Kinetics Chemistry Kinetics Study of reaction rates How




![Rate Law (cont. ) • A+B→ C+D • Rate = k [A]m[B]n • • Rate Law (cont. ) • A+B→ C+D • Rate = k [A]m[B]n • •](https://slidetodoc.com/presentation_image_h/36d292f12f9ae00478e727cbef2cd767/image-13.jpg)

- Slides: 20

Kinetics Chemistry

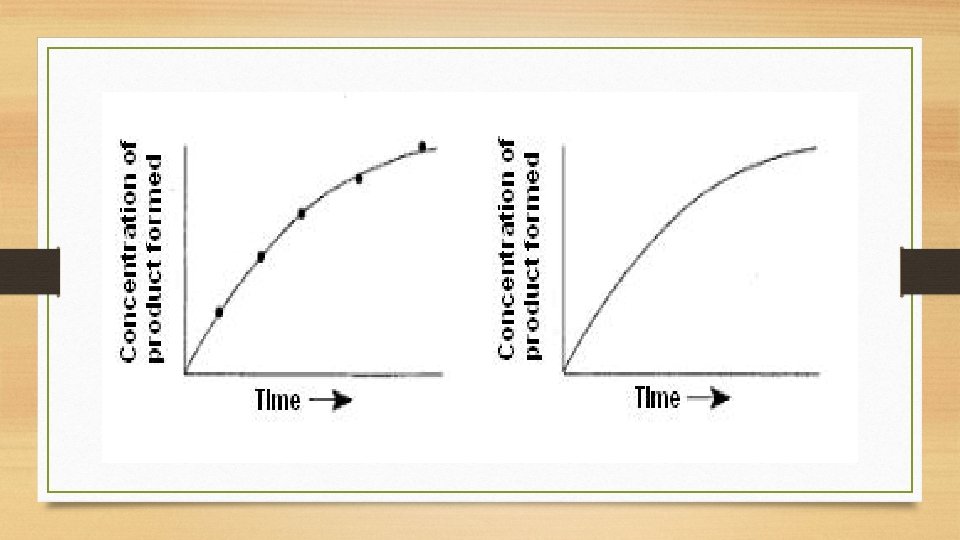
Kinetics • Study of reaction rates • How fast does it happen? What variables influence the rate? What is the path the reaction takes to convert reactants to products? • Rate • Change in an amount over a period of time • Ex. Distance traveled, space travel

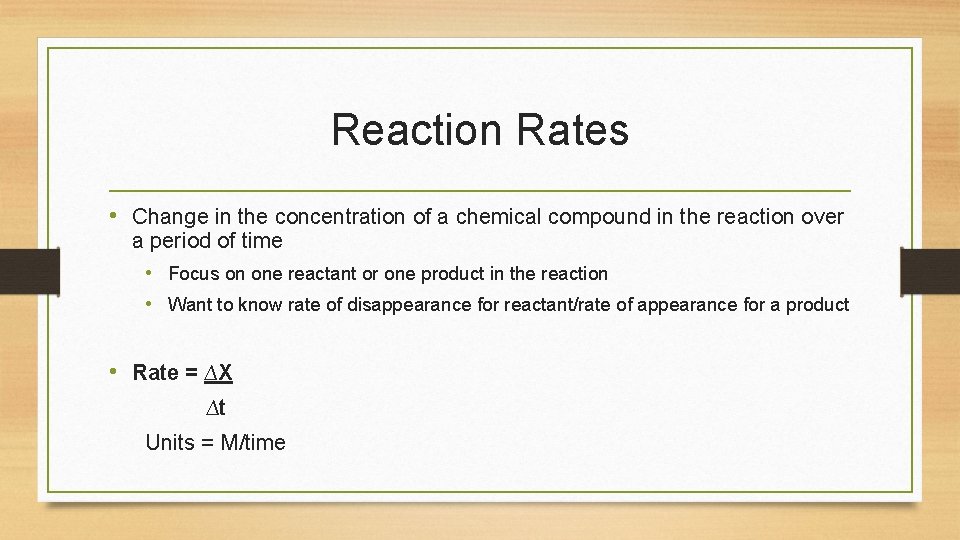
Reaction Rates • Change in the concentration of a chemical compound in the reaction over a period of time • Focus on one reactant or one product in the reaction • Want to know rate of disappearance for reactant/rate of appearance for a product • Rate = ∆X ∆t Units = M/time


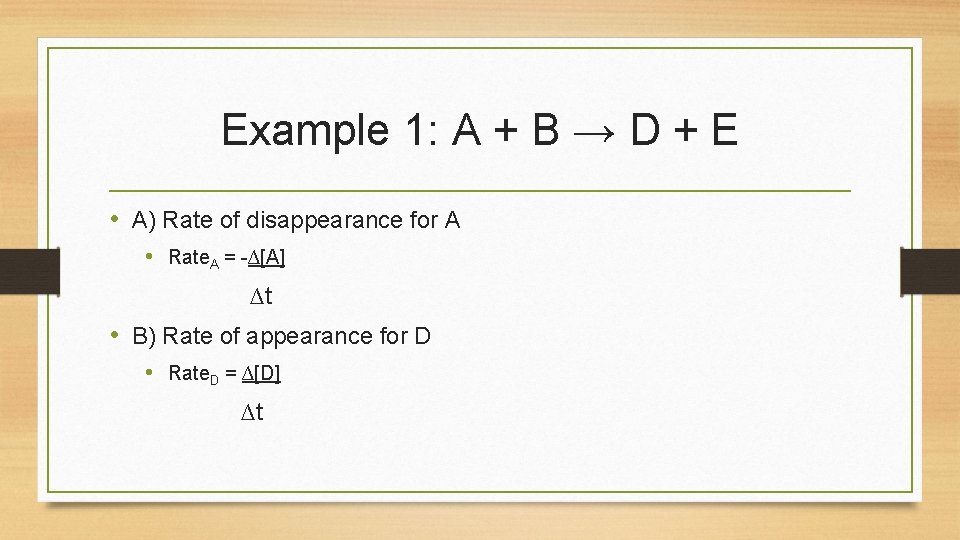
Example 1: A + B → D + E • A) Rate of disappearance for A • Rate. A = -∆[A] ∆t • B) Rate of appearance for D • Rate. D = ∆[D] ∆t

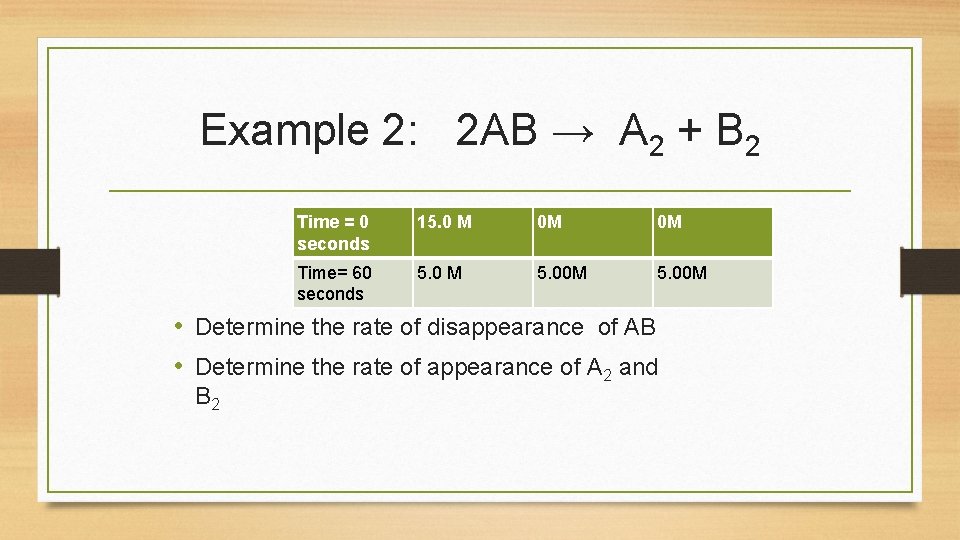
Example 2: 2 AB → A 2 + B 2 Time = 0 seconds 15. 0 M 0 M 0 M Time= 60 seconds 5. 0 M 5. 00 M • Determine the rate of disappearance of AB • Determine the rate of appearance of A 2 and B 2

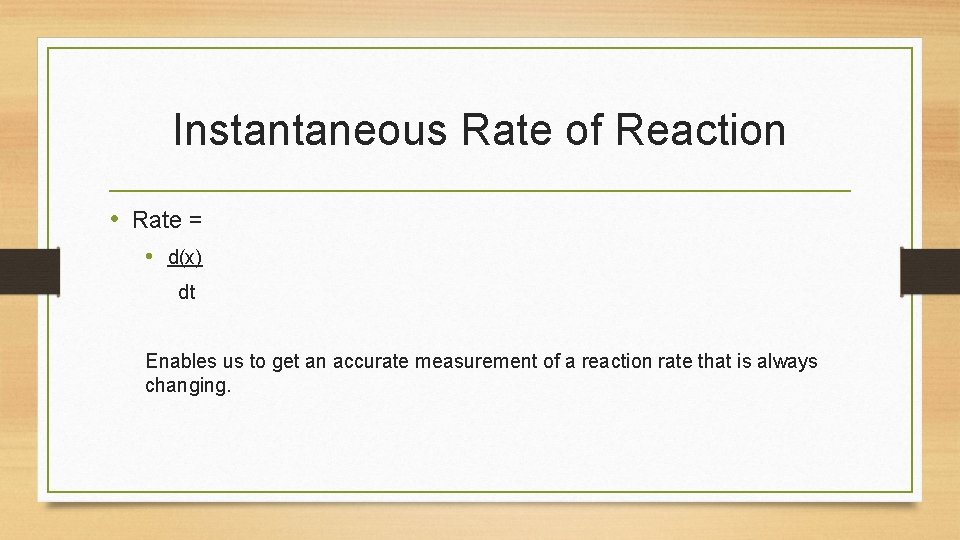
Instantaneous Rate of Reaction • Rate = • d(x) dt Enables us to get an accurate measurement of a reaction rate that is always changing.


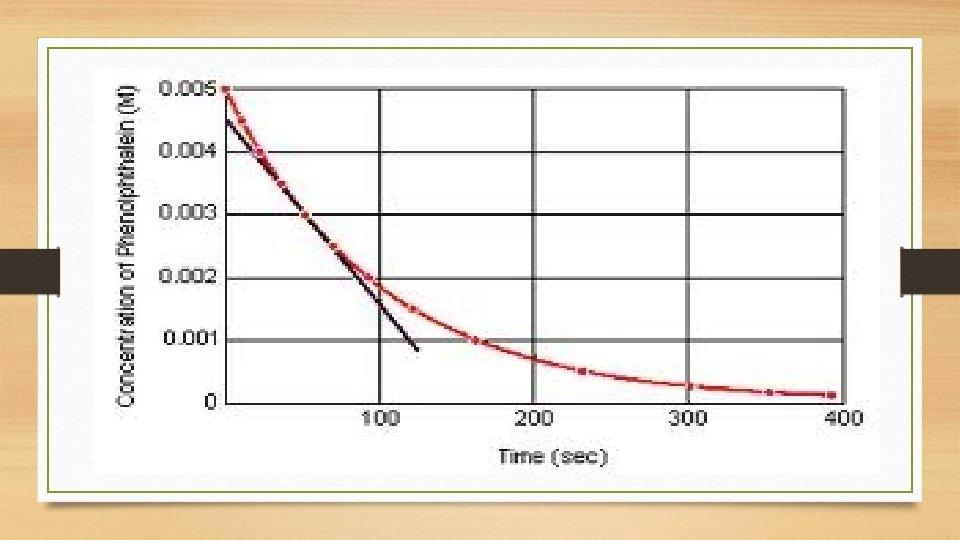
Reaction Rate at ANY moment in time • Slope of line tangent to the reaction curve

Measuring Reaction Rates **Colorimeter/Spectrophotometer • p. H changes • Volume changes • Amount of precipitate formed

What can influence reaction rates? 1) 2) 3) 4) 5) 6) Temperature Concentration Catalyst Surface Area Volume/Pressure Reactant Properties

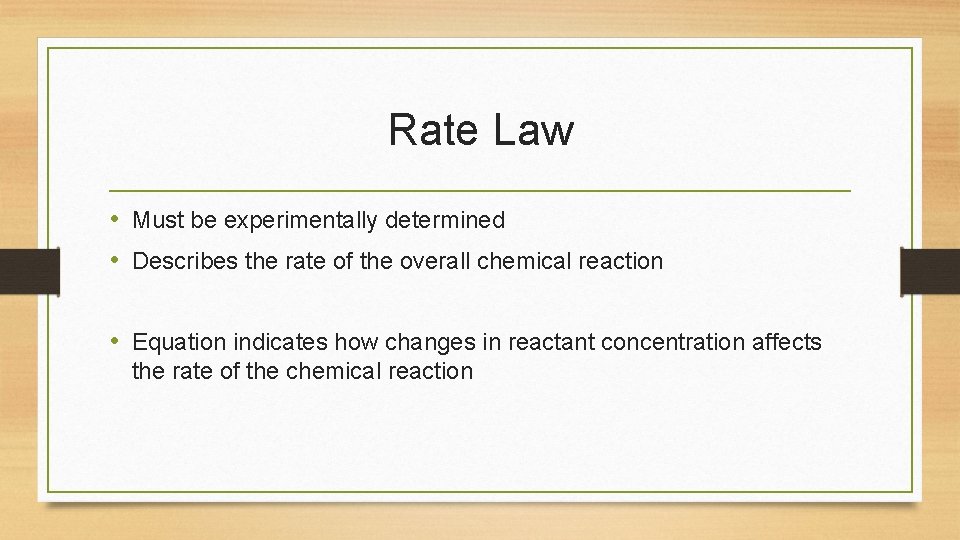
Rate Law • Must be experimentally determined • Describes the rate of the overall chemical reaction • Equation indicates how changes in reactant concentration affects the rate of the chemical reaction
![Rate Law cont AB CD Rate k AmBn Rate Law (cont. ) • A+B→ C+D • Rate = k [A]m[B]n • •](https://slidetodoc.com/presentation_image_h/36d292f12f9ae00478e727cbef2cd767/image-13.jpg)
Rate Law (cont. ) • A+B→ C+D • Rate = k [A]m[B]n • • • Rate = rate of disappearance of reactants k = rate constant, specific to reactions and temperature m = reaction order in terms of A n = reaction order in terms of B m + n = overall reaction order

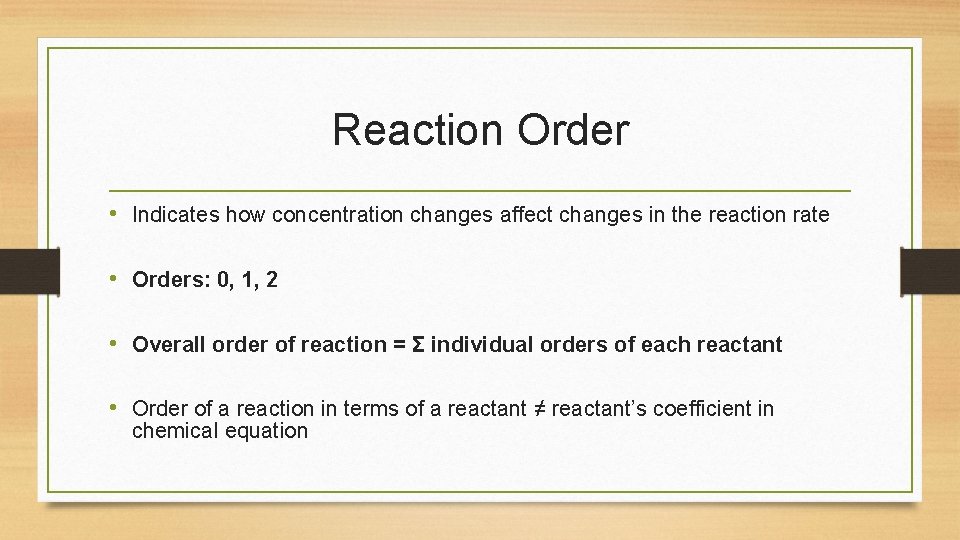
Reaction Order • Indicates how concentration changes affect changes in the reaction rate • Orders: 0, 1, 2 • Overall order of reaction = Σ individual orders of each reactant • Order of a reaction in terms of a reactant ≠ reactant’s coefficient in chemical equation

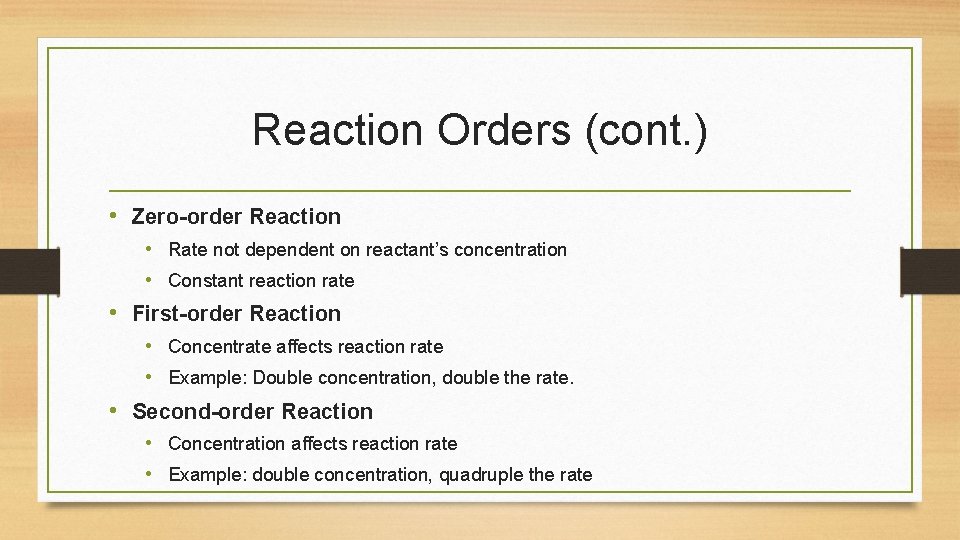
Reaction Orders (cont. ) • Zero-order Reaction • Rate not dependent on reactant’s concentration • Constant reaction rate • First-order Reaction • Concentrate affects reaction rate • Example: Double concentration, double the rate. • Second-order Reaction • Concentration affects reaction rate • Example: double concentration, quadruple the rate

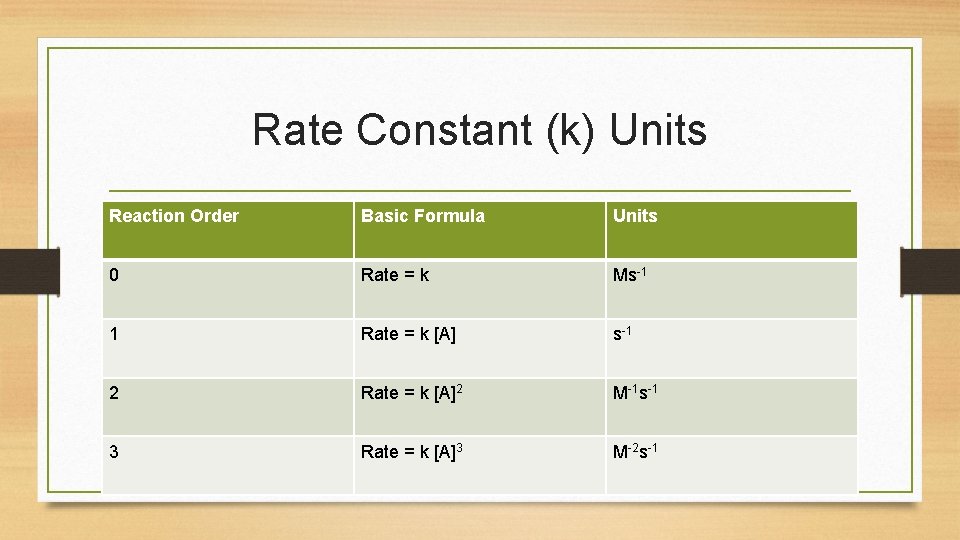
Rate Constant (k) Units Reaction Order Basic Formula Units 0 Rate = k Ms-1 1 Rate = k [A] s-1 2 Rate = k [A]2 M-1 s-1 3 Rate = k [A]3 M-2 s-1

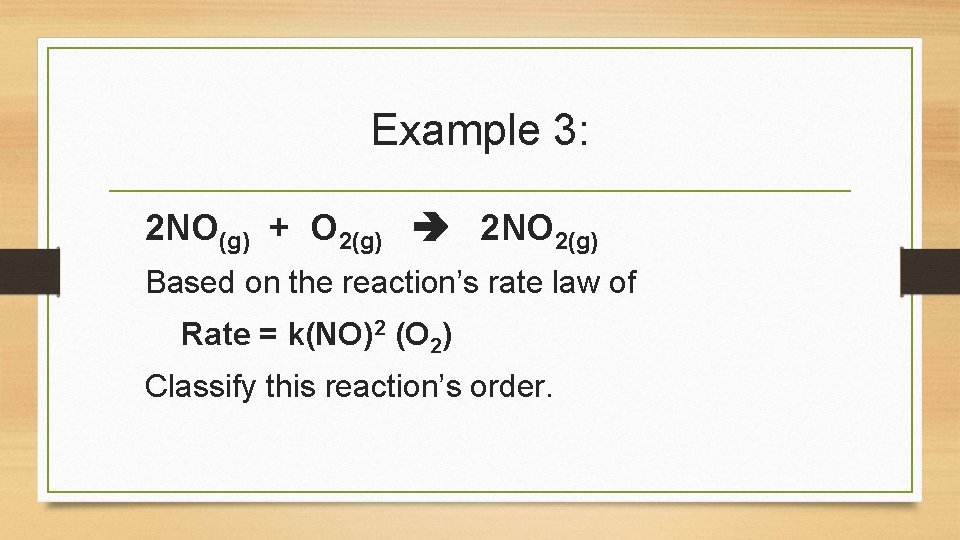
Example 3: 2 NO(g) + O 2(g) 2 NO 2(g) Based on the reaction’s rate law of Rate = k(NO)2 (O 2) Classify this reaction’s order.

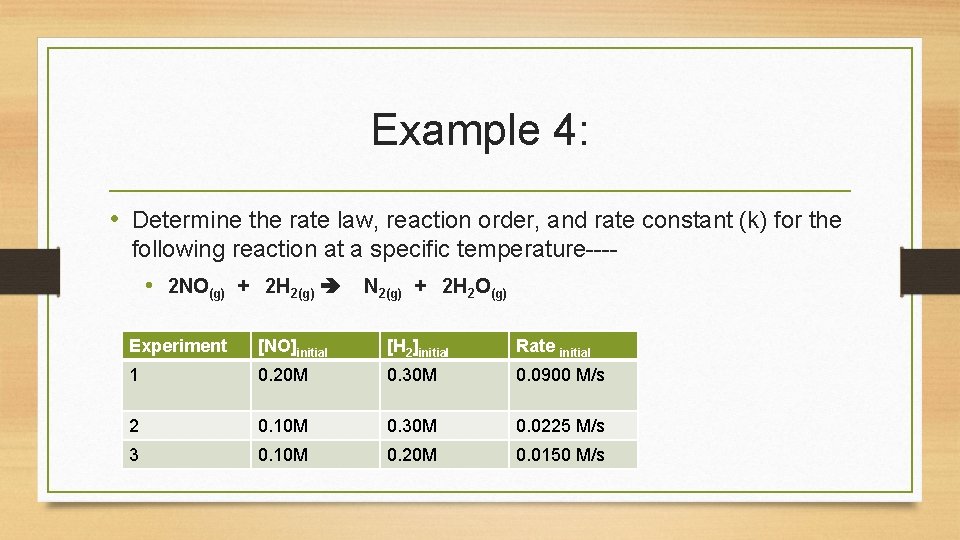
Example 4: • Determine the rate law, reaction order, and rate constant (k) for the following reaction at a specific temperature--- • 2 NO(g) + 2 H 2(g) N 2(g) + 2 H 2 O(g) Experiment [NO]initial [H 2]initial Rate initial 1 0. 20 M 0. 30 M 0. 0900 M/s 2 0. 10 M 0. 30 M 0. 0225 M/s 3 0. 10 M 0. 20 M 0. 0150 M/s

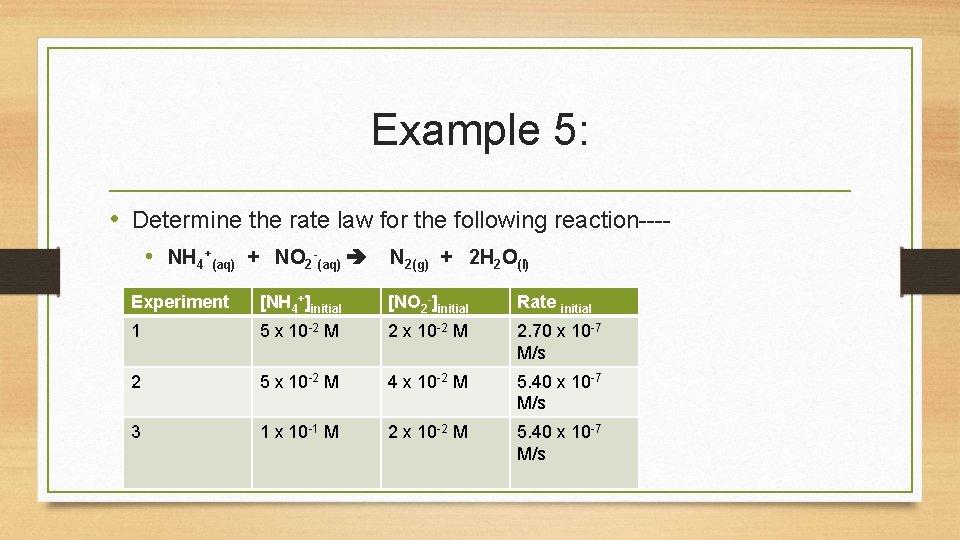
Example 5: • Determine the rate law for the following reaction--- • NH 4+(aq) + NO 2 -(aq) N 2(g) + 2 H 2 O(l) Experiment [NH 4+]initial [NO 2 -]initial Rate initial 1 5 x 10 -2 M 2. 70 x 10 -7 M/s 2 5 x 10 -2 M 4 x 10 -2 M 5. 40 x 10 -7 M/s 3 1 x 10 -1 M 2 x 10 -2 M 5. 40 x 10 -7 M/s

Homework • Kinetics Worksheet #1 -6
 Is a ratio a rate
Is a ratio a rate Ratios guided notes
Ratios guided notes Ratios rates and unit rates
Ratios rates and unit rates Ratios rates and unit rates
Ratios rates and unit rates Kinetics reaction
Kinetics reaction Molecularity
Molecularity Chemistry grade 11 unit 4 chemical kinetics
Chemistry grade 11 unit 4 chemical kinetics Kinetics half life
Kinetics half life Ap chem kinetics practice problems
Ap chem kinetics practice problems Mini unit reaction rates and equilibrium
Mini unit reaction rates and equilibrium Mini unit reaction rates and equilibrium
Mini unit reaction rates and equilibrium Chapter 18 review chemical equilibrium section 3 answer key
Chapter 18 review chemical equilibrium section 3 answer key Section 4 reaction rates and equilibrium
Section 4 reaction rates and equilibrium Chapter 18 reaction rates and equilibrium answer key
Chapter 18 reaction rates and equilibrium answer key Chapter 18 reaction rates and equilibrium
Chapter 18 reaction rates and equilibrium Expressing reaction rates
Expressing reaction rates Rates of reaction can be positive or negative. true false
Rates of reaction can be positive or negative. true false Expressing reaction rates
Expressing reaction rates Reaction rates
Reaction rates Conformity psychology
Conformity psychology Rate law
Rate law






































