Interaction of radiation matter Electromagnetic radiation in different




























- Slides: 55

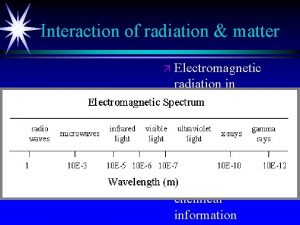
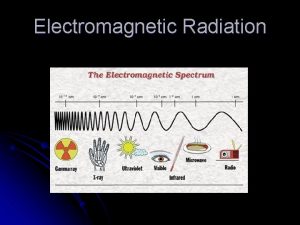
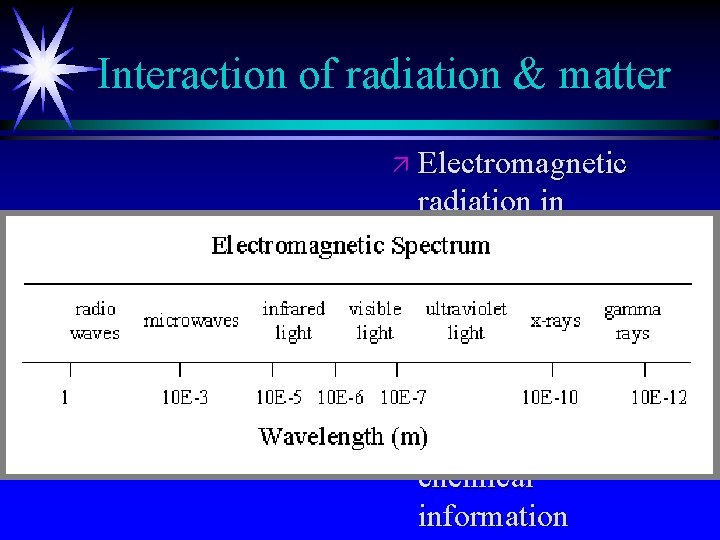
Interaction of radiation & matter ä Electromagnetic radiation in different regions of spectrum can be used for qualitative and quantitative information ä Different types of chemical information

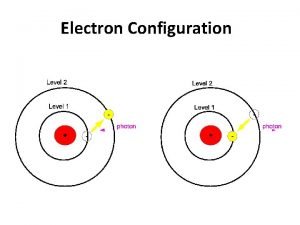
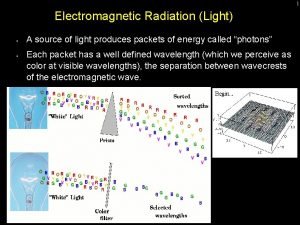
Energy transfer from photon to molecule or atom At room temperature most molecules are at lowest electronic & vibrational state IR radiation can excite vibrational levels that then lose energy quickly in collisions with surroundings

UV Visible Spectrometry äabsorption - specific energy äemission - excited molecule emits äfluorescence äphosphorescence

What happens to molecule after excitation ä collisions deactivate vibrational levels (heat) ä emission of photon (fluorescence) ä intersystem crossover (phosphorescence)

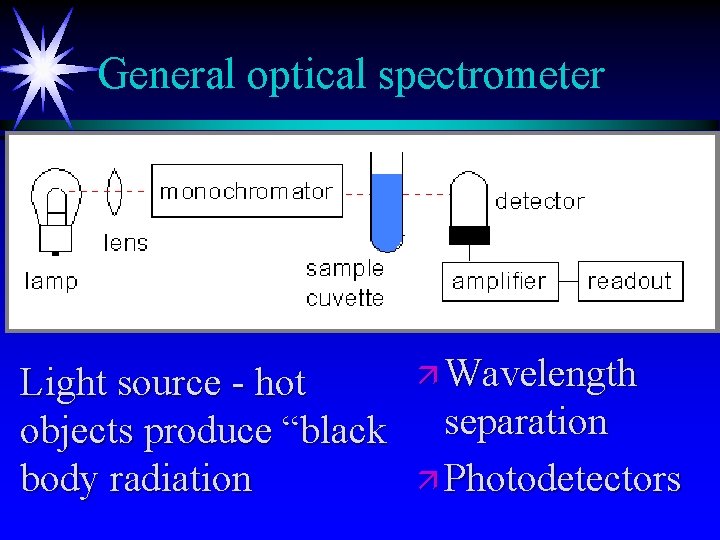
General optical spectrometer Light source - hot objects produce “black body radiation ä Wavelength separation ä Photodetectors

Black body radiation ä Tungsten lamp, Globar, Nernst glower ä Intensity and peak emission wavelength are a function of Temperature ä As T increases the total intensity increases and there is shift to higher energies (toward visible and UV)

UV sources ä Arc discharge lamps with electrical discharge maintained in appropriate gases ä Low pressure hydrogen and deuterium lamps ä Lasers - narrow spectral widths, very high intensity, spatial beam, time resolution, problem with range of wavelengths ä Discrete spectroscopic- metal vapor & hollow cathode lamps

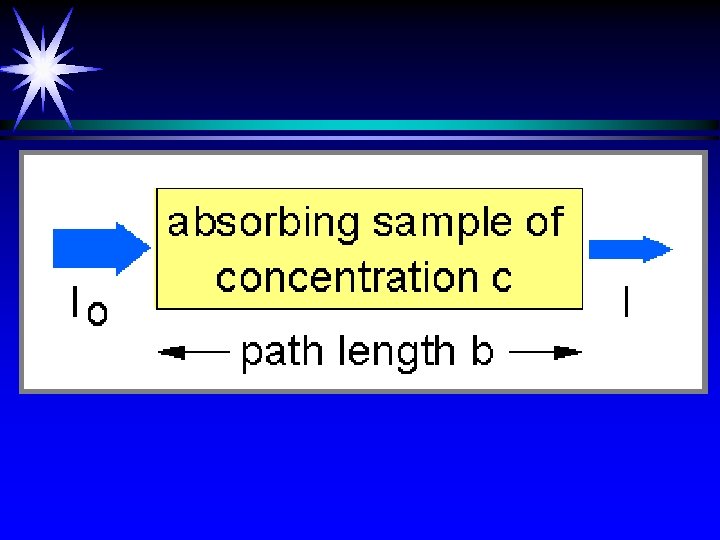
Why separate wavelengths? ä Each compound absorbs different colors (energies) with different probabilities (absorbtivity) ä Selectivity ä Quantitative adherence to Beer’s Law A = abc ä Improves sensitivity

Why are UV-Vis bands broad? ä Electronic energy states give band with no vibrational structure ä Solvent interactions (microenvironments) averaged ä Low temperature gas phase molecules give structure if instrumental resolution is adequate

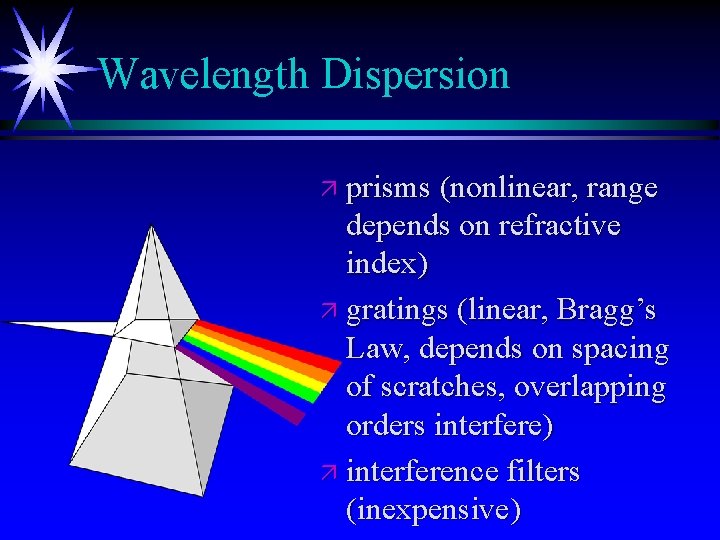
Wavelength Dispersion ä prisms (nonlinear, range depends on refractive index) ä gratings (linear, Bragg’s Law, depends on spacing of scratches, overlapping orders interfere) ä interference filters (inexpensive)

Monochromator ä Entrance slit - provides narrow optical image ä Collimator - makes light hit dispersive element at same angle ä Dispersing element - directional ä Focusing element - image on slit ä Exit slit - isolates desired color to exit

Resolution ä The ability to distinguish different wavelengths of light - R=l/Dl R= ä Linear dispersion - range of wavelengths spread over unit distance at exit slit ä Spectral bandwidth - range of wavelengths included in output of exit slit (FWHM) ä Resolution depends on how widely light is dispersed & how narrow a slice chosen

Filters - inexpensive alternative ä Adsorption type - glass with dyes to adsorb chosen colors ä Interference filters - multiple reflections between 2 parallel reflective surfaces - only certain wavelengths have positive interferences temperature effects spacing between surfaces

Wavelength dependence in spectrometer ä Source ä Monochromator ä Detector ä Sample - We hope so!

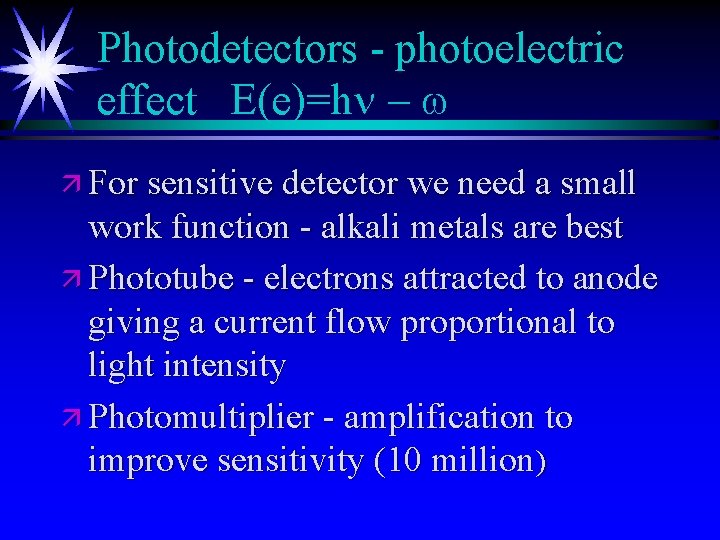
Photodetectors - photoelectric effect E(e)=hn - w ä For sensitive detector we need a small work function - alkali metals are best ä Phototube - electrons attracted to anode giving a current flow proportional to light intensity ä Photomultiplier - amplification to improve sensitivity (10 million)

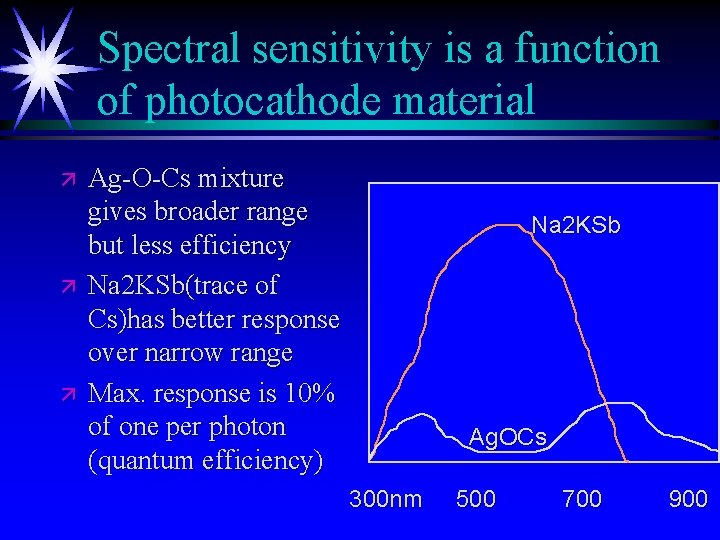
Spectral sensitivity is a function of photocathode material ä ä ä Ag-O-Cs mixture gives broader range but less efficiency Na 2 KSb(trace of Cs)has better response over narrow range Max. response is 10% of one per photon (quantum efficiency) Na 2 KSb Ag. OCs 300 nm 500 700 900

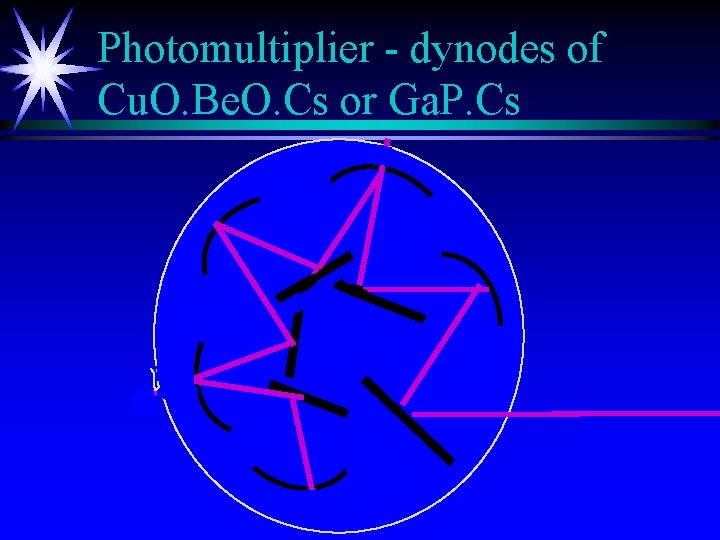
Photomultiplier - dynodes of Cu. O. Be. O. Cs or Ga. P. Cs

Cooled Photomultiplier Tube

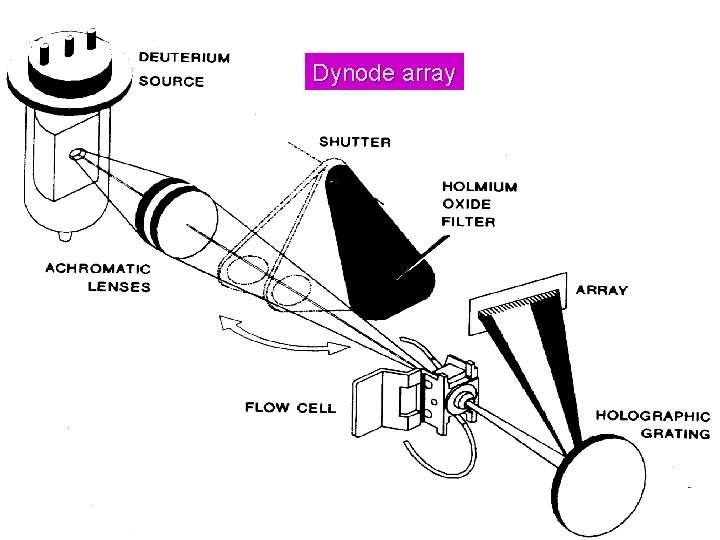
Dynode array

Photodiodes - semiconductor that conducts in one direction only when light is present ä Rugged and small ä Photodiode arrays - allows observation of a number of different locations (wavelengths) simultaneously ä Somewhat less sensitive than PMT


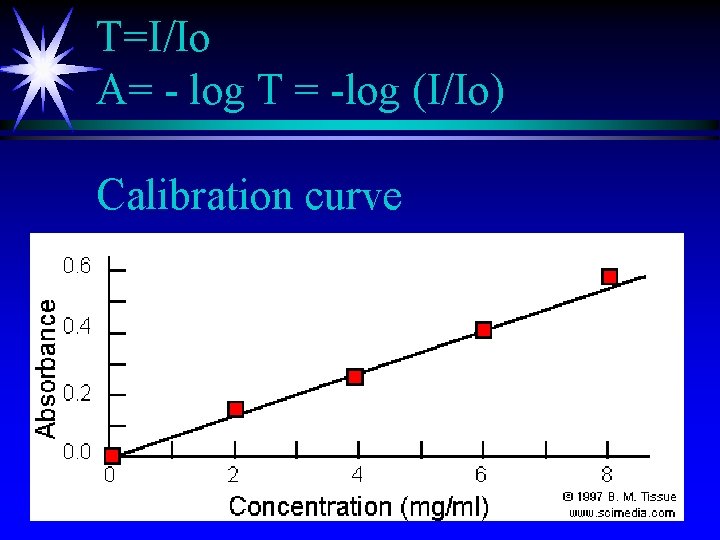
T=I/Io A= - log T = -log (I/Io) Calibration curve


Deviations from Beer’s Law ä High concentrations (0. 01 M) distort each molecules electronic structure & spectra ä Chemical equilibrium ä Stray light ä Polychromatic light ä Interferences

Interpretation - quantitative ä Broad adsorption bands - considerable overlap ä Specral dependence upon solvents ä Resolving mixtures as linear combinations - need to measure as many wavelengths as components ä Beer’s Law. html

Resolving mixtures ä Measure at different wavelengths and solve mathematically ä Use standard additions (measure A and then add known amounts of standard) ä Chemical methods to separate or shift spectrum ä Use time resolution (fluorescence and phosphorescence)

Improving resolution in mixtures ä Instrumental (resolution) ä Mathematical (derivatives) ä Use second parameter (fluorescence) ä Use third parameter (time for phosphorescence) ä Chemical separations (chromatography)

Fluorescence ä Emission at lower energy than absorption ä Greater selectivity but fluorescent yields vary for different molecules ä Detection at right angles to excitation ä S/N is improved so sensitivity is better ä Fluorescent tags

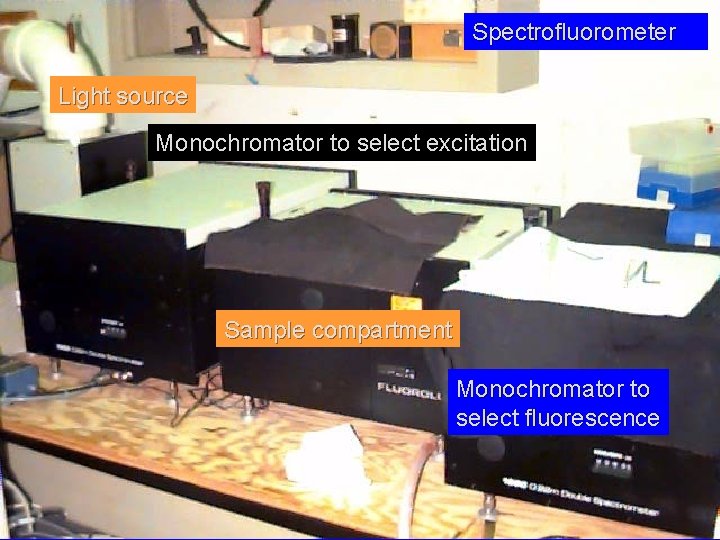
Spectrofluorometer Light source Monochromator to select excitation Sample compartment Monochromator to select fluorescence

Photoacoustic spectroscopy ä Edison’s observations ä If light is pulsed then as gas is excited it can expand (sound)


Principles of IR ä Absorption of energy at various frequencies is detected by IR ä plots the amount of radiation transmitted through the sample as a function of frequency ä compounds have “fingerprint” region of identity

Infrared Spectrometry ä Is especially useful for qualitative analysis ä functional groups ä other structural features ä establishing purity ä monitoring rates ä measuring concentrations ä theoretical studies

How does it work? ä Continuous beam of radiation ä Frequencies display different absorbances ä Beam comes to focus at entrance slit ä molecule absorbs radiation of the energy to excite it to the vibrational state

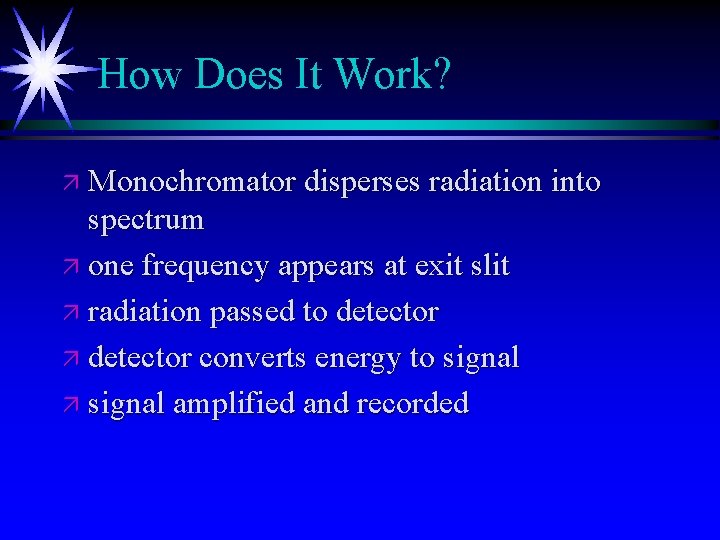
How Does It Work? ä Monochromator disperses radiation into spectrum ä one frequency appears at exit slit ä radiation passed to detector ä detector converts energy to signal ä signal amplified and recorded

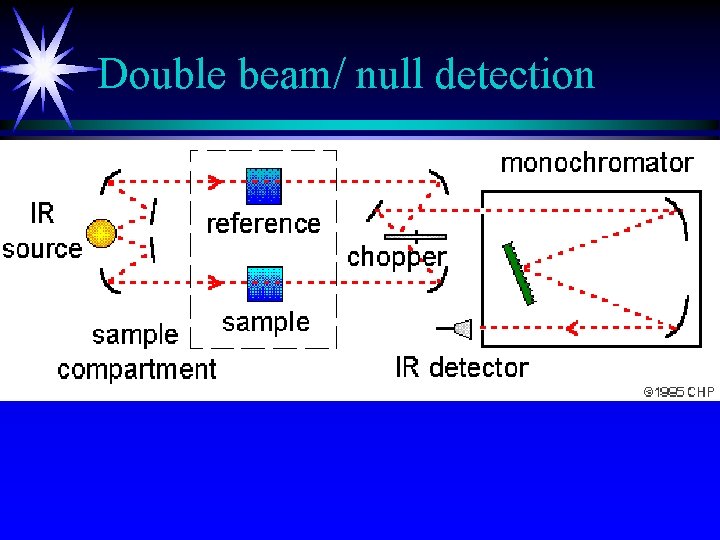
Instrumentation II ä Optical-null double-beam instruments ä Radiation is directed through both cells by mirrors ä sample beam and reference beam ä chopper ä diffraction grating

Double beam/ null detection

Instrumentation III ä Exit slit ä detector ä servo motor ä Resulting spectrum is a plot of the intensity of the transmitted radiation versus the wavelength

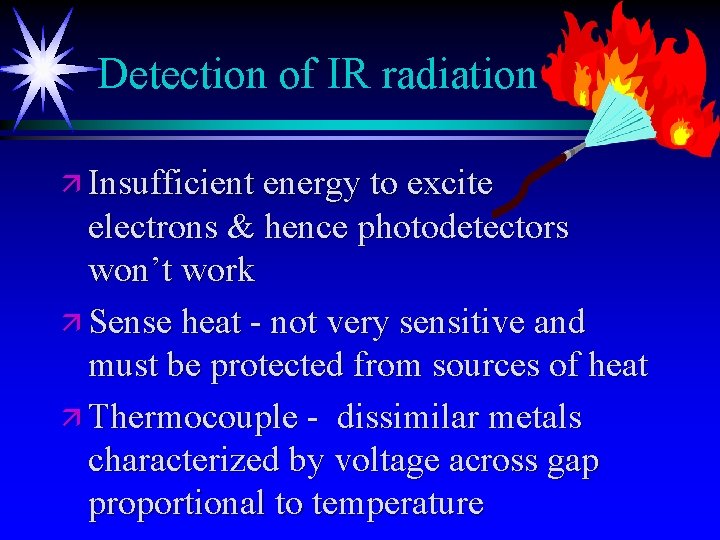
Detection of IR radiation ä Insufficient energy to excite electrons & hence photodetectors won’t work ä Sense heat - not very sensitive and must be protected from sources of heat ä Thermocouple - dissimilar metals characterized by voltage across gap proportional to temperature

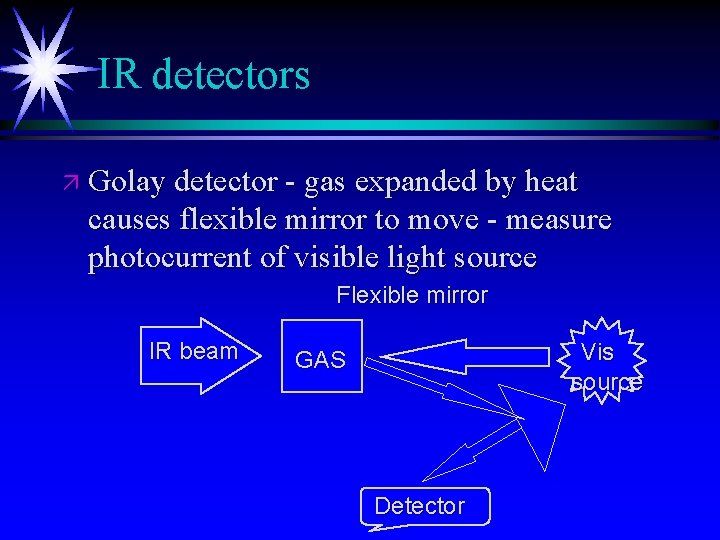
IR detectors ä Golay detector - gas expanded by heat causes flexible mirror to move - measure photocurrent of visible light source Flexible mirror IR beam Vis source GAS Detector

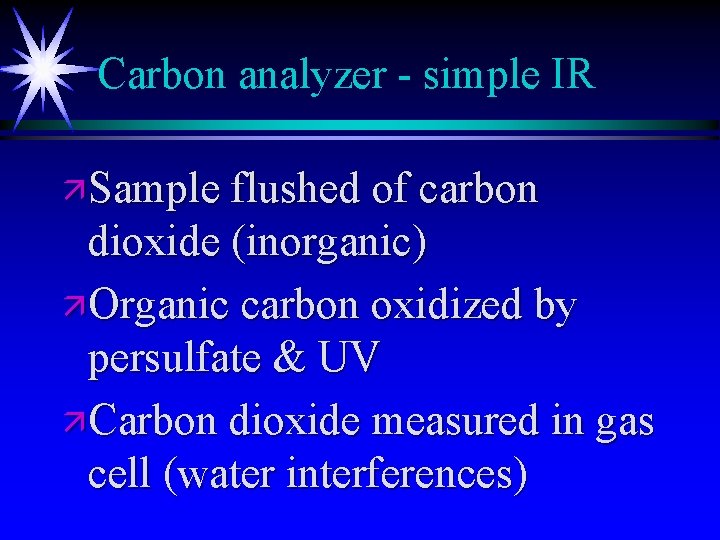
Carbon analyzer - simple IR ä Sample flushed of carbon dioxide (inorganic) ä Organic carbon oxidized by persulfate & UV ä Carbon dioxide measured in gas cell (water interferences)

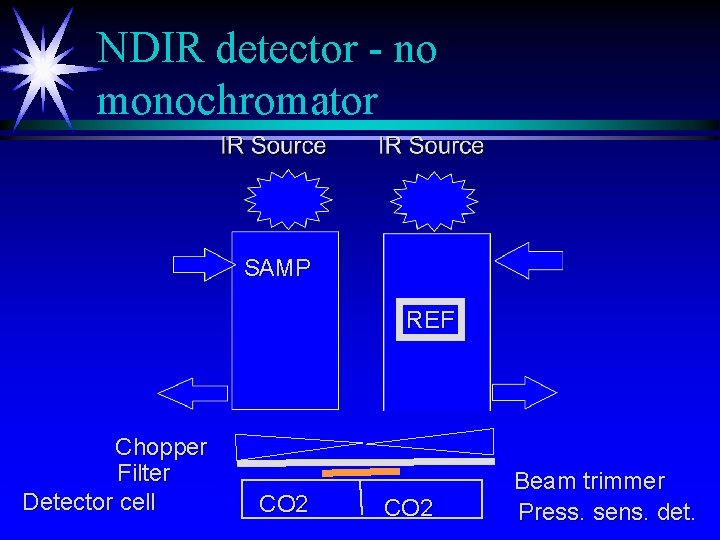
NDIR detector - no monochromator SAMP REF Chopper Filter Detector cell CO 2 Beam trimmer Press. sens. det.

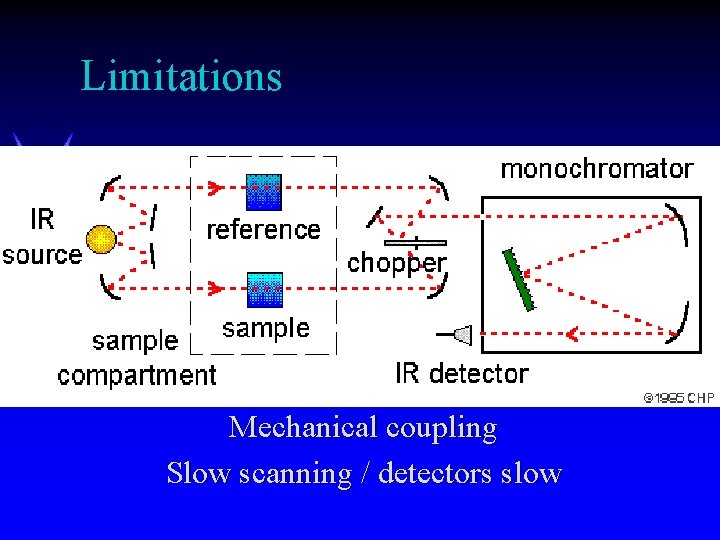
Limitations Mechanical coupling Slow scanning / detectors slow

Limitations of Dispersive IR ä Mechanically complex ä Sensitivity limited ä Requires external calibration ä Tracking errors limit resolution (scanning fast broadens peak, decreases absorbance, shifts peak

Problems with IR ä c no quantitative äH limited resolution ä D not reproducible ä A limited dynamic range ä I limited sensitivity ä E long analysis time ä B functional groups

Limitations ä ä ä Most equipment can measure one wavelength at a time Potentially timeconsuming A solution?

Fourier-Transform Infrared Spectroscopy (FTIR) A Solution!

FTIR ä Analyze all wavelengths simultaneously ä signal decoded to generate complete spectrum ä can be done quickly ä better resolution ä more resolution ä However, . . .

FTIR ä ä A solution, yet an expensive one! FTIR uses sophisticated machinery more complex than generic GCIR

Fourier Transform IR ä Mechanically simple ä Fast, sensitive, accurate ä Internal calibration ä No tracking errors or stray light

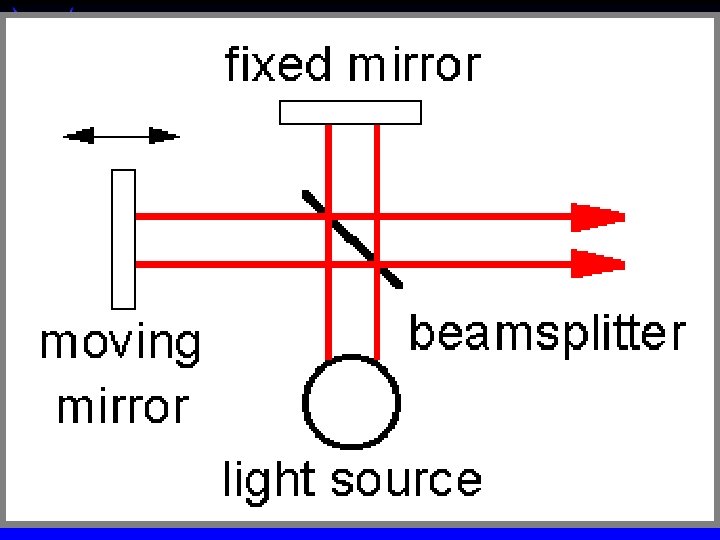
IR Spectroscopy - qualitative Double beam required to correct for blank at each wavelength ä Scan time (sensitivity) Vs resolution ä Michelson interferometer & FTIR

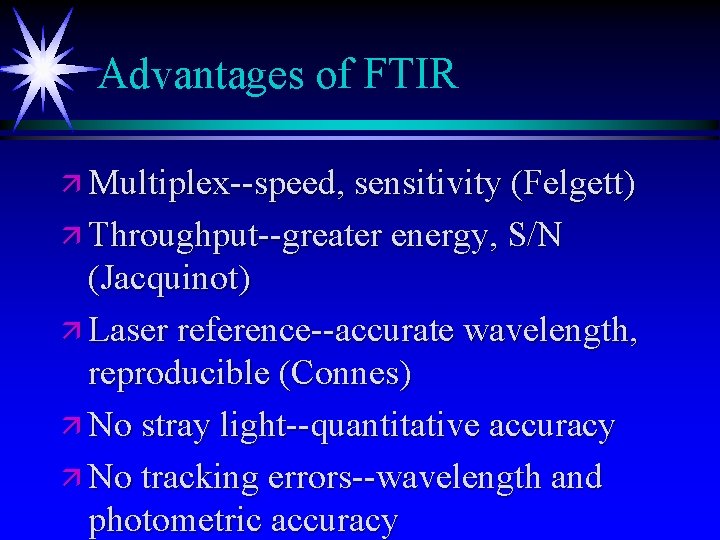
Advantages of FTIR ä Multiplex--speed, sensitivity (Felgett) ä Throughput--greater energy, S/N (Jacquinot) ä Laser reference--accurate wavelength, reproducible (Connes) ä No stray light--quantitative accuracy ä No tracking errors--wavelength and photometric accuracy

New FTIR Applications ä Quality control--speed, accuracy ä Micro, trace analysis--nanogram levels, small samples ä Kinetic studies--milliseconds ä Internal reflection ä Telescopic

Attenuated Internal Reflection ä ä Surface analysis Limited by 75% energy loss

New FTIR Applications ä Quality control--speed, accuracy ä Micro, trace analysis--nanogram levels, small samples ä Kinetic studies--milliseconds ä Internal reflection ä Telescopic
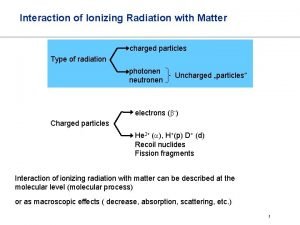
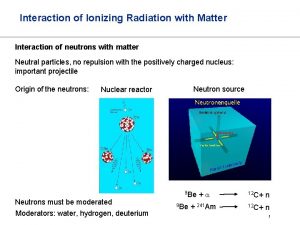
 Ionizing radiation
Ionizing radiation What is interaction of radiation with matter
What is interaction of radiation with matter Transverse wave vs longitudinal wave
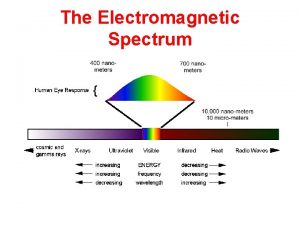
Transverse wave vs longitudinal wave Types of radiation in the electromagnetic spectrum
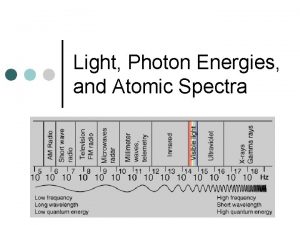
Types of radiation in the electromagnetic spectrum Emag spectrum
Emag spectrum Intensity of em wave
Intensity of em wave Energy density of electromagnetic wave
Energy density of electromagnetic wave When electromagnetic radiation of wavelength 300
When electromagnetic radiation of wavelength 300 Facts about electromagnetic radiation
Facts about electromagnetic radiation Which telescope detects invisible electromagnetic radiation
Which telescope detects invisible electromagnetic radiation Electromagnetic radiation
Electromagnetic radiation Wavelength formula electromagnetic wave
Wavelength formula electromagnetic wave Mechanical waves and electromagnetic waves similarities
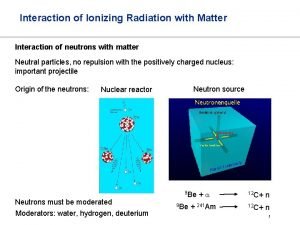
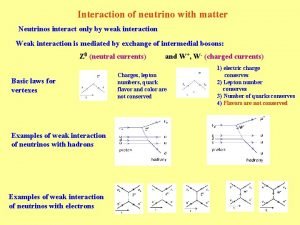
Mechanical waves and electromagnetic waves similarities Neutrino interaction with matter
Neutrino interaction with matter Menu selection interaction styles
Menu selection interaction styles Guinea worm
Guinea worm Arbor vitae
Arbor vitae Energy naturally flows from warmer matter to cooler matter.
Energy naturally flows from warmer matter to cooler matter. Whats gray matter
Whats gray matter Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Composition of matter section 1
Composition of matter section 1 Gray matter and white matter
Gray matter and white matter Chapter 2 section 1 classifying matter answers
Chapter 2 section 1 classifying matter answers Frontal and parietal lobes
Frontal and parietal lobes Section 1 composition of matter
Section 1 composition of matter Sound will travel at different speeds in different mediums.
Sound will travel at different speeds in different mediums. Different angle different story
Different angle different story Argumenterande tal struktur
Argumenterande tal struktur Library.thinkquest.org 19537
Library.thinkquest.org 19537 Acids and bases song lyrics
Acids and bases song lyrics Thermosoftening plastics examples
Thermosoftening plastics examples Different materials have different
Different materials have different Different culture have different moral codes
Different culture have different moral codes Why do different atoms produce different colors
Why do different atoms produce different colors Different people different things
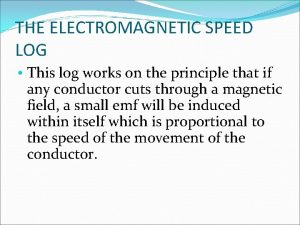
Different people different things Electromagnetic log
Electromagnetic log Electromagnetic speed log principle
Electromagnetic speed log principle Fabrication of electromagnetic braking system
Fabrication of electromagnetic braking system Concept of electromagnetic waves
Concept of electromagnetic waves Electromagnetism equation
Electromagnetism equation Electromagnetic spectrum
Electromagnetic spectrum Doppler gamma
Doppler gamma Electromagnetic waves: seeing objects and color
Electromagnetic waves: seeing objects and color What is the electromagnetic spectrum
What is the electromagnetic spectrum Longest wavelength to shortest wavelength
Longest wavelength to shortest wavelength Poynting vector and intensity
Poynting vector and intensity Light electromagnetic
Light electromagnetic Light electromagnetic
Light electromagnetic Electromagnetic theory
Electromagnetic theory Electromagnetic power formula
Electromagnetic power formula Electromagnetic pickups
Electromagnetic pickups Electromagnetic oscillations and alternating current
Electromagnetic oscillations and alternating current Energy carried by electromagnetic waves
Energy carried by electromagnetic waves Define mutual inductance of 1 henry
Define mutual inductance of 1 henry Radiation powerpoint template free
Radiation powerpoint template free Diffraction ocean waves
Diffraction ocean waves