Unit 1 Reaction Kinetics Lesson 2 Reaction Rates






- Slides: 10

Unit 1: Reaction Kinetics Lesson 2: Reaction Rates and How to Change Them

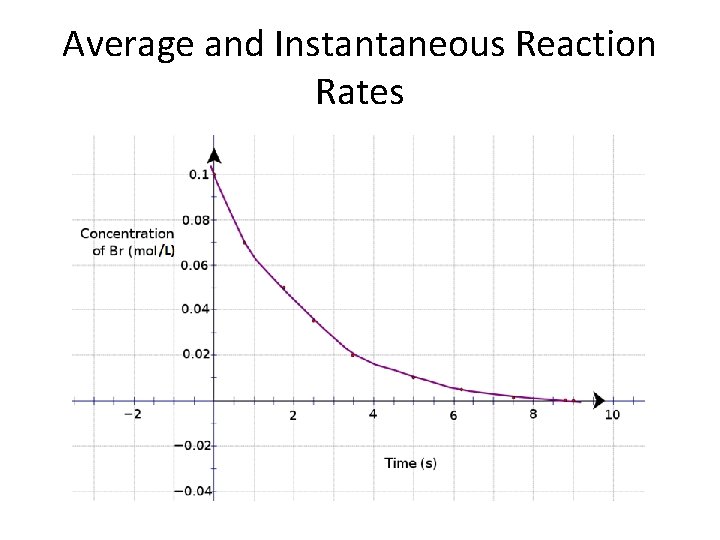
Where we. . . ? • We left off looking at how to measure reaction rates. We graphed mass vs. time and noted that you could also use properties such as temperature, gas pressure, or colour intensity to determine reaction rate. • Today we’ll look more closely at a reaction rate graph. . .

Average and Instantaneous Reaction Rates

Your Turn! • Pg. 11 #18 and 19 a, b.

Brain Break!

Collision Theory • In order to react, two molecules must collide with sufficient energy and correct geometry. • A collision that results in a reaction is referred to as an effective collision. • Collision theory can help us understand why certain factors affect reaction rates. . .

Factors Affecting Reaction Rates 1) Temperature: Temperature is a measure of the average kinetic energy of the particles in a substance. Thus, if temperature increases, the particles have more kinetic energy, so they move faster. This causes them to collide more often and with more energy, which increases the reaction rate.

Factors Affecting Reaction Rates 2) Concentration Recall that concentration (usually mol/L) is a measure of the number of particles in a given amount of space. If the concentration of a reactant increases, then there are more particles in a given amount of space. Thus the particles will collide more often, so the reaction rate increases.

Factors Affecting Reaction Rates 3) Pressure is really just the concentration of a gas – the number of gas particles in a given amount of space. Thus pressure has the same effect on reaction rate as concentration does – when the pressure increases, the reaction rate increases (and vice versa).

Homework: • Pg. 11 #18 and 19 a, b (if not already finished) and pg. 10 #17.