Kinetics Chapter 12 Reaction Rates Kinetics is concerned




![Calculating Activation Energy § Arrhenius Equation: k = [A]e-Ea/RT § Natural log of Arrhenius Calculating Activation Energy § Arrhenius Equation: k = [A]e-Ea/RT § Natural log of Arrhenius](https://slidetodoc.com/presentation_image_h2/36ca8485d92c17796be50f230192ee95/image-17.jpg)




- Slides: 22

Kinetics Chapter 12

Reaction Rates § Kinetics is concerned with studying the reaction mechanism of a reaction. § An average reaction rate describes how fast (not how spontaneous) a given reaction is overall. § Only positive rates are considered. In the event of a decrease in concentration, we insert a negative sign into the equation.

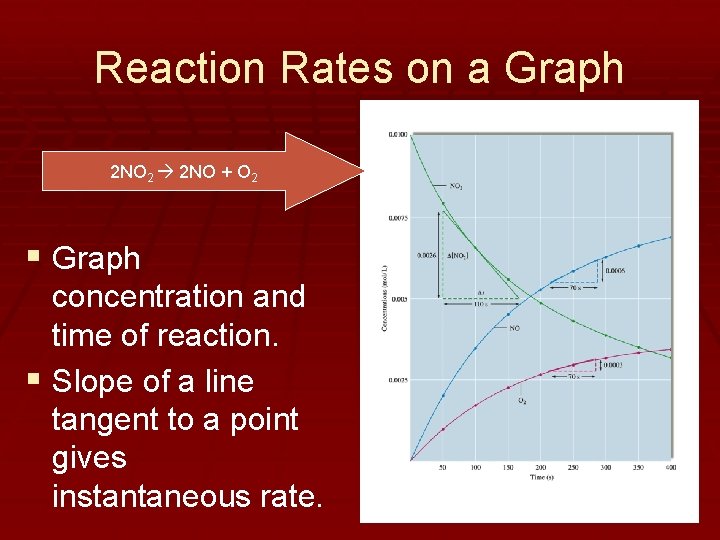
Reaction Rates on a Graph 2 NO 2 2 NO + O 2 § Graph concentration and time of reaction. § Slope of a line tangent to a point gives instantaneous rate.

Reaction Rate Summary § Average rate is not always equal to instantaneous rate. § Concentration of the products and reactants change over time, producing logarithmic curves. § Because concentration changes, rate is changing. § Coefficients in the balanced reaction indicate the relationship between the rates of reactions AND the concentrations.

Rate Laws Intro § All chemical reactions are reversible. § When the rate of the forward and reverse reactions are equal, it is called equilibrium. § Having the reverse reaction occur simultaneously with the forward messes with concentration. § We manipulate conditions to inhibit the progress of the reverse reaction when measuring rates.

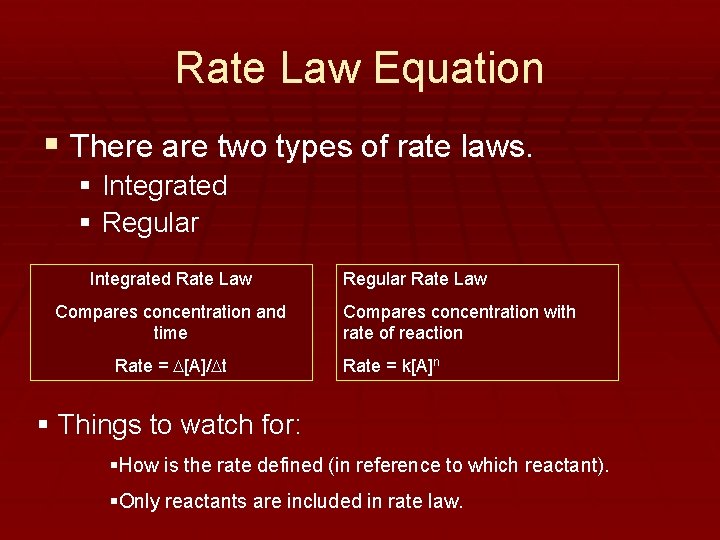
Rate Law Equation § There are two types of rate laws. § Integrated § Regular Integrated Rate Law Compares concentration and time Rate = D[A]/Dt Regular Rate Law Compares concentration with rate of reaction Rate = k[A]n § Things to watch for: §How is the rate defined (in reference to which reactant). §Only reactants are included in rate law.

Determining the Order of Each Reactant in the Rate Law § The order is the superscript on the rate law. § These values are determined from data that is derived from several experiments that measure initial concentration and initial rate of reaction. § You must scrutinize the relationship between concentration and rate to determine the order of a given reactant. § Each reactant can have a different order.

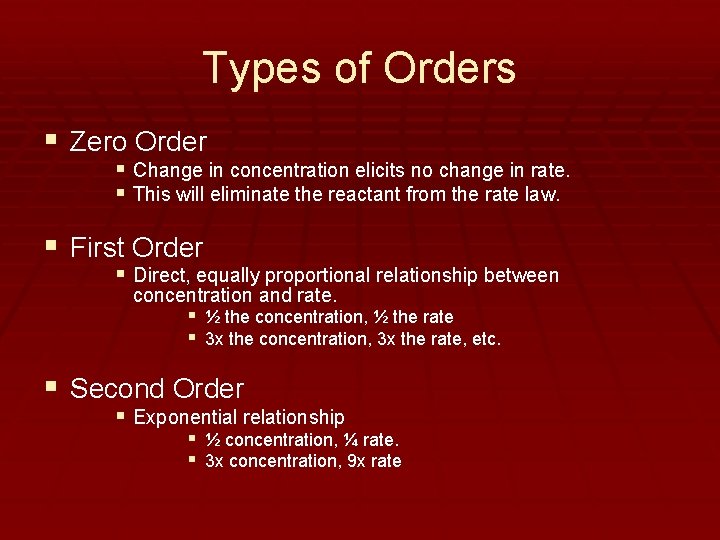
Types of Orders § Zero Order § Change in concentration elicits no change in rate. § This will eliminate the reactant from the rate law. § First Order § Direct, equally proportional relationship between concentration and rate. § ½ the concentration, ½ the rate § 3 x the concentration, 3 x the rate, etc. § Second Order § Exponential relationship § ½ concentration, ¼ rate. § 3 x concentration, 9 x rate

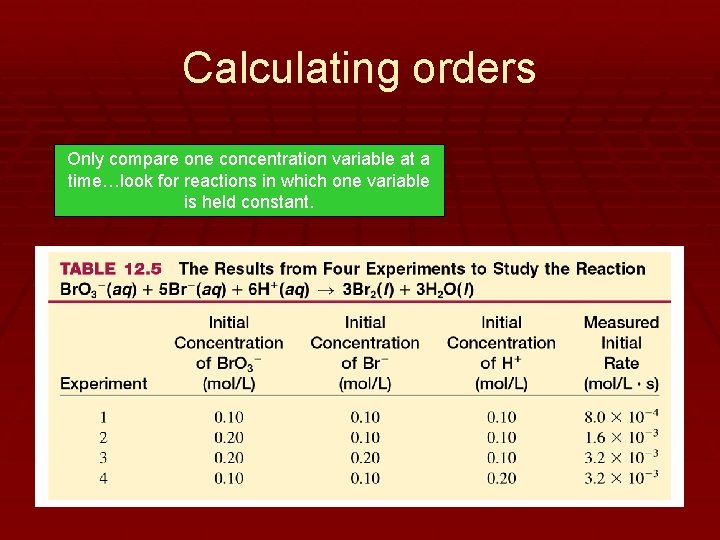
Calculating orders Only compare one concentration variable at a time…look for reactions in which one variable is held constant.

Reaction Mechanisms § A reaction mechanism is a series of smaller reactions by which the overall reaction occurs. § The smaller reactions must add together to give the complete balanced overall reaction. § The experimentally derived rate law must agree with the rate law given in the slowest step of the reaction mechanism.

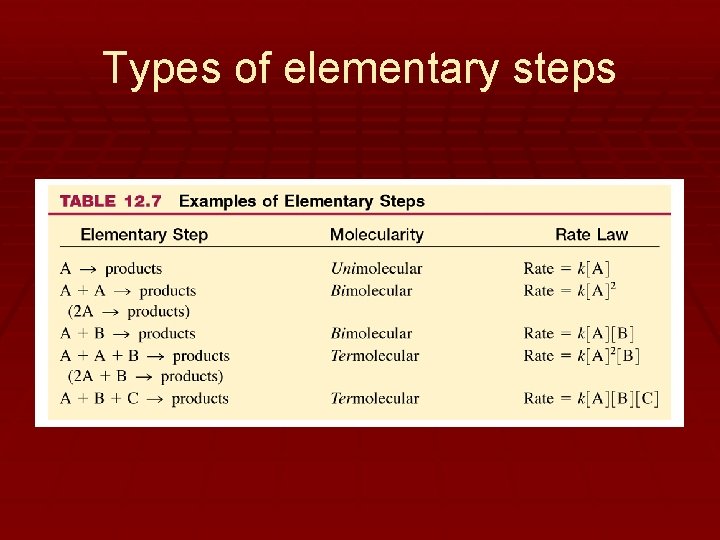
Types of elementary steps

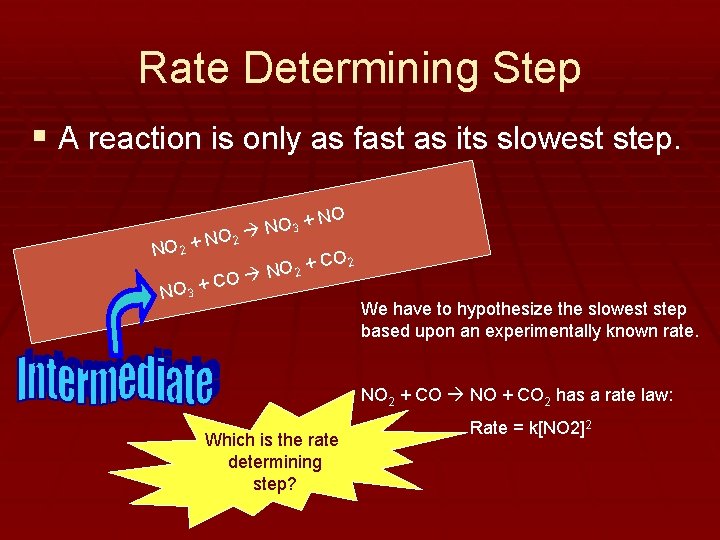
Rate Determining Step § A reaction is only as fast as its slowest step. O 2 N NO 2 + NO 3 + + NO 3 O 2 + N O NO CO 2 C We have to hypothesize the slowest step based upon an experimentally known rate. NO 2 + CO NO + CO 2 has a rate law: Which is the rate determining step? Rate = k[NO 2]2

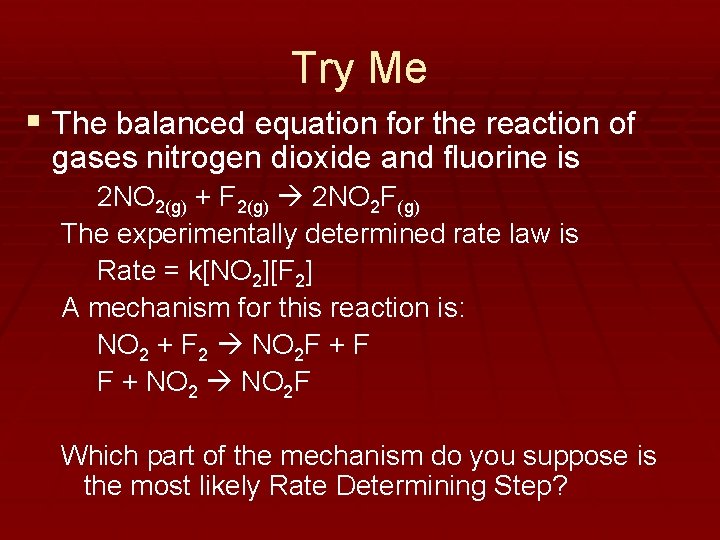
Try Me § The balanced equation for the reaction of gases nitrogen dioxide and fluorine is 2 NO 2(g) + F 2(g) 2 NO 2 F(g) The experimentally determined rate law is Rate = k[NO 2][F 2] A mechanism for this reaction is: NO 2 + F 2 NO 2 F + F F + NO 2 F Which part of the mechanism do you suppose is the most likely Rate Determining Step?

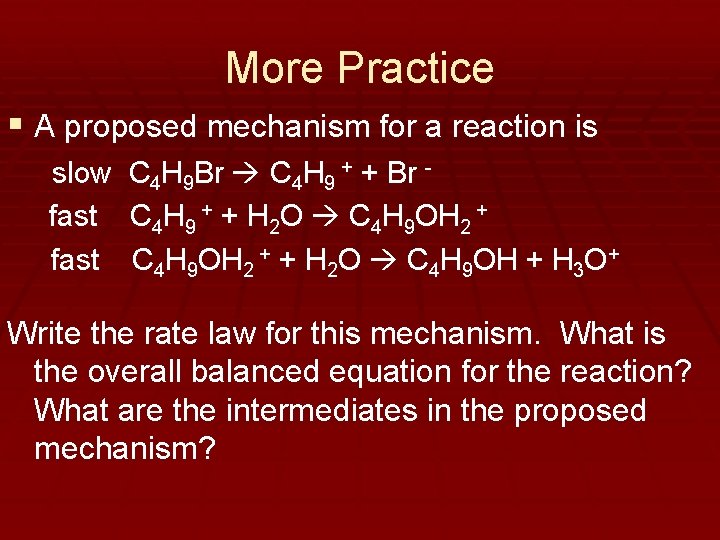
More Practice § A proposed mechanism for a reaction is slow fast C 4 H 9 Br C 4 H 9 + + Br C 4 H 9 + + H 2 O C 4 H 9 OH 2 + + H 2 O C 4 H 9 OH + H 3 O+ Write the rate law for this mechanism. What is the overall balanced equation for the reaction? What are the intermediates in the proposed mechanism?

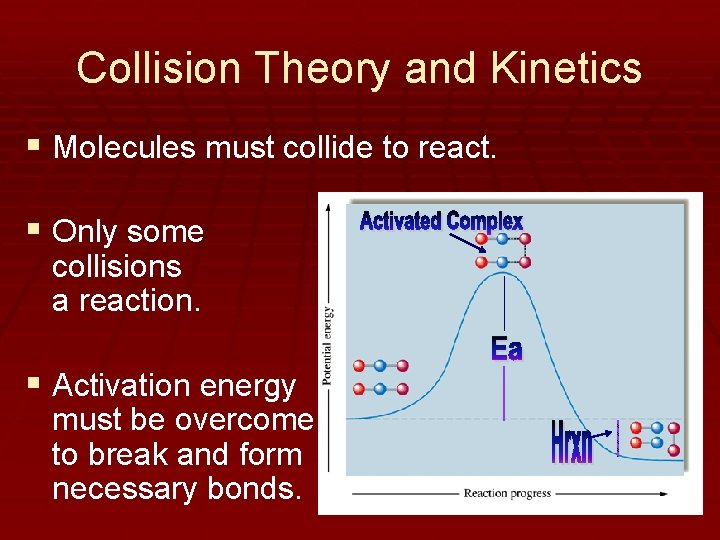
Collision Theory and Kinetics § Molecules must collide to react. § Only some collisions a reaction. § Activation energy must be overcome to break and form necessary bonds. yield

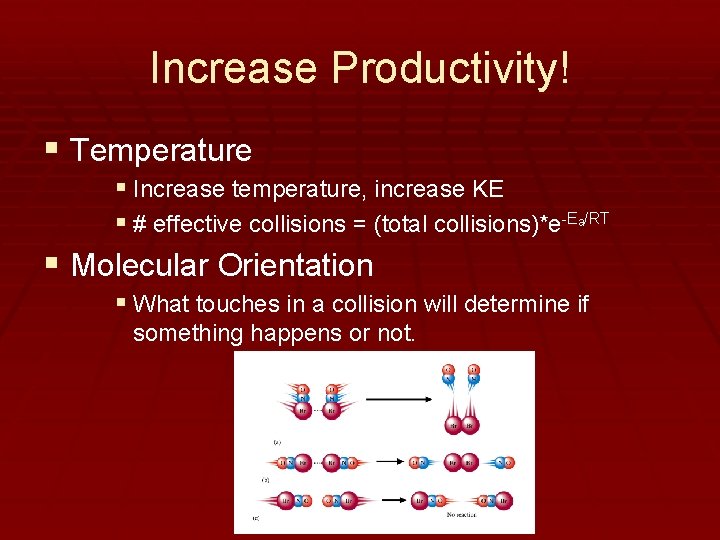
Increase Productivity! § Temperature § Increase temperature, increase KE § # effective collisions = (total collisions)*e-Ea/RT § Molecular Orientation § What touches in a collision will determine if something happens or not.
![Calculating Activation Energy Arrhenius Equation k AeEaRT Natural log of Arrhenius Calculating Activation Energy § Arrhenius Equation: k = [A]e-Ea/RT § Natural log of Arrhenius](https://slidetodoc.com/presentation_image_h2/36ca8485d92c17796be50f230192ee95/image-17.jpg)
Calculating Activation Energy § Arrhenius Equation: k = [A]e-Ea/RT § Natural log of Arrhenius Equation: ln(k) = -(Ea/R)(1/T)+ ln[A]

Try Me!! § The Reaction: 2 N 2 O 5 4 NO 2 + O 2 yielded the following data. Calculate Ea. k (s-1) 2. 0 x 10 -5 7. 3 x 10 -5 2. 7 x 10 -4 9. 1 x 10 -4 2. 9 x 10 -3 T (o. C) 20 30 40 50 60

Derive This Equation: ln(k 2/k 1) = (Ea/R)(1/T 1 – 1//T 2) From These Equations: ln(k 1) = -(Ea/R)(1/T 1)+ ln(A) ln(k 2) = -(Ea/R)(1/T 2)+ ln(A)

Catalysis § Catalyst: a substance that speeds up a reaction without consumed during What do youbeing already know about the process. how a catalyst works? § How? § The presence of a catalyst causes the reaction to take a different reaction mechanism. § The new reaction mechanism has a different slow step. § The new slow step has a lower activation energy.


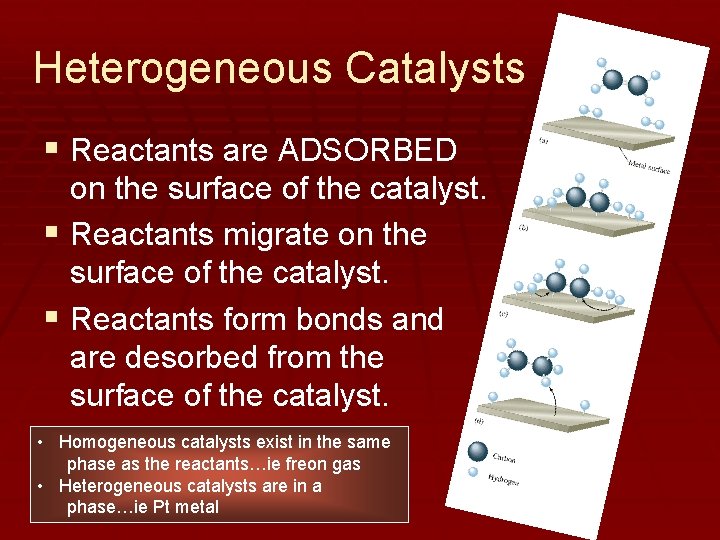
Heterogeneous Catalysts § Reactants are ADSORBED on the surface of the catalyst. § Reactants migrate on the surface of the catalyst. § Reactants form bonds and are desorbed from the surface of the catalyst. • Homogeneous catalysts exist in the same phase as the reactants…ie freon gas • Heterogeneous catalysts are in a phase…ie Pt metal