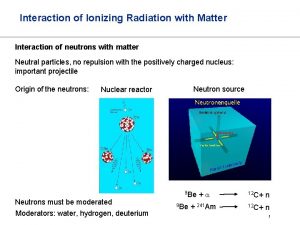
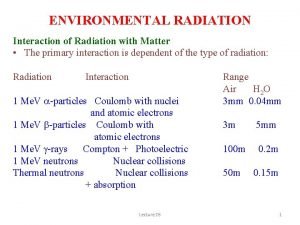
Lecture on Interaction of Electromagnetic Radiation with Matter


- Slides: 21

Lecture on Interaction of Electromagnetic Radiation with Matter By Dr. Pragati Kumar Department of Nano Sciences and Materials Central University of Jammu

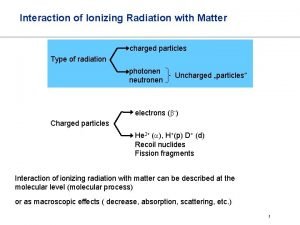
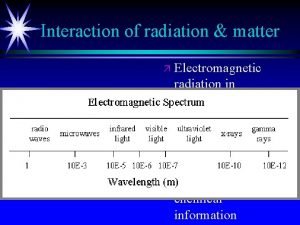
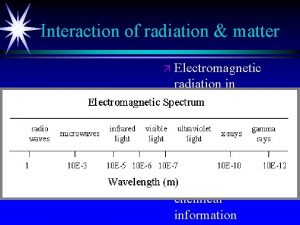
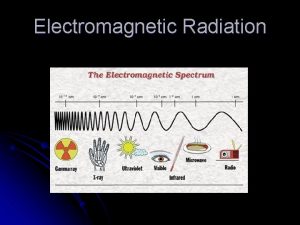
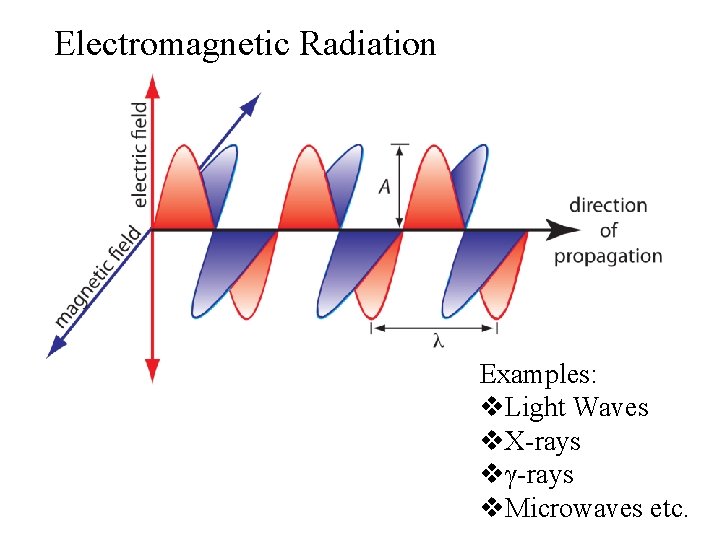
Electromagnetic Radiation Examples: v. Light Waves v. X-rays vγ-rays v. Microwaves etc.

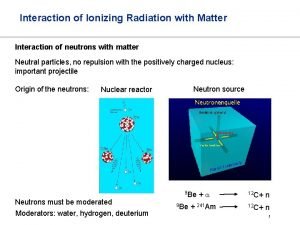
Interaction of Electromagnetic Waves with Matter Particles EM waves may interact with matter particle in different ways, the most important interactions are; ØEM waves may give entire energy to matter particle. (Photoelectric Effect) ØEM waves may give a part of their energy to matter particles. (Compton Scattering) ØEM waves may materialize into electron and positron. (Pair Production)

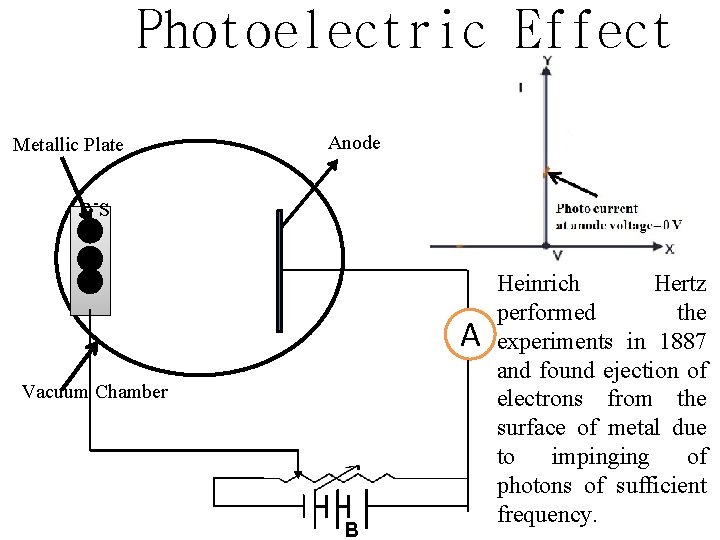
Photoelectric Effect Metallic Plate Anode e -s A Vacuum Chamber B Heinrich Hertz performed the experiments in 1887 and found ejection of electrons from the surface of metal due to impinging of photons of sufficient frequency.

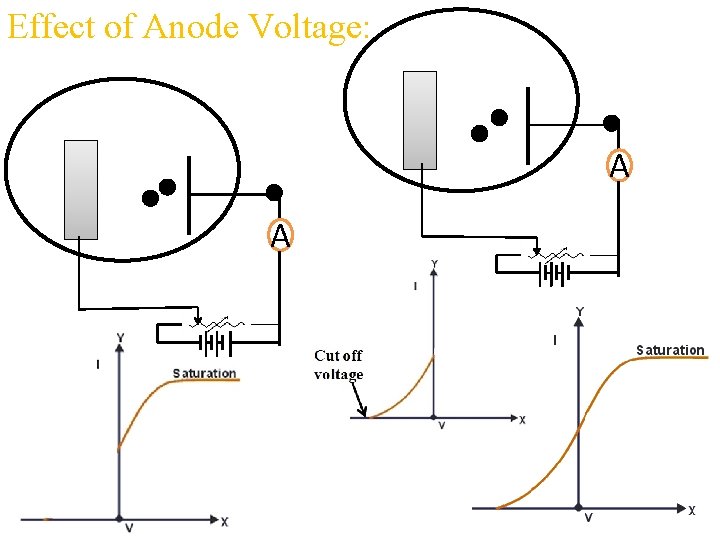
Effect of Anode Voltage: A A

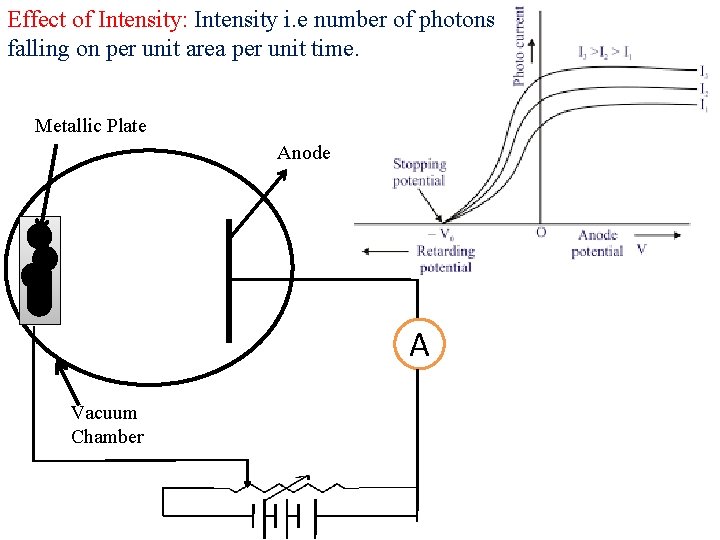
Effect of Intensity: Intensity i. e number of photons falling on per unit area per unit time. Metallic Plate Anode A Vacuum Chamber

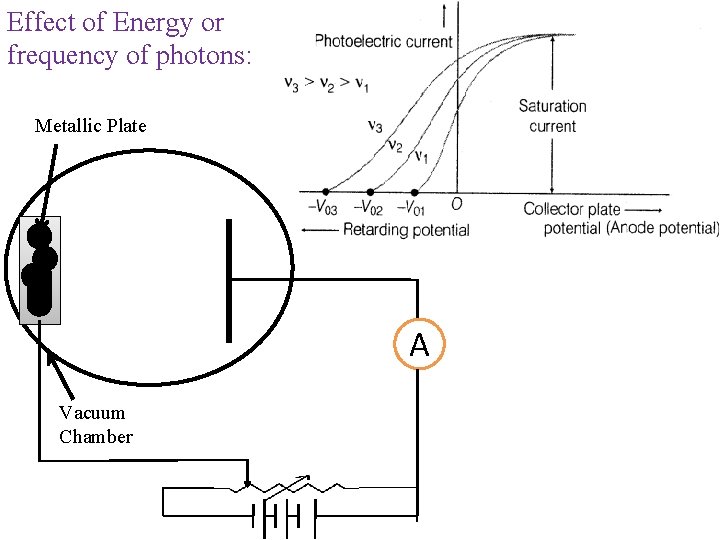
Effect of Energy or frequency of photons: Metallic Plate A Vacuum Chamber

i Cs K Na

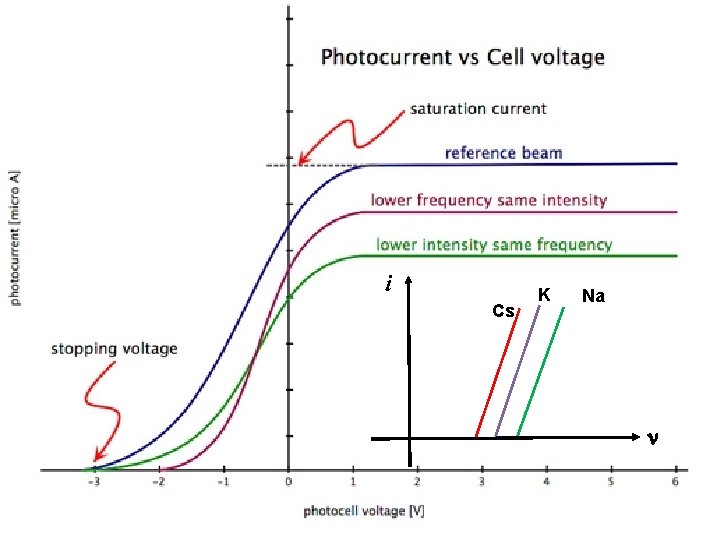
Laws of Photoelectric Effect: ØThere is no time lag between incident radiation (photon) and ejected photoelectron. Ø The rate of photo-emission is directly proportional to intensity of incident radiation (light). ØThe velocity and hence the kinetic energy of photoelectrons is independent of intensity of incident light. ØThe velocity and hence the kinetic energy of photoelectrons is directly proportional to frequency of incident radiation. ØThe emission of electron take place above a certain frequency known as threshold frequency. This frequency is characteristic frequency of photo-metal used.

Failures of Classical theory: According to classical theory: v. Intensity of a wave is the energy incident per unit area per unit time and given by. Where n & a are frequency and amplitude of wave. v Higher intensity of light, greater the energies of the photoelectrons. Classical wave theory cannot explain the first 3 observations of photoelectric effect. 1. Existence of the threshold frequency. 2. Almost immediate emission of photoelectrons as the energy of em waves spread across the wavefronts, a period of time should be lapsed before an electron accumulates enough energy to leave the metal. 3. Higher frequencyof light, greater the energies of the electrons.

Quantum Theory of Photoelectric Effect On the bases of Planck’s quantum which predicts that emission of radiation takes place in small packets of energy, known as quanta or photon or bundle, Einstein argued that light is not only emitted in quanta but travels as quanta i. e the photons preserve their identity throughout their life. He postulated that during interaction of photon with matter, either it may give its entire energy to material’s electron or none at all. When photon gives its energy to electron A part of this energy is used to overcome the binding forces of nucleus, the used energy for above is known as work function of that metal. Remaining energy is used to impart kinetic energy to same electron. If ν is the frequency of incident photon and v be the velocity of photoelectron then h ν=W+mv 2/2 Where W is work function of metal.

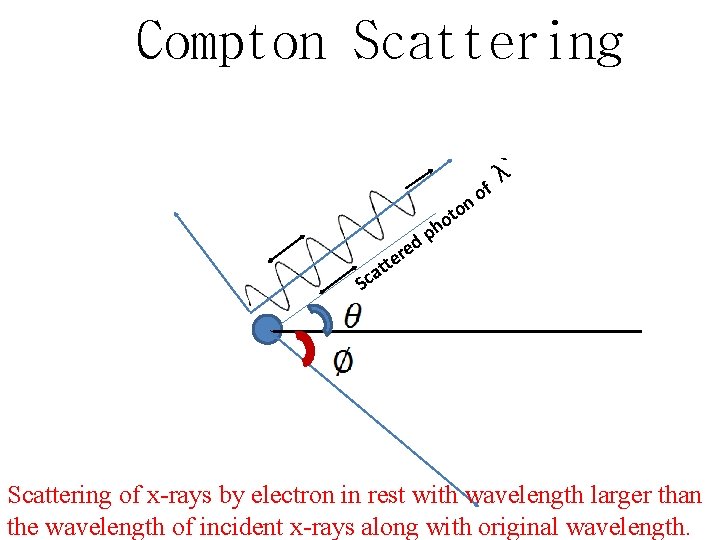
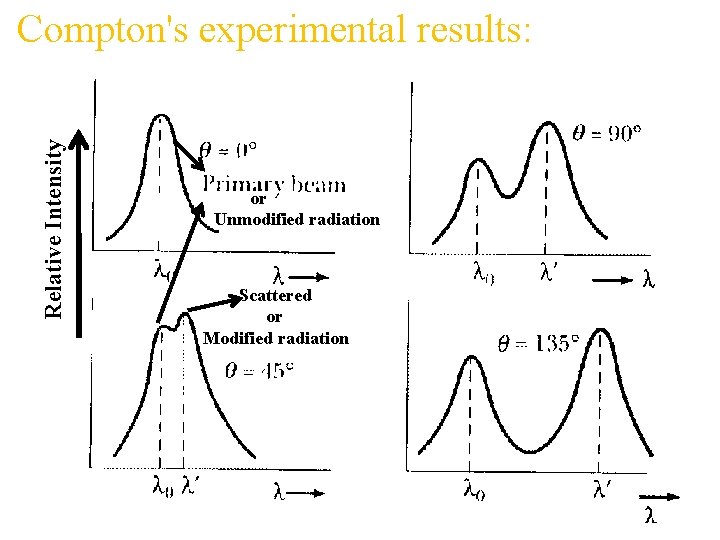
Compton Scattering ` λ f o n o t Sc ed r tte o h p a Scattering of x-rays by electron in rest with wavelength larger than the wavelength of incident x-rays along with original wavelength.

Relative Intensity Compton's experimental results: or Unmodified radiation Scattered or Modified radiation

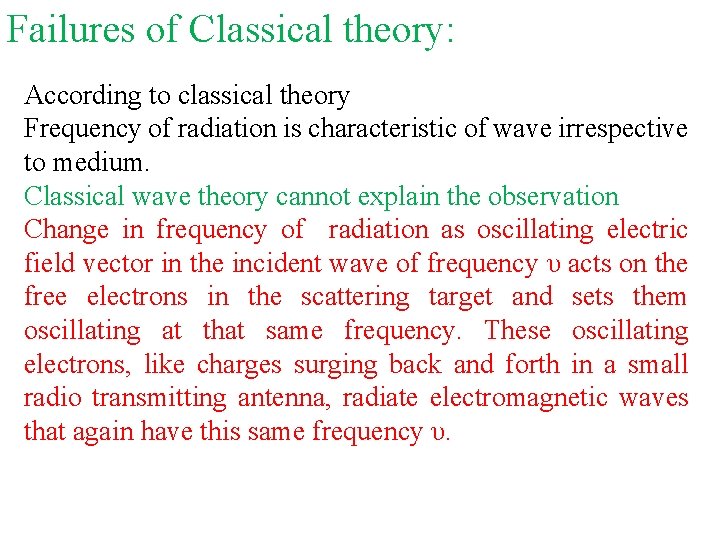
Failures of Classical theory: According to classical theory Frequency of radiation is characteristic of wave irrespective to medium. Classical wave theory cannot explain the observation Change in frequency of radiation as oscillating electric field vector in the incident wave of frequency υ acts on the free electrons in the scattering target and sets them oscillating at that same frequency. These oscillating electrons, like charges surging back and forth in a small radio transmitting antenna, radiate electromagnetic waves that again have this same frequency υ.

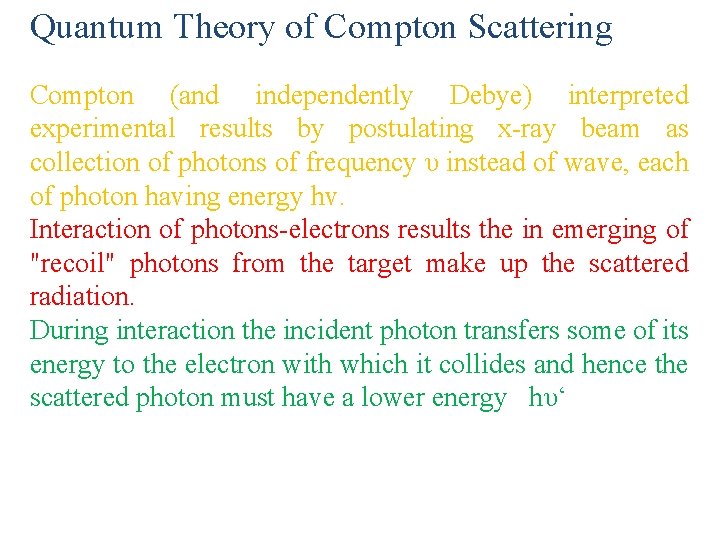
Quantum Theory of Compton Scattering Compton (and independently Debye) interpreted experimental results by postulating x-ray beam as collection of photons of frequency υ instead of wave, each of photon having energy hv. Interaction of photons-electrons results the in emerging of "recoil" photons from the target make up the scattered radiation. During interaction the incident photon transfers some of its energy to the electron with which it collides and hence the scattered photon must have a lower energy hυ‘

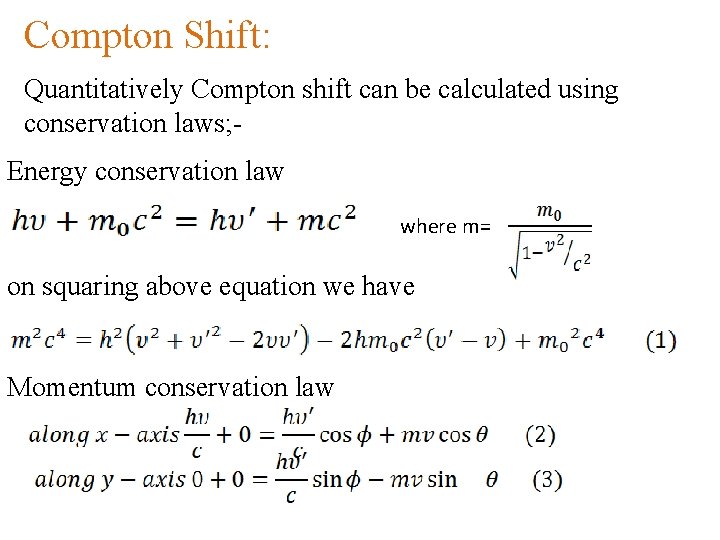
Compton Shift: Quantitatively Compton shift can be calculated using conservation laws; Energy conservation law where m= on squaring above equation we have Momentum conservation law


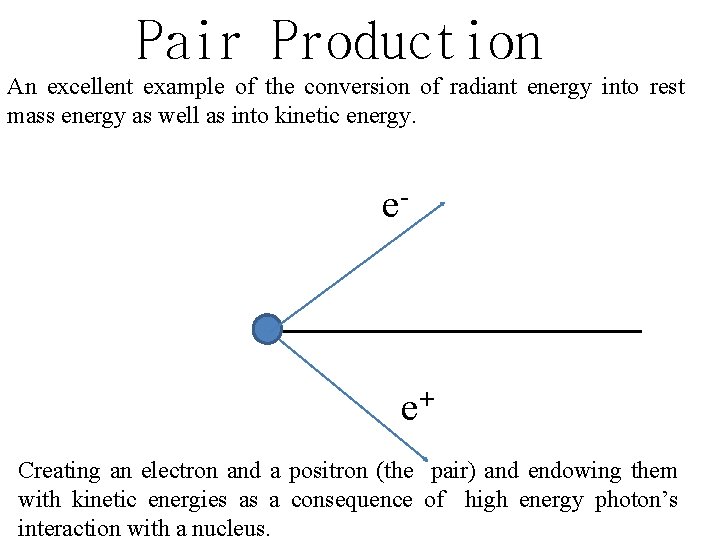
Pair Production An excellent example of the conversion of radiant energy into rest mass energy as well as into kinetic energy. e- e+ Creating an electron and a positron (the pair) and endowing them with kinetic energies as a consequence of high energy photon’s interaction with a nucleus.

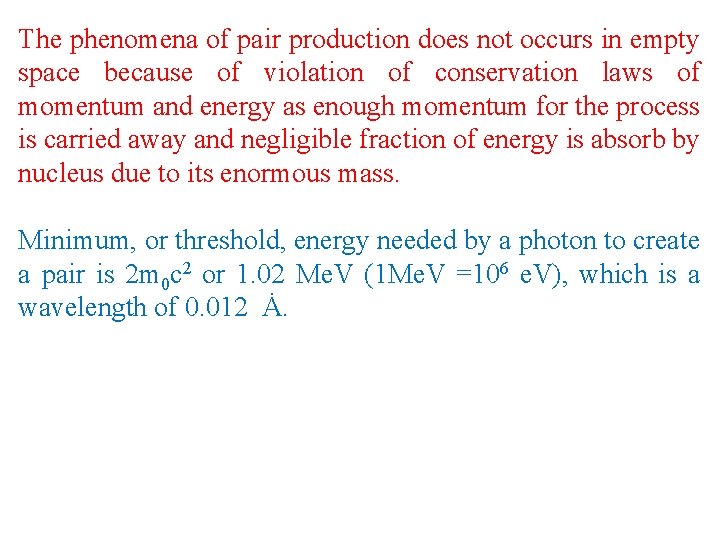
The phenomena of pair production does not occurs in empty space because of violation of conservation laws of momentum and energy as enough momentum for the process is carried away and negligible fraction of energy is absorb by nucleus due to its enormous mass. Minimum, or threshold, energy needed by a photon to create a pair is 2 m 0 c 2 or 1. 02 Me. V (1 Me. V =106 e. V), which is a wavelength of 0. 012 Ȧ.

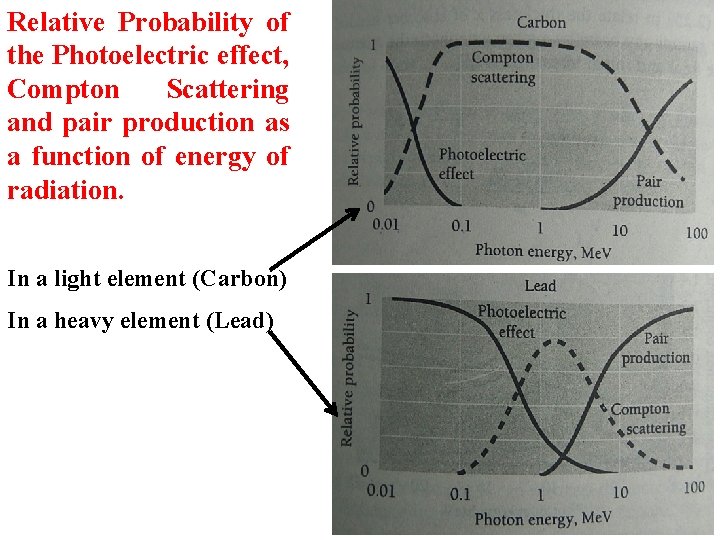
Relative Probability of the Photoelectric effect, Compton Scattering and pair production as a function of energy of radiation. In a light element (Carbon) In a heavy element (Lead)

Thanks for your attention…!
 Ionizing radiation
Ionizing radiation What is interaction of radiation with matter
What is interaction of radiation with matter Light is an electromagnetic wave true or false
Light is an electromagnetic wave true or false Types of radiation in the electromagnetic spectrum
Types of radiation in the electromagnetic spectrum Electromagnetic radiation chart
Electromagnetic radiation chart Intensity of electromagnetic wave
Intensity of electromagnetic wave Intensity of electromagnetic radiation
Intensity of electromagnetic radiation When electromagnetic radiation of wavelength 300
When electromagnetic radiation of wavelength 300 Facts about electromagnetic radiation
Facts about electromagnetic radiation Which telescope detects invisible electromagnetic radiation
Which telescope detects invisible electromagnetic radiation Em waves
Em waves Electromagnetic spectrum conclusion
Electromagnetic spectrum conclusion Difference between matter waves and electromagnetic waves
Difference between matter waves and electromagnetic waves 01:640:244 lecture notes - lecture 15: plat, idah, farad
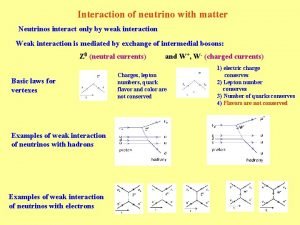
01:640:244 lecture notes - lecture 15: plat, idah, farad Neutrino interaction with matter
Neutrino interaction with matter Composition of matter section 1
Composition of matter section 1 Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Gray matter and white matter
Gray matter and white matter Chapter 2 section 1 classifying matter answer key
Chapter 2 section 1 classifying matter answer key Rhinencephalon
Rhinencephalon Section 1 composition of matter
Section 1 composition of matter What makes up the diencephalon
What makes up the diencephalon