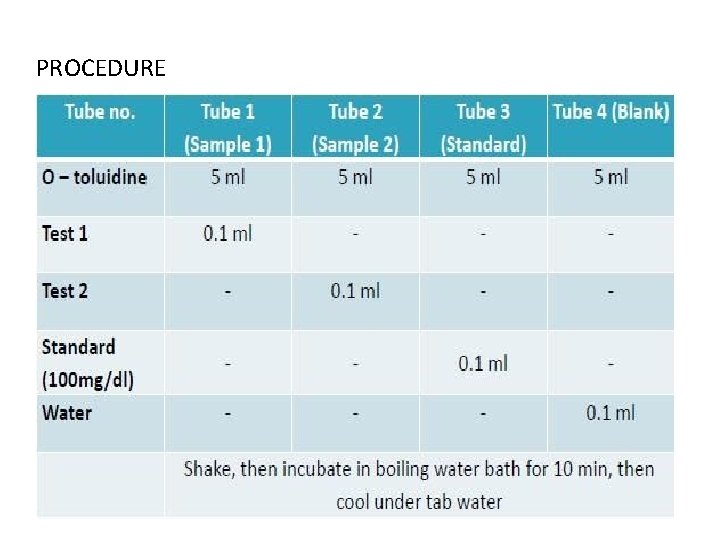
PROCEDURE

calculation The concentration of glucose in the standard solution is 100 mg/100 ml. The concentration of glucose in urine is given by: O. D. TEST ––––––– 100 = mg Glucose /100 ml blood O. D. STANDARD

ADVANTAGES DISADVANTAGES It is Non-Specific Method And Orthotoluidine is carcinogenic, so not utilized nowadays.

5. Folin Vui Method • Time consuming method • Non specific method , also measure fructose.

Glucometer Blood is placed onto a test strip & insert into the glucometer to measure blood sugar levels It is only type of dry chemistry Advantage : Can do from capillary collection method. E. g. Heal Pick, Pinna Pick Gives result with in second. Disadvantage : Costly. Slightly high result than actual.

MEASUREMENT OF GLUCOSE IN URINE METHOD: 1. Qualitative 2. Quantitative 3. Semi- quantitative 1) QUALITATIVE METHOD: • It is determination by Benedict test

2) QUANTITATIVE MATHOD: • It Is Determination By Hexokinase & Glucose Dehydrogenase 3)SEMI QUANTITATIVE MATHOD: • It is determination by Glucose Oxidase strip test • E. g. Urine strip

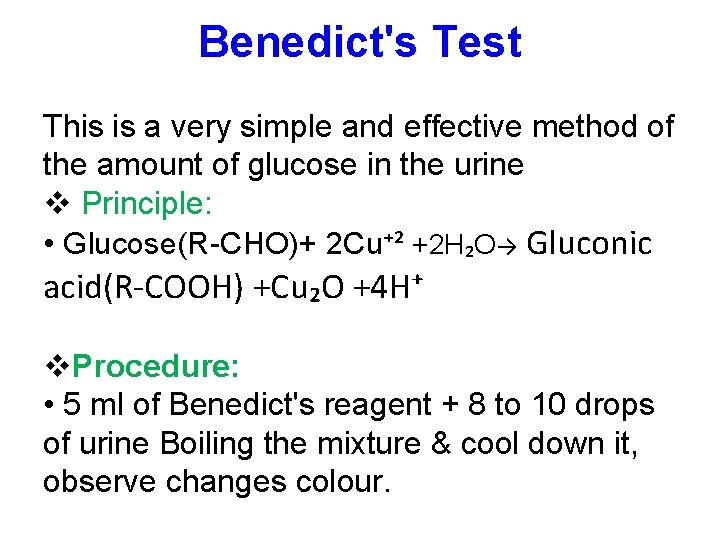
Benedict's Test This is a very simple and effective method of the amount of glucose in the urine Principle: • Glucose(R-CHO)+ 2 Cu⁺² +2 H₂O→ Gluconic acid(R-COOH) +Cu₂O +4 H⁺ Procedure: • 5 ml of Benedict's reagent + 8 to 10 drops of urine Boiling the mixture & cool down it, observe changes colour.

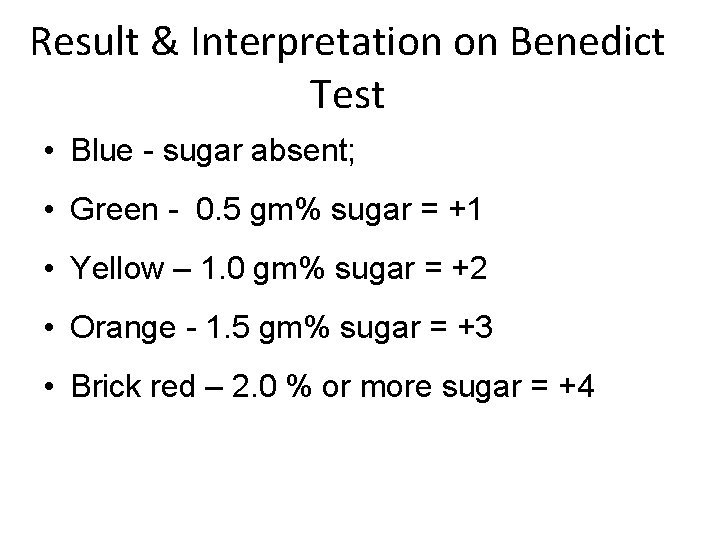
Result & Interpretation on Benedict Test • Blue - sugar absent; • Green - 0. 5 gm% sugar = +1 • Yellow – 1. 0 gm% sugar = +2 • Orange - 1. 5 gm% sugar = +3 • Brick red – 2. 0 % or more sugar = +4

• Click to edit Master text styles – Second level – Third level • Fourth level – Fifth level

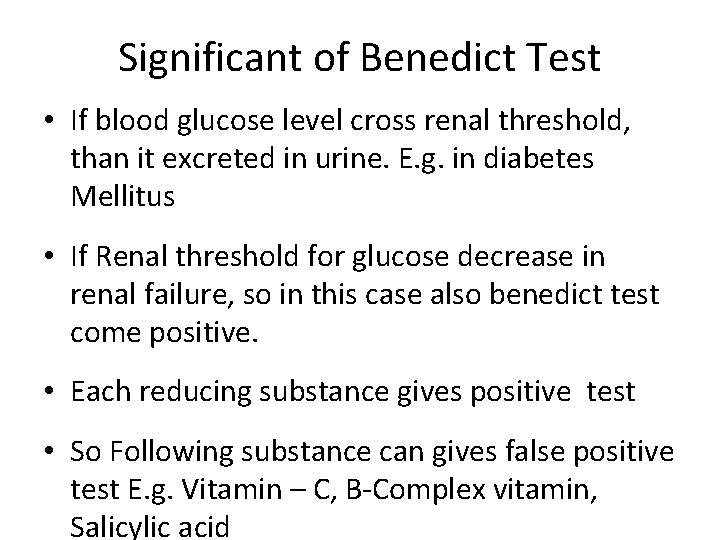
Significant of Benedict Test • If blood glucose level cross renal threshold, than it excreted in urine. E. g. in diabetes Mellitus • If Renal threshold for glucose decrease in renal failure, so in this case also benedict test come positive. • Each reducing substance gives positive test • So Following substance can gives false positive test E. g. Vitamin – C, B-Complex vitamin, Salicylic acid

Glucose Oxidase Test • Paper or plastic strips, called diastix. • A color-chart is provided with the strips. • Strip contain dye are O-tolidine, tetramethylbenzidine , potassium iodide, 4 - amino phynazome, phenol. • The dye changes colour on coming in contact with the urine. • After 30 to 60 seconds the colour of the strip matched with the colours of the provided

Oxidase Strip • Click to edit Master text styles – Second level – Third level • Fourth level – Fifth level

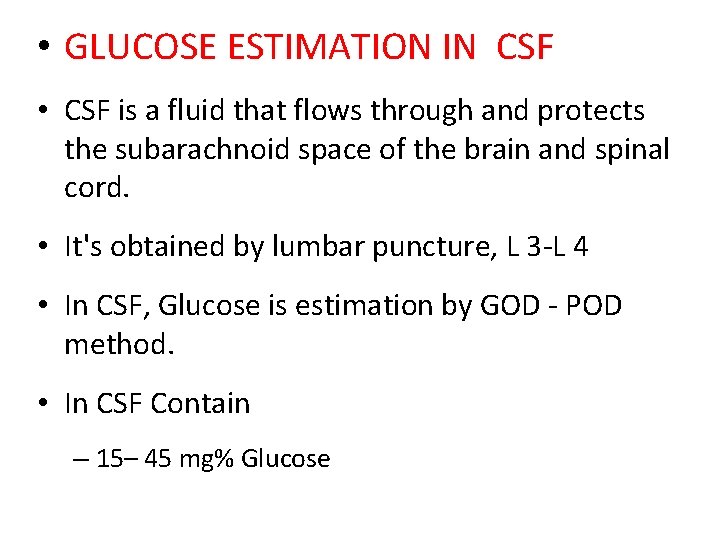
• GLUCOSE ESTIMATION IN CSF • CSF is a fluid that flows through and protects the subarachnoid space of the brain and spinal cord. • It's obtained by lumbar puncture, L 3 -L 4 • In CSF, Glucose is estimation by GOD - POD method. • In CSF Contain – 15– 45 mg% Glucose

Clinical interpretation: An increased CSF glucose level is seen in hyperglycemia. Decreased CSF glucose in 1. Bacterial Infection 2. Hypoglycemia

CLINICAL SIGNIFICANCE Increased glucose : (hyper glycemia) • Diabetes mellitus, • Hyper thyroidism, • Hyper pituitarism, • Adrenocortical hyper activity, Decreased glucose: (hypo glycemia) • Hypo thyroidism,

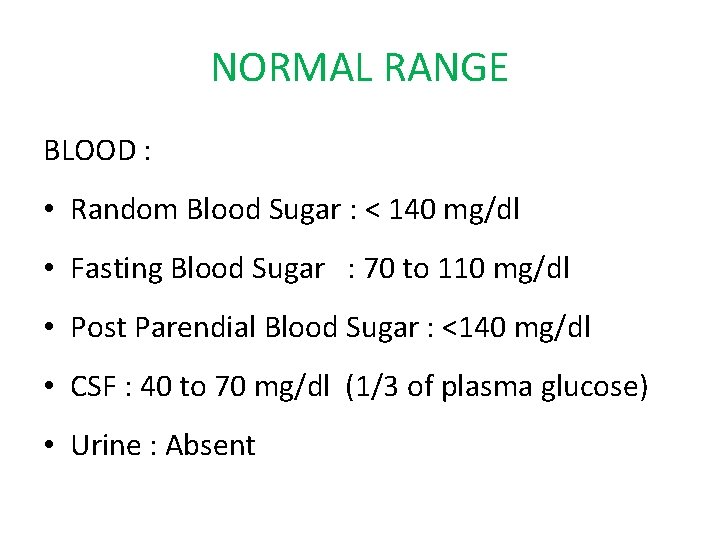
NORMAL RANGE BLOOD : • Random Blood Sugar : < 140 mg/dl • Fasting Blood Sugar : 70 to 110 mg/dl • Post Parendial Blood Sugar : <140 mg/dl • CSF : 40 to 70 mg/dl (1/3 of plasma glucose) • Urine : Absent

GTT (Glucose Tolerance Test)

INTRODUCTION Glucose tolerance means ability of the body to utilize (tolerate) glucose in blood circulation. The effect of ingested carbohydrate can be studied under reasonably standard condition by means of the Glucose Tolerance Test. It is indicated by the nature of blood glucose curve following the

Temporary rise of blood sugar after food intake for few hours. Extent and duration of rise depends on type of food (Glycemic index). Glucose If level returns to normal within 2 -3 hrs. it take >2 hours =Decrease glucose tolerance.

Types of GTT 1) Oral GTT (OGTT) 2) INTRAVENOUS GTT (IVGTT)

PRINCIPLE Following the standard oral dose of glucose, plasma and urine glucose levels are monitored at regular intervals, in order to measure tolerance under defined conditions.

INDICATIONS FOR GTT Having symptoms like diabetes mellitus, but fasting blood sugar value is inconclusive(between 100 -126 mg/dl) During pregnancy and past history of miscarriage. To rule out benign renal glucosuria.

CONTRA-INDICATION Person with confiemed Diabetic patients. Mal-absorption Test disease(OGTT). should not done in acutely ill patients.

PRE-CAUTION Normal Avoid heavy exercise. Report hrs. Avoid diet intake in last 3 days. to lab after fasting for 12 -16 drug that change glucose level. e. g. steroid , Insulin , Oral Hypo Glycemic Drug. Addiction : Alcohol & smoking.

METHOD OF THE TEST Collection of fasting urine & blood (in fluoride) sample. Give 75 gm or 100 gm of glucose dissolved in lemon water to the patient. Note the time of oral glucose administration. In pediatric patient 1. 5 - 1. 75 gm/kg glucose/dextrose powder. Collect five sample of venous blood and urine are collected at the half hourly intervals. Determine blood glucose by the specific method. e. g. GOD-POD method. Urine glucose = Semi-Quantitative Method – Benedict's Test Prepare a glucose tolerance curve(plasma glucose level - time).

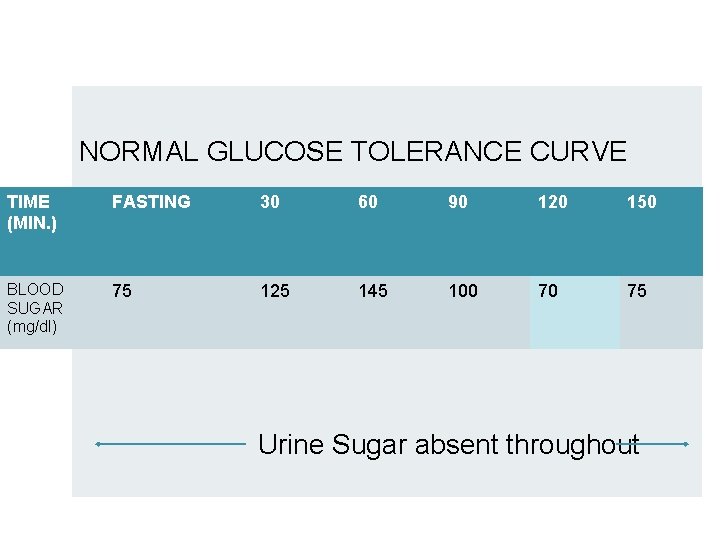
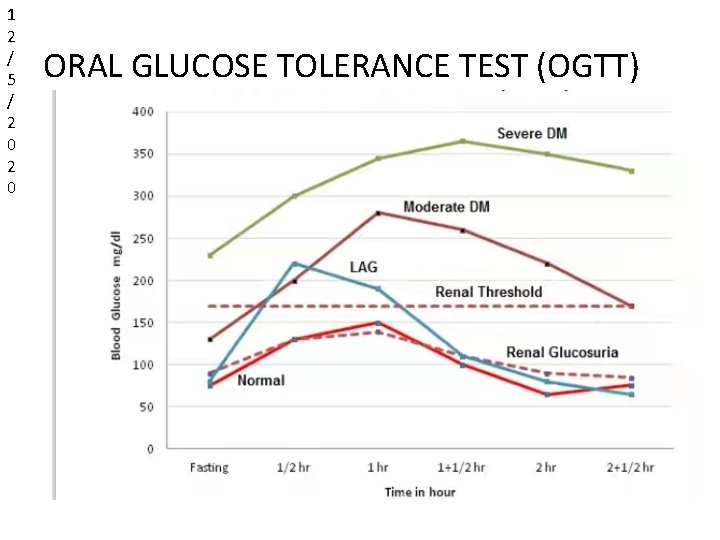
NORMAL GLUCOSE TOLERANCE CURVE TIME (MIN. ) FASTING 30 60 90 120 150 BLOOD SUGAR (mg/dl) 75 125 145 100 70 75 Urine Sugar absent throughout

1 2 / 5 / 2 0 ORAL GLUCOSE TOLERANCE TEST (OGTT)

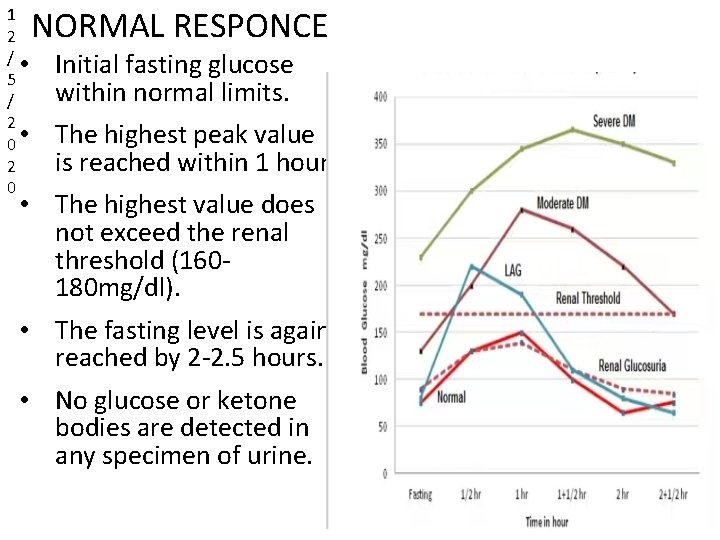
1 2 / • 5 / 2 0 • 2 0 NORMAL RESPONCE Initial fasting glucose within normal limits. The highest peak value is reached within 1 hour. • The highest value does not exceed the renal threshold (160180 mg/dl). • The fasting level is again reached by 2 -2. 5 hours. • No glucose or ketone bodies are detected in any specimen of urine.

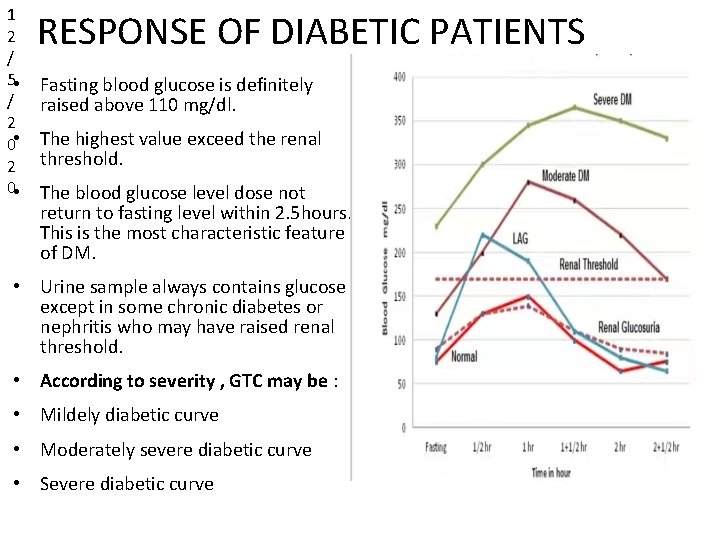
1 2 / 5 • / 2 0 • RESPONSE OF DIABETIC PATIENTS Fasting blood glucose is definitely raised above 110 mg/dl. The highest value exceed the renal threshold. The blood glucose level dose not return to fasting level within 2. 5 hours. This is the most characteristic feature of DM. • Urine sample always contains glucose except in some chronic diabetes or nephritis who may have raised renal threshold. • According to severity , GTC may be : • Mildely diabetic curve • Moderately severe diabetic curve • Severe diabetic curve

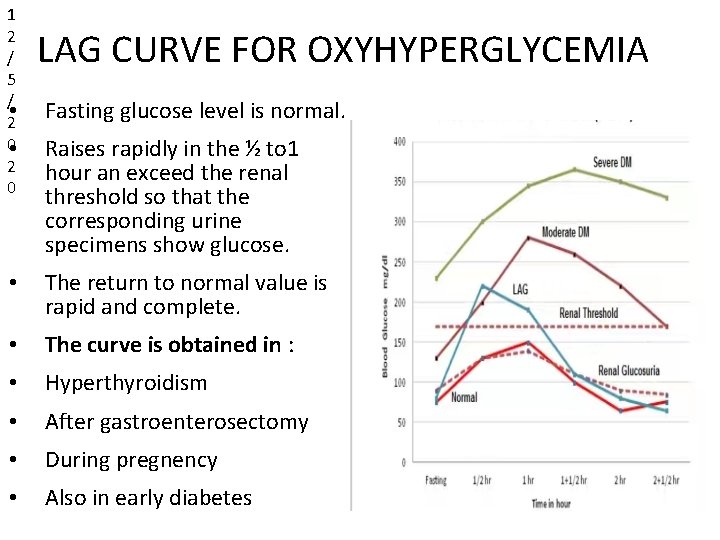
1 2 / 5 / • 2 0 LAG CURVE FOR OXYHYPERGLYCEMIA Fasting glucose level is normal. Raises rapidly in the ½ to 1 hour an exceed the renal threshold so that the corresponding urine specimens show glucose. • The return to normal value is rapid and complete. • The curve is obtained in : • Hyperthyroidism • After gastroenterosectomy • During pregnency • Also in early diabetes

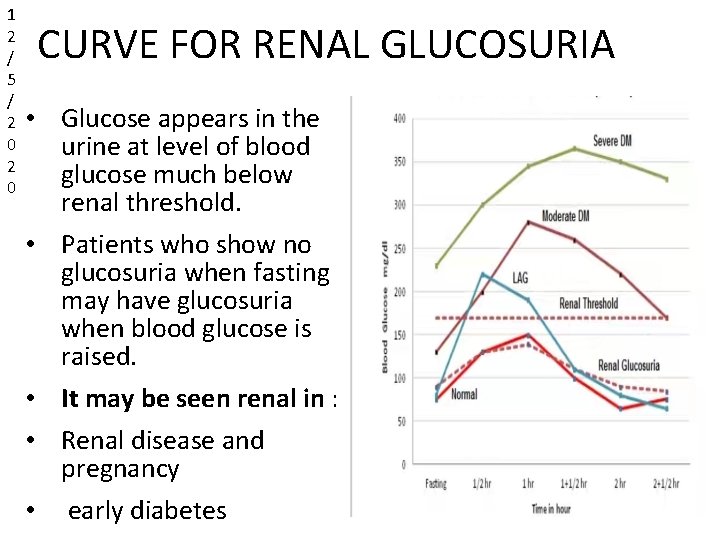
1 2 / 5 / 2 0 CURVE FOR RENAL GLUCOSURIA • Glucose appears in the urine at level of blood glucose much below renal threshold. • Patients who show no glucosuria when fasting may have glucosuria when blood glucose is raised. • It may be seen renal in : • Renal disease and pregnancy • early diabetes

ADVANTAGES It is useful in recognizing of border line cases of diabetes. GTT is useful in early diagnosis diabetes malitus.

DISADVANTAGES GTT is not necessary in known cases of hyperglycemic patient. Oral GTT is also not necessary in know cases of mal -absorption. This time I-V glucose tolerance test is

1 2 / 5 / 2 0 PRINCIPAL OF CARBOHYDRATE CHEMISTRY TEST

Molisch test This test is specific for all carbohydrates. Monosaccharide gives a rapid positive test, Disaccharides and polysaccharides react slower. Objective: To identify the carbohydrate from other macromolecules lipids and proteins.

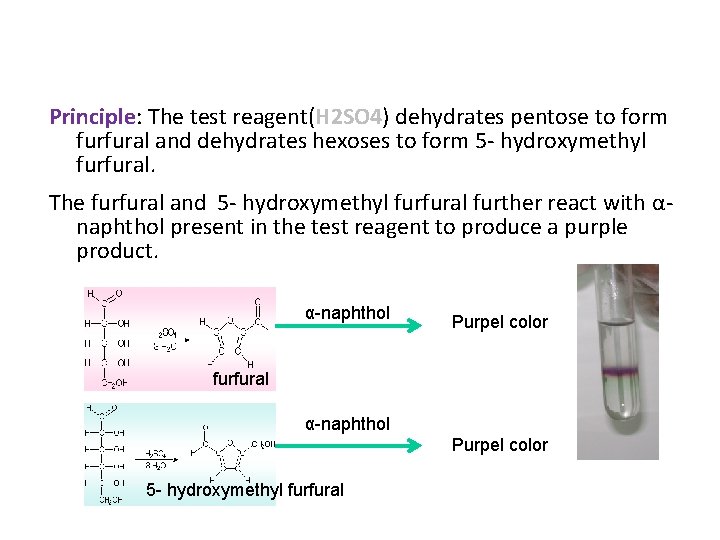
Principle: The test reagent(H 2 SO 4) dehydrates pentose to form furfural and dehydrates hexoses to form 5 - hydroxymethyl furfural. The furfural and 5 - hydroxymethyl furfural further react with αnaphthol present in the test reagent to produce a purple product. α-naphthol Purpel color furfural α-naphthol Purpel color 5 - hydroxymethyl furfural

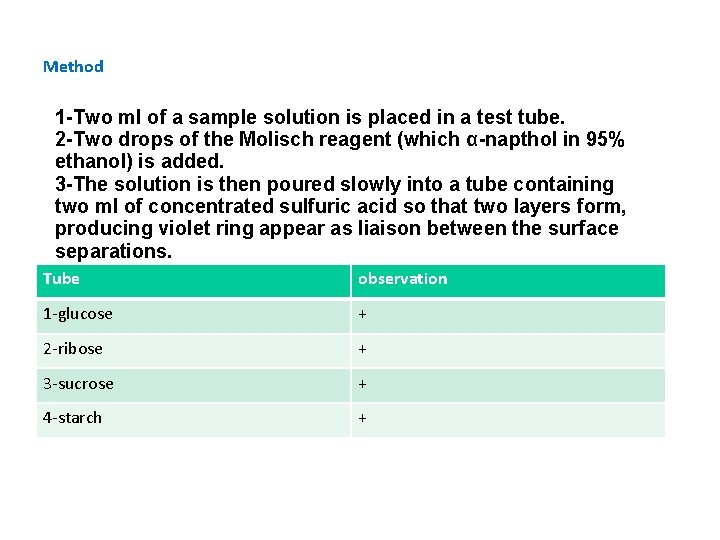
Method 1 -Two ml of a sample solution is placed in a test tube. 2 -Two drops of the Molisch reagent (which α-napthol in 95% ethanol) is added. 3 -The solution is then poured slowly into a tube containing two ml of concentrated sulfuric acid so that two layers form, producing violet ring appear as liaison between the surface separations. Tube observation 1 -glucose + 2 -ribose + 3 -sucrose + 4 -starch +

Benedict's test Benedict's reagent is used as a test for the presence of reducing sugars. All monosaccharides are reducing sugars; they all have a free reactive carbonyl group. Some disaccharides have exposed carbonyl groups and are also reducing sugars. Other disaccharides such as sucrose are nonreducing sugars and will not react with Benedict's solution. Large polymers of glucose, such as starch, are not reducing sugars Objective: To distinguish between the reducing and non-reducing sugars.

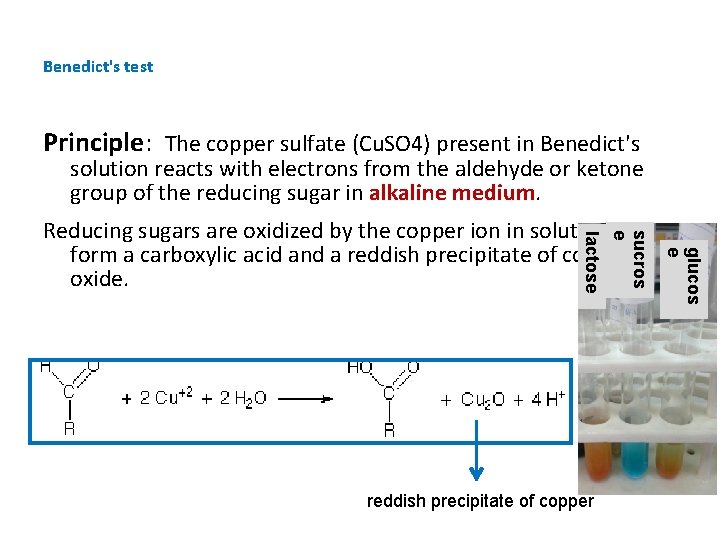
Benedict's test Principle: The copper sulfate (Cu. SO 4) present in Benedict's solution reacts with electrons from the aldehyde or ketone group of the reducing sugar in alkaline medium. glucos e reddish precipitate of copper sucros e lactose Reducing sugars are oxidized by the copper ion in solution to form a carboxylic acid and a reddish precipitate of copper oxide.

Other suger

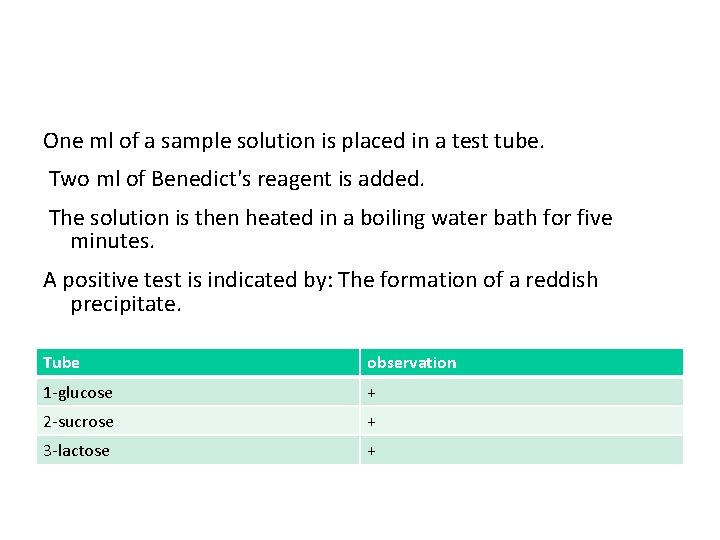
One ml of a sample solution is placed in a test tube. Two ml of Benedict's reagent is added. The solution is then heated in a boiling water bath for five minutes. A positive test is indicated by: The formation of a reddish precipitate. Tube observation 1 -glucose + 2 -sucrose + 3 -lactose +

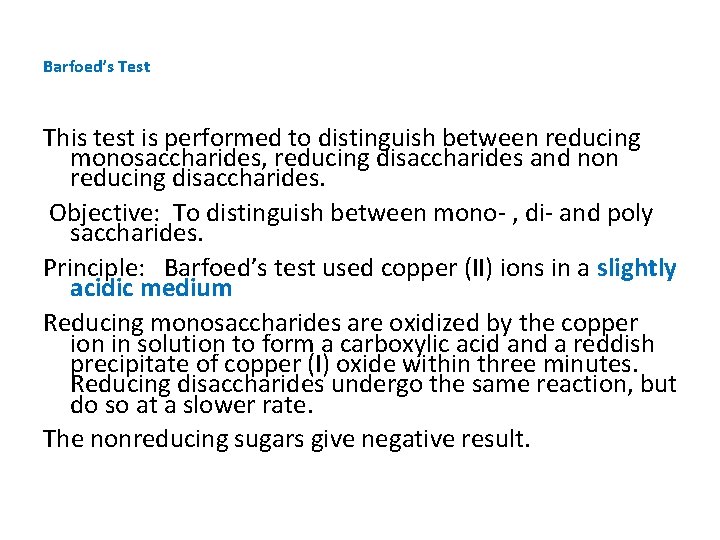
Barfoed’s Test This test is performed to distinguish between reducing monosaccharides, reducing disaccharides and non reducing disaccharides. Objective: To distinguish between mono- , di- and poly saccharides. Principle: Barfoed’s test used copper (II) ions in a slightly acidic medium Reducing monosaccharides are oxidized by the copper ion in solution to form a carboxylic acid and a reddish precipitate of copper (I) oxide within three minutes. Reducing disaccharides undergo the same reaction, but do so at a slower rate. The nonreducing sugars give negative result.

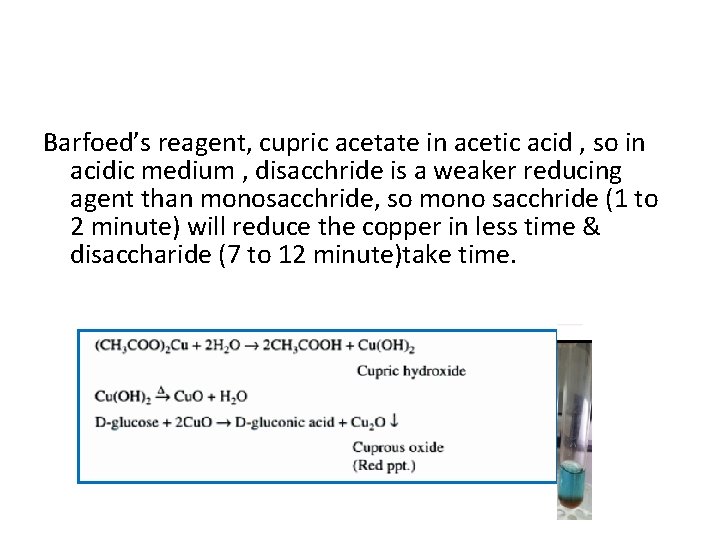
Barfoed’s reagent, cupric acetate in acetic acid , so in acidic medium , disacchride is a weaker reducing agent than monosacchride, so mono sacchride (1 to 2 minute) will reduce the copper in less time & disaccharide (7 to 12 minute)take time.

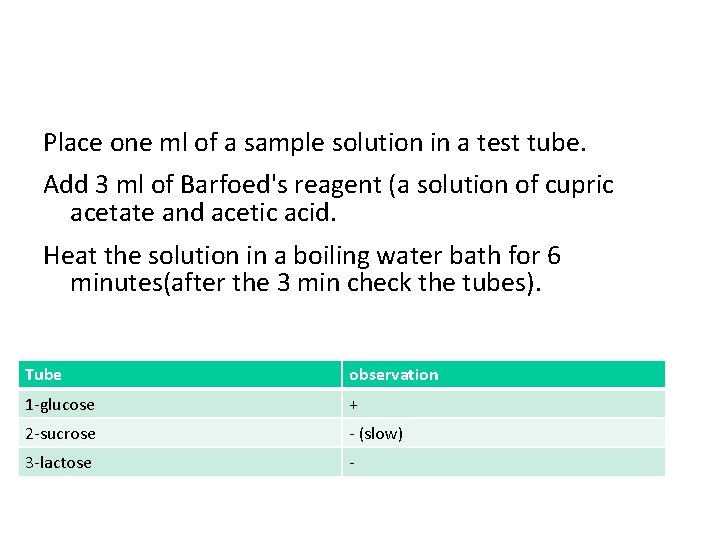
Place one ml of a sample solution in a test tube. Add 3 ml of Barfoed's reagent (a solution of cupric acetate and acetic acid. Heat the solution in a boiling water bath for 6 minutes(after the 3 min check the tubes). Tube observation 1 -glucose + 2 -sucrose - (slow) 3 -lactose -

Seliwanoff's Test This test is used to distinguish between aldoses (like glucose) and ketoses (like fructose). Objective: To distinguish between aldose and ketone sucrose.

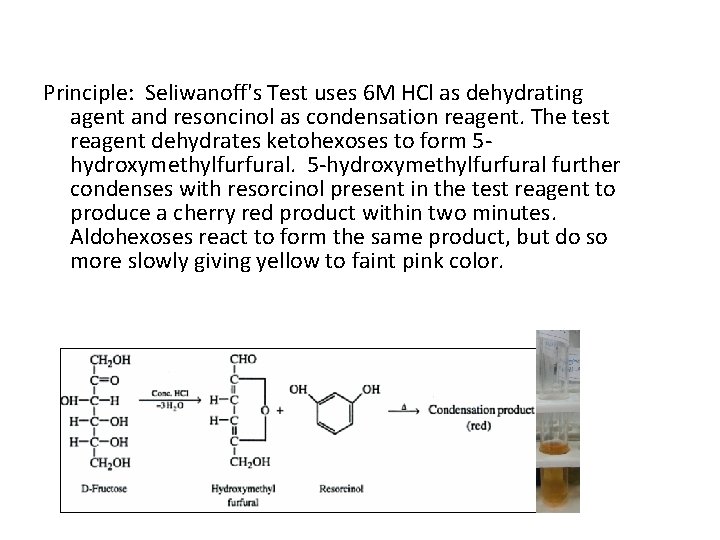
Principle: Seliwanoff's Test uses 6 M HCl as dehydrating agent and resoncinol as condensation reagent. The test reagent dehydrates ketohexoses to form 5 hydroxymethylfurfural. 5 -hydroxymethylfurfural further condenses with resorcinol present in the test reagent to produce a cherry red product within two minutes. Aldohexoses react to form the same product, but do so more slowly giving yellow to faint pink color.

One half ml of a sample solution is placed in a test tube. Two ml of Seliwanoff's reagent (a solution of resorcinol and HCl) is added. The solution is then heated in a boiling water Tube bath for two minutes. observation 1 -glucose + 2 -fructose +

THANK YOU
 Mention different methods of glucose estimation
Mention different methods of glucose estimation Quantitative estimation of glucose by which method
Quantitative estimation of glucose by which method Normal range of blood sugar
Normal range of blood sugar A vs b glucose
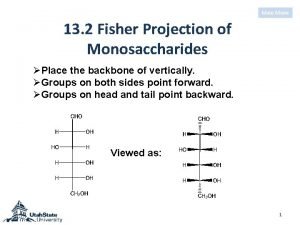
A vs b glucose Sorbose fischer projection
Sorbose fischer projection Protein in csf
Protein in csf Iv pump rate formula
Iv pump rate formula How to calculate gtt/min
How to calculate gtt/min Mat 119
Mat 119 How to calculate iv flow rate
How to calculate iv flow rate Iv fluid computation
Iv fluid computation Gtt resep
Gtt resep Gtt unit of measurement
Gtt unit of measurement Criteria and constraints clipart
Criteria and constraints clipart Gtt design process
Gtt design process Gtt design process
Gtt design process Calcolo percorsi gtt
Calcolo percorsi gtt Gtt technologies
Gtt technologies 1 gtt bid
1 gtt bid Unit 7 architecture
Unit 7 architecture Auric dalam resep
Auric dalam resep Protein estimation by lowry method
Protein estimation by lowry method Rna estimation by orcinol method
Rna estimation by orcinol method Quantitative estimation of amino acids by ninhydrin
Quantitative estimation of amino acids by ninhydrin Comparative graphical method
Comparative graphical method Lowry method
Lowry method Scs method
Scs method Find the cube root of 110592 by prime factorization method
Find the cube root of 110592 by prime factorization method What is symposium in education
What is symposium in education What is the purpose of a sauce? *
What is the purpose of a sauce? * Glucose to carbon dioxide and water
Glucose to carbon dioxide and water Galactose and glucose
Galactose and glucose Galactose and glucose
Galactose and glucose Water and glucose
Water and glucose Insulin subcutaneous order and blood glucose record
Insulin subcutaneous order and blood glucose record Blood glucose record
Blood glucose record Glucose ups and downs
Glucose ups and downs Contrived experiences and their various forms
Contrived experiences and their various forms 5 techniques in summarizing a text
5 techniques in summarizing a text Nims management characteristics
Nims management characteristics Draw impedance triangle and show various quantities on it
Draw impedance triangle and show various quantities on it In the bilingual method
In the bilingual method Difference between dwf and wwf
Difference between dwf and wwf Demand estimation in managerial economics
Demand estimation in managerial economics Open wiring design
Open wiring design Demand estimation and forecasting
Demand estimation and forecasting Sampling and estimation methods in business analytics
Sampling and estimation methods in business analytics Demand estimation and forecasting
Demand estimation and forecasting Shell and tube heat exchanger cost estimation
Shell and tube heat exchanger cost estimation One and two sample estimation problems
One and two sample estimation problems One and two sample estimation problems
One and two sample estimation problems Cost theory and estimation
Cost theory and estimation Parameter estimation and inverse problems
Parameter estimation and inverse problems What is demand forecasting and estimation
What is demand forecasting and estimation Cash flow estimation and risk analysis
Cash flow estimation and risk analysis Production theory and estimation
Production theory and estimation Production theory and estimation
Production theory and estimation Glucose breakdown
Glucose breakdown Non reducing sugar examples
Non reducing sugar examples Examples of external stimuli
Examples of external stimuli Benedict indicator
Benedict indicator Glucose-oxidase-test reaktionsgleichung
Glucose-oxidase-test reaktionsgleichung Metabolism of glucose in erythrocytes
Metabolism of glucose in erythrocytes What is reducing sugar
What is reducing sugar Bial's test for carbohydrates
Bial's test for carbohydrates Qualitative analysis of carbohydrates
Qualitative analysis of carbohydrates Glucose found in
Glucose found in Le transport actif secondaire
Le transport actif secondaire