Infrared Spectra of Anionic Coinage MetalWater Complexes J






![Experimental Method: IR Photodissociation h [A·B] M-·H 2 O·Arn + h [A]- + B Experimental Method: IR Photodissociation h [A·B] M-·H 2 O·Arn + h [A]- + B](https://slidetodoc.com/presentation_image/2cea833fa06bf33b7039591b75abdd10/image-8.jpg)






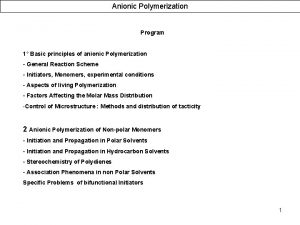
![E [me. V] Potential of the water rocking motion Calculated barriers (CCSD(T)/aug-pc-2; ECP-MCDF-aug-p. VTZ): E [me. V] Potential of the water rocking motion Calculated barriers (CCSD(T)/aug-pc-2; ECP-MCDF-aug-p. VTZ):](https://slidetodoc.com/presentation_image/2cea833fa06bf33b7039591b75abdd10/image-25.jpg)


- Slides: 31

Infrared Spectra of Anionic Coinage Metal-Water Complexes J. Mathias Weber JILA and Department of Chemistry and Biochemistry University of Colorado at Boulder

Dramatis Personae Experiment: Holger Schneider (now CU Boulder) Calculations: A. Daniel Boese (Institute for Nanotechnology, Forschungszentrum Karlsruhe, Germany) €€€ DFG (Emmy-Noether-Program), Universität Karlsruhe

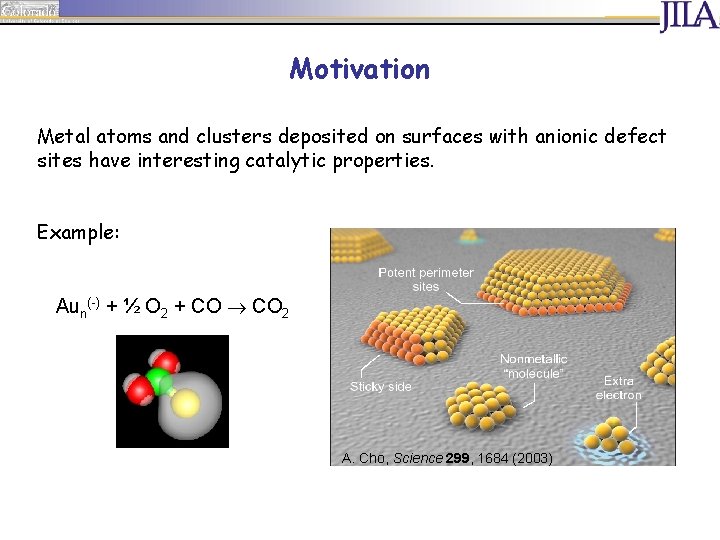
Motivation Metal atoms and clusters deposited on surfaces with anionic defect sites have interesting catalytic properties. Example: Aun(-) + ½ O 2 + CO 2 A. Cho, Science 299, 1684 (2003)

Motivation Metal atoms and clusters deposited on surfaces with anionic defect sites have interesting catalytic properties. The presence of water has been seen to strongly influence the catalytic process. How do water molecules and noble metal anions interact?

Possible Approach: Vibrational Spectroscopy OH groups equivalent symmetric and antisymmetric stretch vibrations in free H 2 O: 3657 cm-1 3756 cm-1

Possible Approach: Vibrational Spectroscopy OH groups equivalent symmetric and antisymmetric stretch vibrations in free H 2 O: 3657 cm-1 3756 cm-1

Possible Approach: Vibrational Spectroscopy OH groups equivalent symmetric and antisymmetric stretch vibrations in free H 2 O: 3657 cm-1 3756 cm-1 in clusters: H bonds with ion and other ligands Þ stretching of the H bonding OH groups Þ breaking of symmetry Þ red shift of H bonded oscillators
![Experimental Method IR Photodissociation h AB MH 2 OArn h A B Experimental Method: IR Photodissociation h [A·B] M-·H 2 O·Arn + h [A]- + B](https://slidetodoc.com/presentation_image/2cea833fa06bf33b7039591b75abdd10/image-8.jpg)
Experimental Method: IR Photodissociation h [A·B] M-·H 2 O·Arn + h [A]- + B [M-·H 2 O·Arn ]* M-·H 2 O·Arm + (n-m) Ar

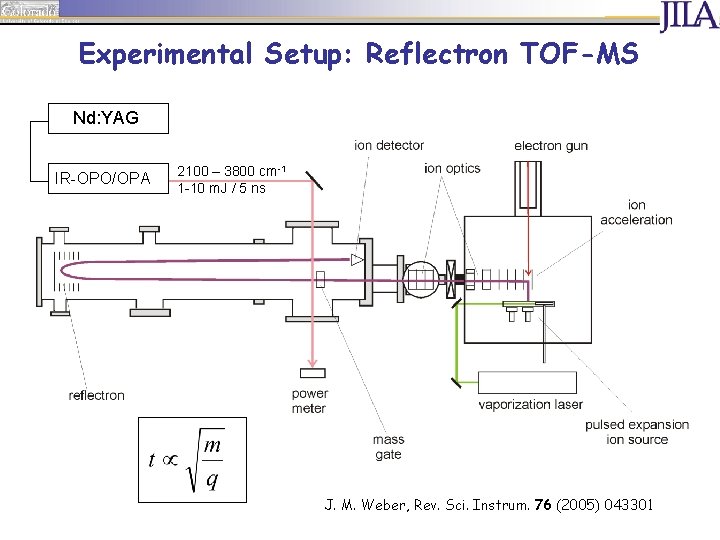
Experimental Setup: Reflectron TOF-MS Nd: YAG IR-OPO/OPA 2100 – 3800 cm-1 1 -10 m. J / 5 ns J. M. Weber, Rev. Sci. Instrum. 76 (2005) 043301

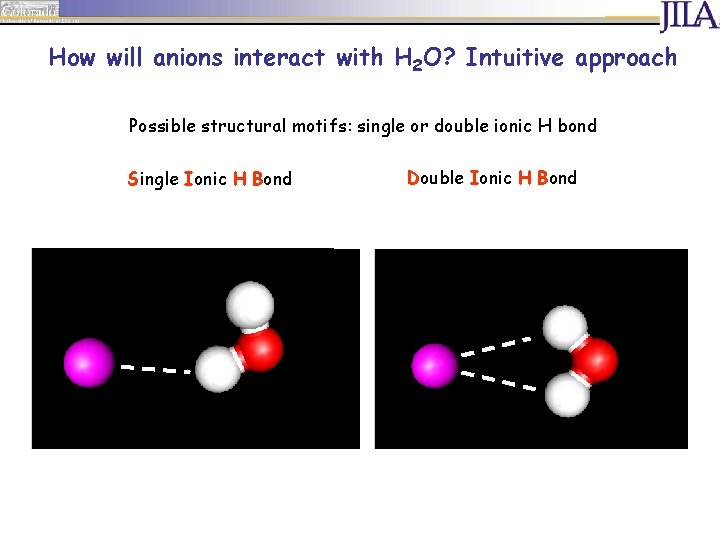
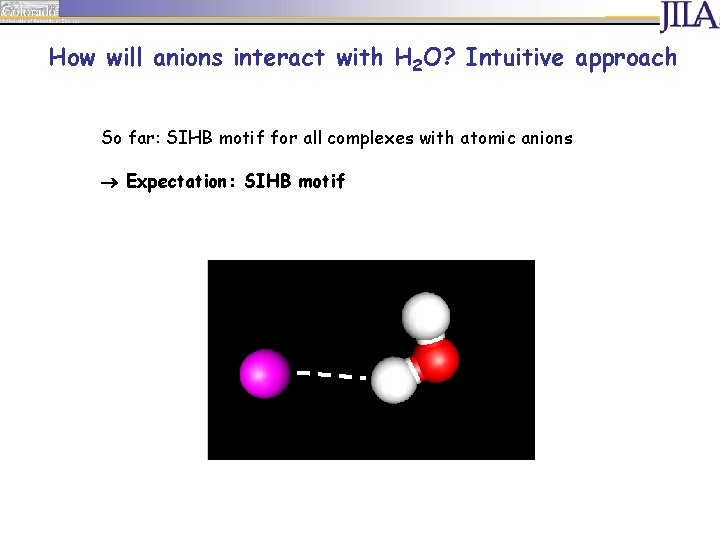
How will anions interact with H 2 O? Intuitive approach Possible structural motifs: single or double ionic H bond Single Ionic H Bond Double Ionic H Bond

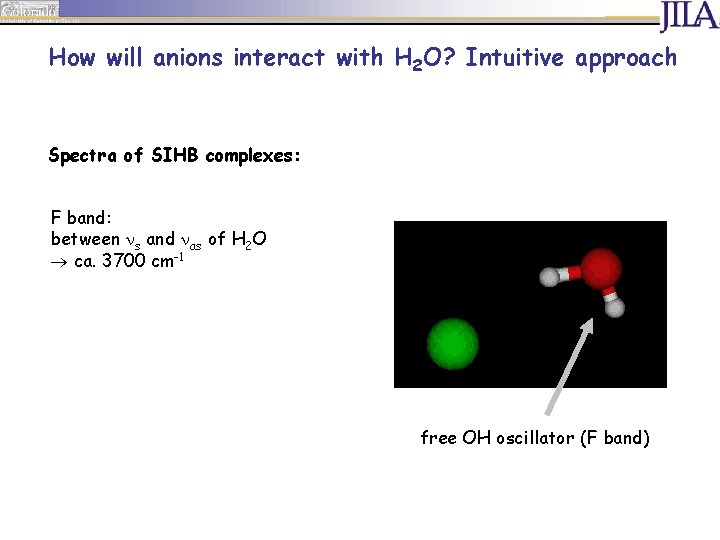
How will anions interact with H 2 O? Intuitive approach Spectra of SIHB complexes: F band: between s and as of H 2 O ca. 3700 cm-1 free OH oscillator (F band)

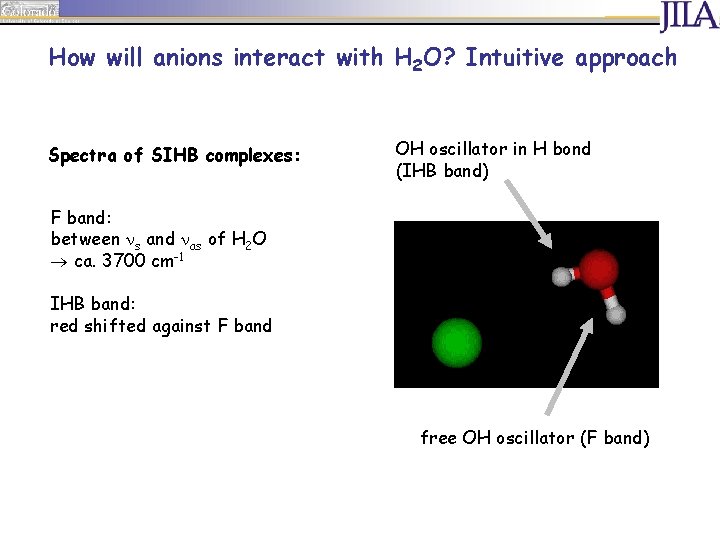
How will anions interact with H 2 O? Intuitive approach Spectra of SIHB complexes: OH oscillator in H bond (IHB band) F band: between s and as of H 2 O ca. 3700 cm-1 IHB band: red shifted against F band free OH oscillator (F band)

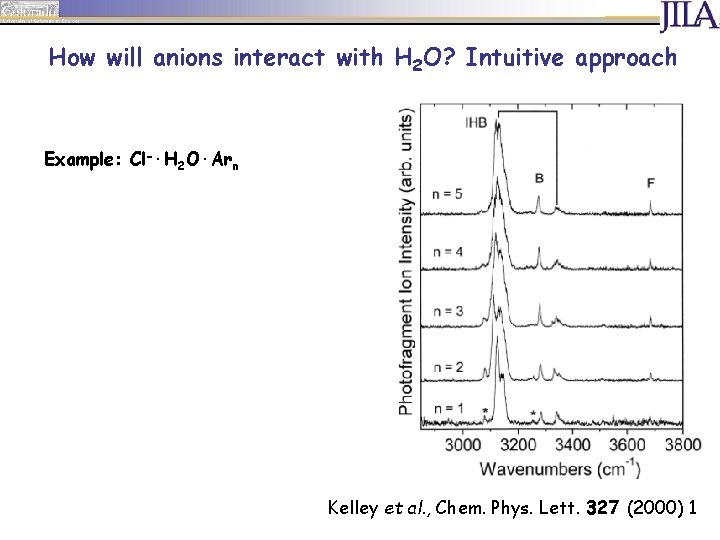
How will anions interact with H 2 O? Intuitive approach Example: Cl-·H 2 O·Arn Kelley et al. , Chem. Phys. Lett. 327 (2000) 1

How will anions interact with H 2 O? Intuitive approach Example: Cl-·H 2 O·Arn F band Kelley et al. , Chem. Phys. Lett. 327 (2000) 1

How will anions interact with H 2 O? Intuitive approach Example: Cl-·H 2 O·Arn F band IHB band Kelley et al. , Chem. Phys. Lett. 327 (2000) 1

How will anions interact with H 2 O? Intuitive approach Example: Cl-·H 2 O·Arn F band IHB band Fermi resonance of IHB with bend overtone, combination band with ion-molecule stretch vibration Kelley et al. , Chem. Phys. Lett. 327 (2000) 1

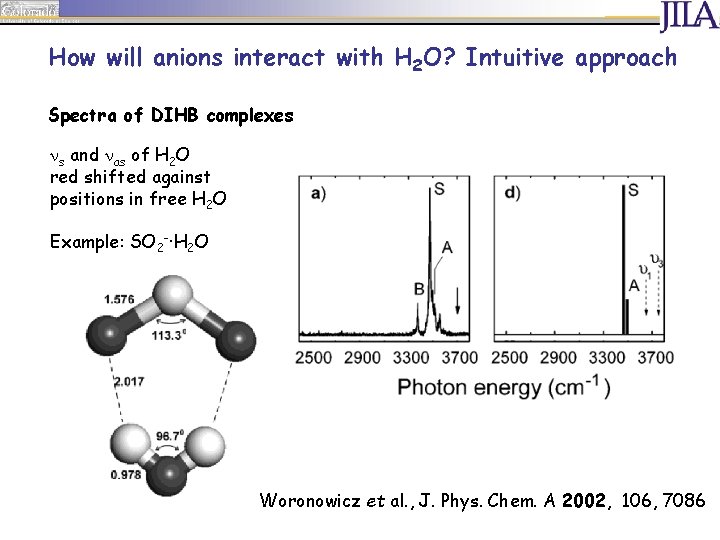
How will anions interact with H 2 O? Intuitive approach Spectra of DIHB complexes s and as of H 2 O red shifted against positions in free H 2 O Example: SO 2 -·H 2 O Woronowicz et al. , J. Phys. Chem. A 2002, 106, 7086

How will anions interact with H 2 O? Intuitive approach So far: SIHB motif for all complexes with atomic anions Expectation: SIHB motif

Red shift and anion proton affinity (SIHB motif)

Expectation for IHB bands of M-·H 2 O in SIHB configuration

IR spectra of M-·H 2 O·Ar 2 + h M-·H 2 O + 2 Ar

IR spectra of M-·H 2 O Expectation for IHB: D Cu > D Ag > D Au

IR spectra of M-·H 2 O Expectation for IHB: D Cu > D Ag > D Au Result: D Au > D Cu > D Ag Moreover: F band red shifted !!!

Comparing SIHB / DIHB data SIHB DIHB
![E me V Potential of the water rocking motion Calculated barriers CCSDTaugpc2 ECPMCDFaugp VTZ E [me. V] Potential of the water rocking motion Calculated barriers (CCSD(T)/aug-pc-2; ECP-MCDF-aug-p. VTZ):](https://slidetodoc.com/presentation_image/2cea833fa06bf33b7039591b75abdd10/image-25.jpg)
E [me. V] Potential of the water rocking motion Calculated barriers (CCSD(T)/aug-pc-2; ECP-MCDF-aug-p. VTZ): • Au-·H 2 O: 42 me. V • Ag-·H 2 O: 16 me. V • Cu-·H 2 O: 17 me. V Very low barriers! Cl-·H 2 O: 80 me. V

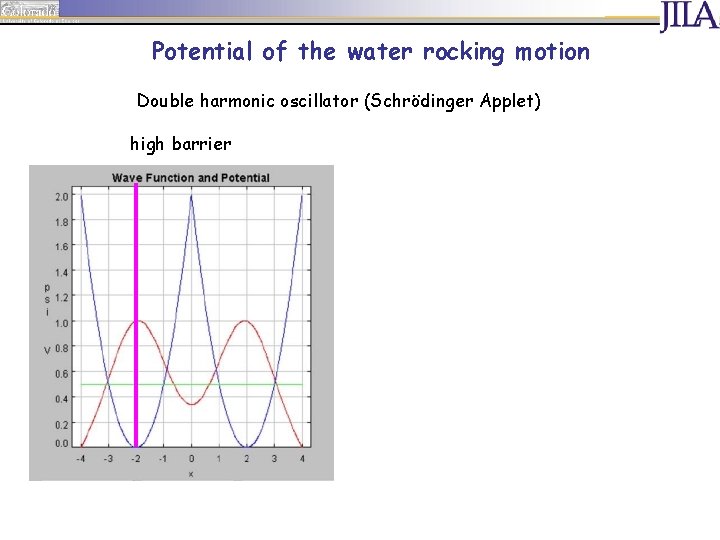
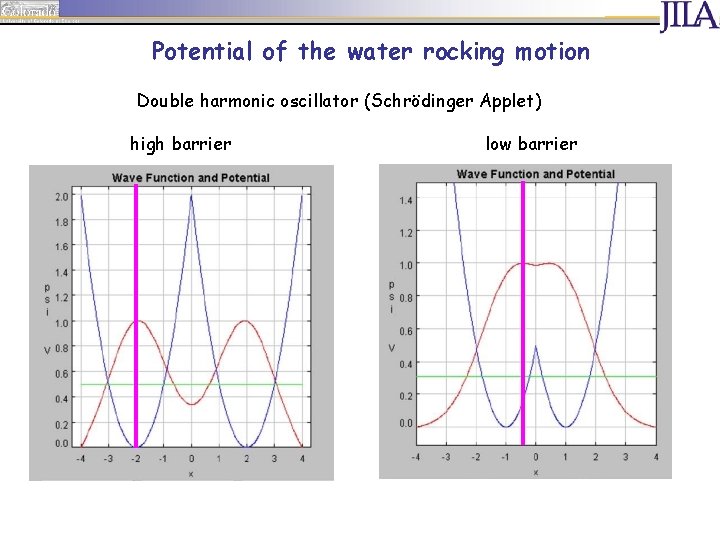
Potential of the water rocking motion Double harmonic oscillator (Schrödinger Applet) high barrier

Potential of the water rocking motion Double harmonic oscillator (Schrödinger Applet) high barrier low barrier

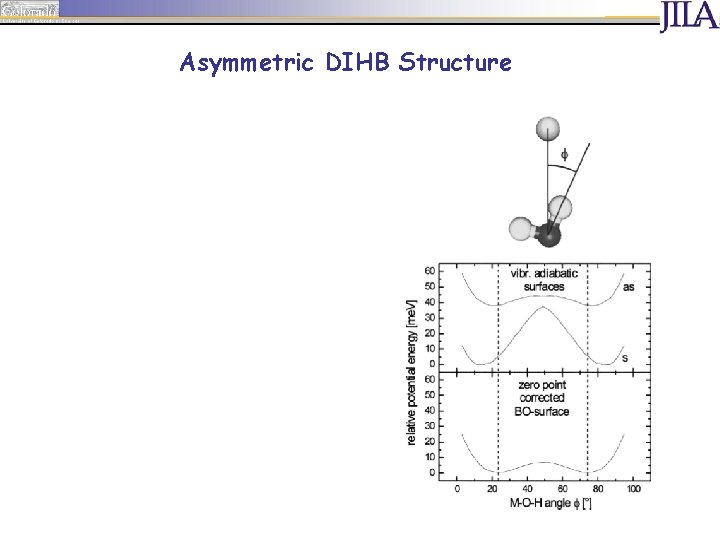
Asymmetric DIHB Structure Cs equilibrium structure, but complex explores geometries near C 2 v transition state due to zero point motion „free“ OH group contributes to binding red shift of F band red shift of IHB band reduced New H-bonding behavior: dynamic asymmetric DIHB due to ground state zero point motion H. Schneider, A. D. Boese, J. M. Weber, J. Chem. Phys. 123, 084307 (2005)

The End

Argon Effects

Asymmetric DIHB Structure
 Limitations of orgel diagram
Limitations of orgel diagram Nephelauxetic effect slideshare
Nephelauxetic effect slideshare Anionic polymerization mechanism
Anionic polymerization mechanism Tratament hipernatremie
Tratament hipernatremie Sian fain
Sian fain Lanthanides have poor tendency to form complexes
Lanthanides have poor tendency to form complexes Subordination phrase complexe
Subordination phrase complexe K complex eeg
K complex eeg Texas industrialized housing and buildings program
Texas industrialized housing and buildings program Slidetodoc.com
Slidetodoc.com Optical isomers of mabcdef
Optical isomers of mabcdef Ligand spectrochemical series
Ligand spectrochemical series Freud complexes
Freud complexes A ________ is formed from beadlike histone-dna complexes.
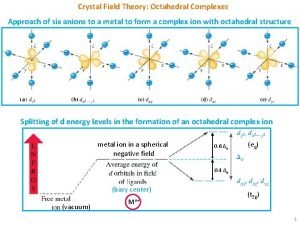
A ________ is formed from beadlike histone-dna complexes. Splitting in octahedral complexes
Splitting in octahedral complexes Spherical complexes of emulsified fats are known as
Spherical complexes of emulsified fats are known as La roda maquina simple
La roda maquina simple Examples of inert and labile complexes
Examples of inert and labile complexes Inert and labile complexes
Inert and labile complexes Radius ratio
Radius ratio Activation complex
Activation complex Whats morpheme
Whats morpheme Coinage
Coinage Nosebleed
Nosebleed Althea rodriguez
Althea rodriguez Clipping english
Clipping english Coinage lexicology
Coinage lexicology Words and word formation processes
Words and word formation processes Word coinage nedir
Word coinage nedir Vibronic spectra
Vibronic spectra Atomic emission spectra periodic table
Atomic emission spectra periodic table Amine group ir spectrum
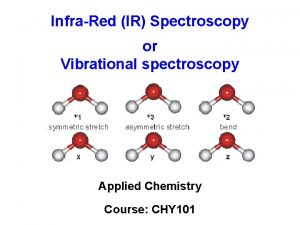
Amine group ir spectrum