Kinetics II Reaction Rates Continued Lecture 14 Reaction








- Slides: 14

Kinetics II: Reaction Rates Continued Lecture 14

Reaction Rates • Recall that we defined the rate of reaction as the rate of production of the products, or equivalently, the rate of consumption of the reactants. • Reaction rates for elementary reactions depend on: o Concentrations of reactants o A rate constant, which reflects: • Frequency of opportunity for reaction • Thermal energy available to reactants; i. e. , temperature

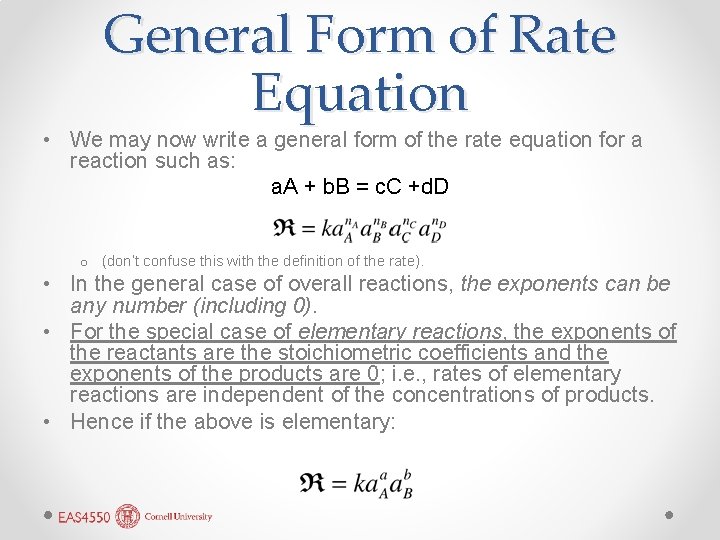
General Form of Rate Equation • We may now write a general form of the rate equation for a reaction such as: a. A + b. B = c. C +d. D o (don’t confuse this with the definition of the rate). • In the general case of overall reactions, the exponents can be any number (including 0). • For the special case of elementary reactions, the exponents of the reactants are the stoichiometric coefficients and the exponents of the products are 0; i. e. , rates of elementary reactions are independent of the concentrations of products. • Hence if the above is elementary:

Order of the Reaction • The order of the reaction is the sum of the exponents of the activities in the rate equation. • For example, formation of carbonic acid CO 2 + H 2 O = H 2 CO 3 • Rate should be (assuming ideality, and an elementary reaction): • So this is a second order reaction. • In this case, however, concentration of water will not change appreciably, so it is a pseudo-first order reaction:

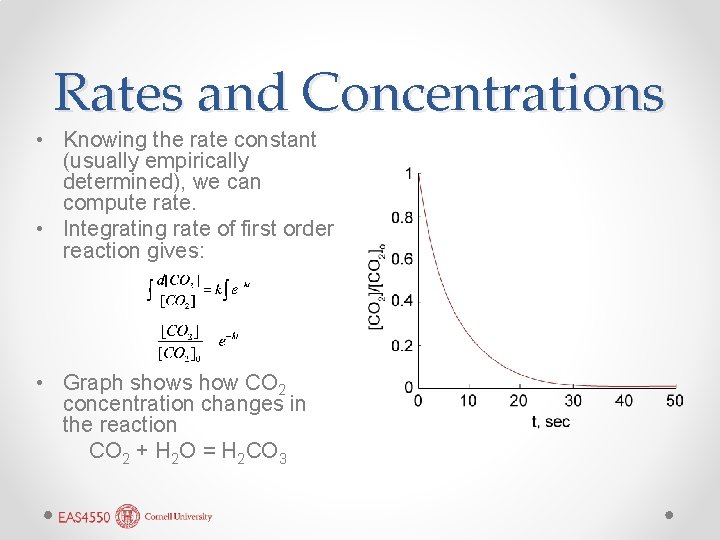
Rates and Concentrations • Knowing the rate constant (usually empirically determined), we can compute rate. • Integrating rate of first order reaction gives: • Graph shows how CO 2 concentration changes in the reaction CO 2 + H 2 O = H 2 CO 3

Distinguishing Elementary from Complex Reactions

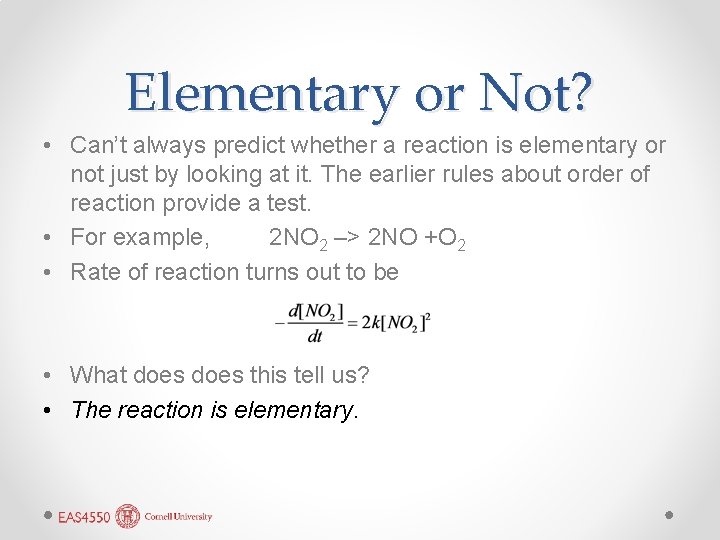
Elementary or Not? • Can’t always predict whether a reaction is elementary or not just by looking at it. The earlier rules about order of reaction provide a test. • For example, 2 NO 2 –> 2 NO +O 2 • Rate of reaction turns out to be • What does this tell us? • The reaction is elementary.

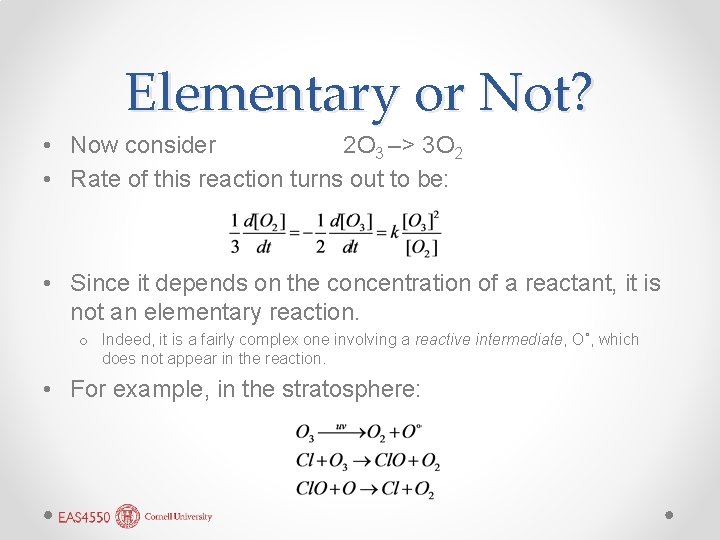
Elementary or Not? • Now consider 2 O 3 –> 3 O 2 • Rate of this reaction turns out to be: • Since it depends on the concentration of a reactant, it is not an elementary reaction. o Indeed, it is a fairly complex one involving a reactive intermediate, O˚, which does not appear in the reaction. • For example, in the stratosphere:

Rates of Complex Reactions • Complex reactions that involve a series of steps that must occur in sequence are called chain reactions. • In a chain reaction when one step is much slower than the others, the overall rate will be determined by that step, which is known as the rate-determining step. • Complex reactions can involve alternate routes or branches (read about H 2 combustion in book). o In a branch, the rate of the fastest branch will determine that step. • Another example of a branched reaction is stratospheric ozone.

Stratospheric Ozone Cycle • Normally, stratospheric ozone is created by photolysis reactions in the stratosphere where photons of sufficient energy (UV) are abundant: • Ozone is then destroyed by a similar photolysis reaction: • • The overall rate is then the sum of the two rates. But if Normally, the rates of these reactions are such that it produces a steady-state the rate of one reaction concentration of ozone. (Ozone is far more likely to be photolyzed (higher rateis far constant), but it is far less abundant, so the rates balance). faster, the rate of the slower The ozone hole has developed because Cl in the stratosphere provides an reaction becomes irrelevant alternate pathway for ozone destruction: and the overall rate is governed by the rate of the fast reaction.

Relating Thermodynamics and Kinetics

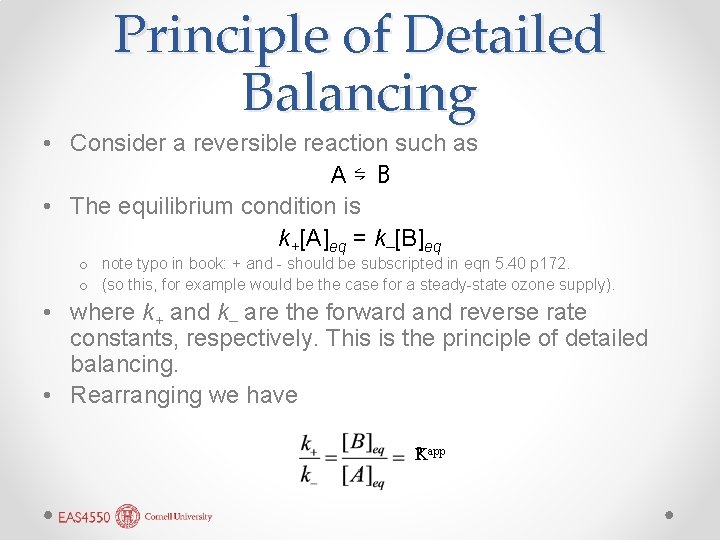
Principle of Detailed Balancing • Consider a reversible reaction such as A⇋ B • The equilibrium condition is k+[A]eq = k–[B]eq o note typo in book: + and - should be subscripted in eqn 5. 40 p 172. o (so this, for example would be the case for a steady-state ozone supply). • where k+ and k– are the forward and reverse rate constants, respectively. This is the principle of detailed balancing. • Rearranging we have ? app K

Relating k and K • We can write the temperature dependence of K as: • For constant ∆Sr: o where C is a constant. • See anything familiar? • Then

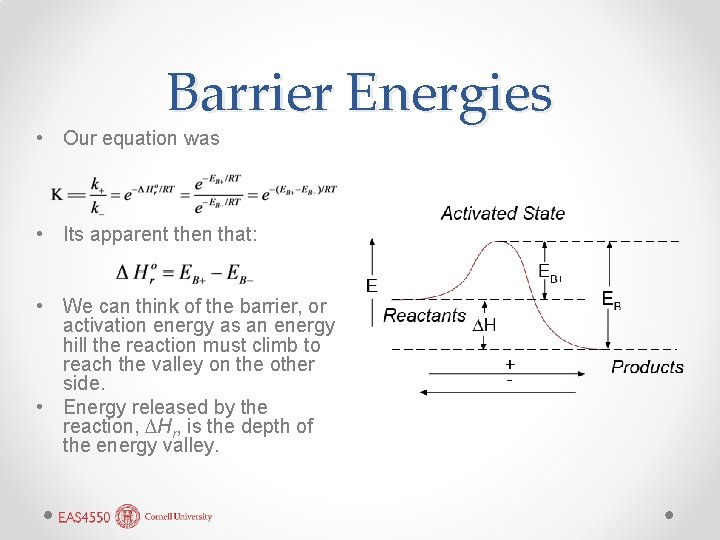
Barrier Energies • Our equation was • Its apparent then that: • We can think of the barrier, or activation energy as an energy hill the reaction must climb to reach the valley on the other side. • Energy released by the reaction, ∆Hr, is the depth of the energy valley.
 Is a ratio a rate
Is a ratio a rate Equivalent ratios
Equivalent ratios Ratios rates and unit rates
Ratios rates and unit rates Ratios rates and unit rates
Ratios rates and unit rates Kinetics reaction
Kinetics reaction 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad Expressing reaction rates
Expressing reaction rates Mini unit reaction rates and equilibrium
Mini unit reaction rates and equilibrium Chapter 18 reaction rates and equilibrium
Chapter 18 reaction rates and equilibrium Chapter 18 review chemical equilibrium section 3 answer key
Chapter 18 review chemical equilibrium section 3 answer key Expressing reaction rates
Expressing reaction rates Section 4 reaction rates and equilibrium
Section 4 reaction rates and equilibrium Reaction rate
Reaction rate Mini unit reaction rates and equilibrium
Mini unit reaction rates and equilibrium Chapter 18 reaction rates and equilibrium
Chapter 18 reaction rates and equilibrium