Complexes Complexes Compounds in which a metal atom


![Structure 1. [Hexaammineplatinum(IV)] chloride [Pt(NH 3)6]Cl 4 central ion coordination number ligand complex cation Structure 1. [Hexaammineplatinum(IV)] chloride [Pt(NH 3)6]Cl 4 central ion coordination number ligand complex cation](https://slidetodoc.com/presentation_image_h2/9683a4af1273358f85277427c531d824/image-3.jpg)
![2. Potassium hexachloroplatinate(IV) K 2[Pt. Cl 6] central ion coordination number ligand complex anion 2. Potassium hexachloroplatinate(IV) K 2[Pt. Cl 6] central ion coordination number ligand complex anion](https://slidetodoc.com/presentation_image_h2/9683a4af1273358f85277427c531d824/image-4.jpg)
![3. Iron(O)pentacarbonyl [Fe(CO)5] central atom coordination number ligand complex molecule 3. Iron(O)pentacarbonyl [Fe(CO)5] central atom coordination number ligand complex molecule](https://slidetodoc.com/presentation_image_h2/9683a4af1273358f85277427c531d824/image-5.jpg)

![Complexes with various coordination numbers Coordination Complex number 2 [Ag(NH 3)2]+, [Cu. Cl 2]3 Complexes with various coordination numbers Coordination Complex number 2 [Ag(NH 3)2]+, [Cu. Cl 2]3](https://slidetodoc.com/presentation_image_h2/9683a4af1273358f85277427c531d824/image-7.jpg)
![Dissociation of complexes Conductance* Number of ions Number of chloride ions [Pt(NH 3)6]Cl 4 Dissociation of complexes Conductance* Number of ions Number of chloride ions [Pt(NH 3)6]Cl 4](https://slidetodoc.com/presentation_image_h2/9683a4af1273358f85277427c531d824/image-14.jpg)
![Cr. Cl 3·6 H 2 O [Cr(H 2 O)6]Cl 3 [Cr(H 2 O)4 Cl Cr. Cl 3·6 H 2 O [Cr(H 2 O)6]Cl 3 [Cr(H 2 O)4 Cl](https://slidetodoc.com/presentation_image_h2/9683a4af1273358f85277427c531d824/image-17.jpg)
![cis trans NH 3 Cl Co NH 3 Cl NH 3 [dichloro-tetraammine-cobalt(III)] ion cis trans NH 3 Cl Co NH 3 Cl NH 3 [dichloro-tetraammine-cobalt(III)] ion](https://slidetodoc.com/presentation_image_h2/9683a4af1273358f85277427c531d824/image-18.jpg)
![Cl Cl Cl en Cl Co Co en en [dichloro-bis(etylenediammine)cobalt(III)] ion en = etylenediammine Cl Cl Cl en Cl Co Co en en [dichloro-bis(etylenediammine)cobalt(III)] ion en = etylenediammine](https://slidetodoc.com/presentation_image_h2/9683a4af1273358f85277427c531d824/image-19.jpg)

- Slides: 20

Complexes

Complexes Compounds in which a metal atom or ion is surrounded by a number of oppositely charged ions or by neutral molecules. All possessing lone pairs of electrons which are available for donation to vacant orbitals of the metal atom or ion.
![Structure 1 HexaammineplatinumIV chloride PtNH 36Cl 4 central ion coordination number ligand complex cation Structure 1. [Hexaammineplatinum(IV)] chloride [Pt(NH 3)6]Cl 4 central ion coordination number ligand complex cation](https://slidetodoc.com/presentation_image_h2/9683a4af1273358f85277427c531d824/image-3.jpg)
Structure 1. [Hexaammineplatinum(IV)] chloride [Pt(NH 3)6]Cl 4 central ion coordination number ligand complex cation
![2 Potassium hexachloroplatinateIV K 2Pt Cl 6 central ion coordination number ligand complex anion 2. Potassium hexachloroplatinate(IV) K 2[Pt. Cl 6] central ion coordination number ligand complex anion](https://slidetodoc.com/presentation_image_h2/9683a4af1273358f85277427c531d824/image-4.jpg)
2. Potassium hexachloroplatinate(IV) K 2[Pt. Cl 6] central ion coordination number ligand complex anion
![3 IronOpentacarbonyl FeCO5 central atom coordination number ligand complex molecule 3. Iron(O)pentacarbonyl [Fe(CO)5] central atom coordination number ligand complex molecule](https://slidetodoc.com/presentation_image_h2/9683a4af1273358f85277427c531d824/image-5.jpg)
3. Iron(O)pentacarbonyl [Fe(CO)5] central atom coordination number ligand complex molecule

Central ions, atoms: Transition metals Alkaline earth metals Alkaline metals Coordination numbers: Number of ligands in the first coordination sphere
![Complexes with various coordination numbers Coordination Complex number 2 AgNH 32 Cu Cl 23 Complexes with various coordination numbers Coordination Complex number 2 [Ag(NH 3)2]+, [Cu. Cl 2]3](https://slidetodoc.com/presentation_image_h2/9683a4af1273358f85277427c531d824/image-7.jpg)
Complexes with various coordination numbers Coordination Complex number 2 [Ag(NH 3)2]+, [Cu. Cl 2]3 4 5 6 7 8 [Hg. I 3][Zn(NH 3)4]2+, [Ni(CN)4]2[Ni(CN)5]3 -, Fe(CO)5 [Cr(H 2 O)6]3+, [Fe(CN)6]3[Zr. F 7]3[Mo(CN)8]4 -

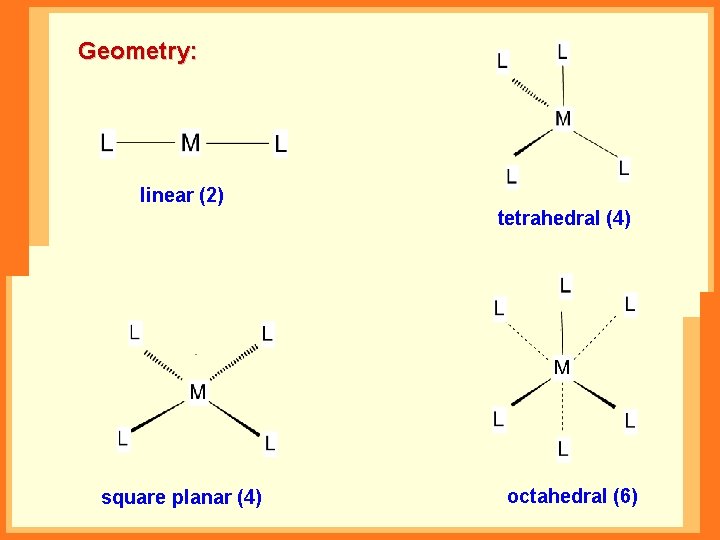
Geometry: linear (2) tetrahedral (4) square planar (4) octahedral (6)

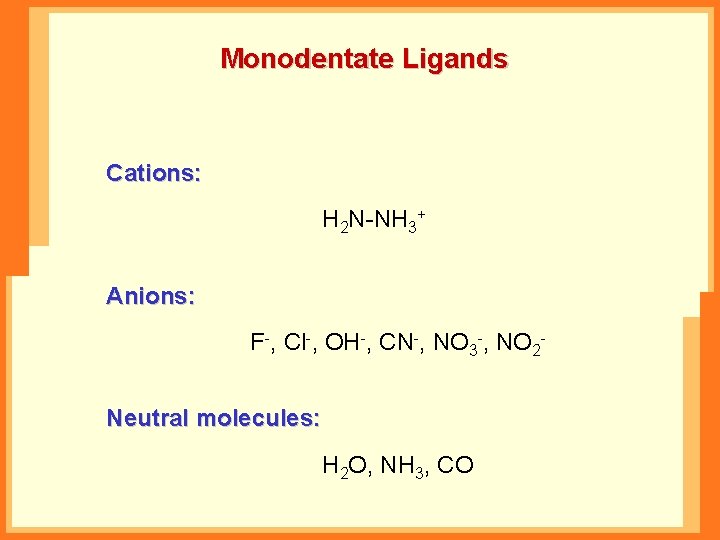
Monodentate Ligands Cations: H 2 N-NH 3+ Anions: F-, Cl-, OH-, CN-, NO 3 -, NO 2 Neutral molecules: H 2 O, NH 3, CO

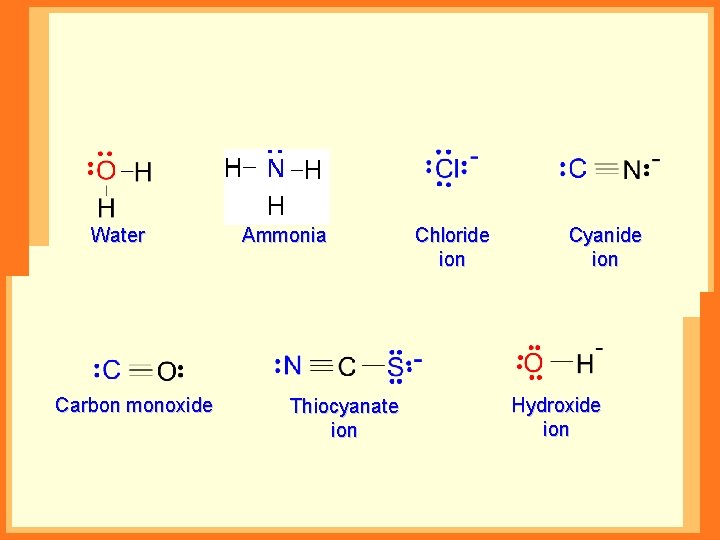
Water Carbon monoxide Ammonia Thiocyanate ion Chloride ion Cyanide ion Hydroxide ion

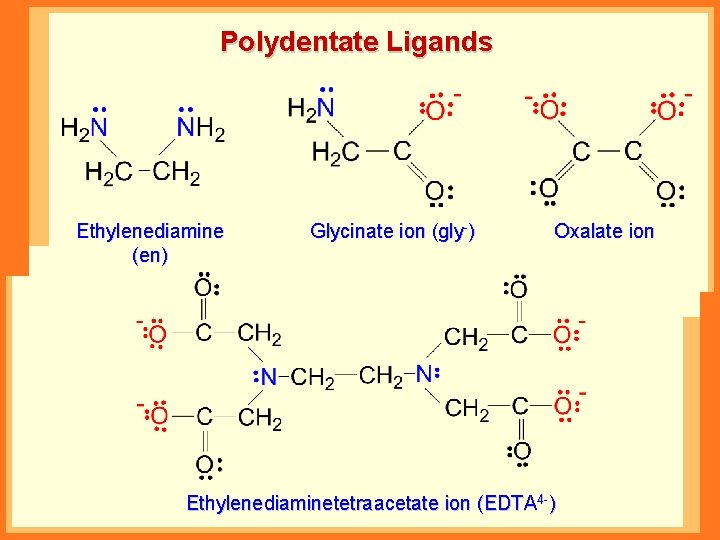
Polydentate Ligands Ethylenediamine (en) Glycinate ion (gly-) Oxalate ion Ethylenediaminetetraacetate ion (EDTA 4 -)

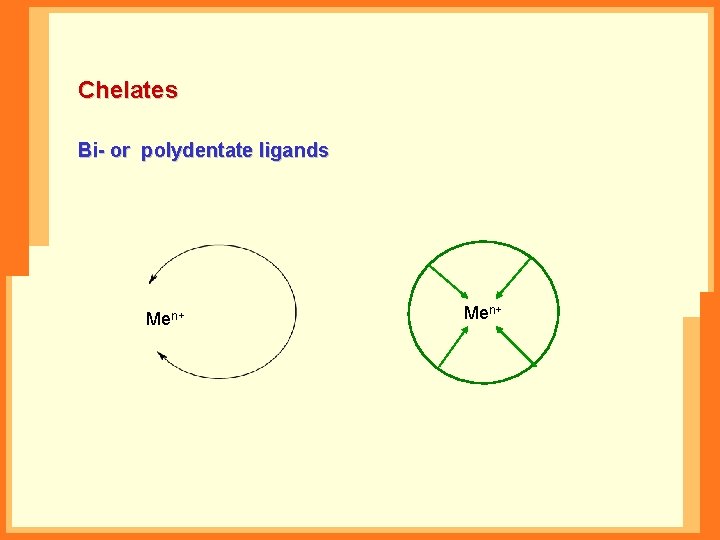
Chelates Bi- or polydentate ligands Men+n+ Me Me

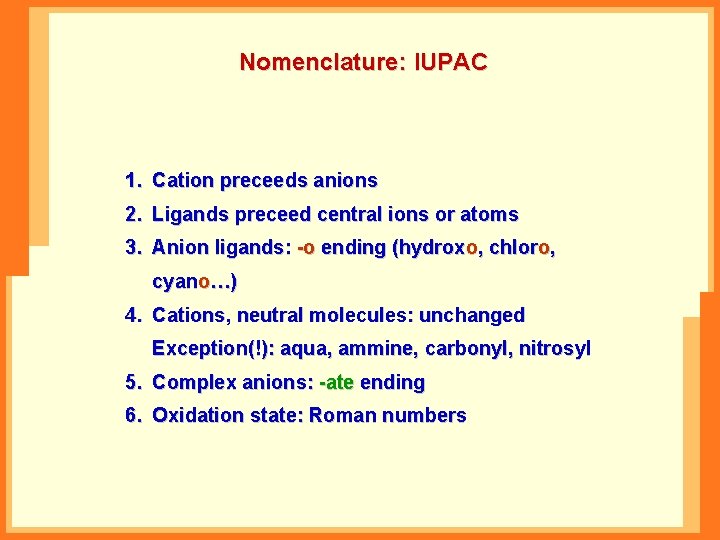
Nomenclature: IUPAC 1. Cation preceeds anions 2. Ligands preceed central ions or atoms 3. Anion ligands: -o ending (hydroxo, chloro, cyano…) 4. Cations, neutral molecules: unchanged Exception(!): aqua, ammine, carbonyl, nitrosyl 5. Complex anions: -ate ending 6. Oxidation state: Roman numbers
![Dissociation of complexes Conductance Number of ions Number of chloride ions PtNH 36Cl 4 Dissociation of complexes Conductance* Number of ions Number of chloride ions [Pt(NH 3)6]Cl 4](https://slidetodoc.com/presentation_image_h2/9683a4af1273358f85277427c531d824/image-14.jpg)
Dissociation of complexes Conductance* Number of ions Number of chloride ions [Pt(NH 3)6]Cl 4 523 5 4 [Pt(NH 3)5 Cl]Cl 3 404 4 3 [Pt(NH 3)4 Cl 2]Cl 2 228 3 2 [Pt(NH 3)3 Cl 3]Cl 97 2 1 [Pt(NH 3)2 Cl 4] 0 0 0 K[Pt(NH 3)Cl 5] 108 2 0 K 2[Pt. Cl 6] 256 3 0 Formula *Molar conductance: cm 2/ ·mól, 0, 001 M, 25°C

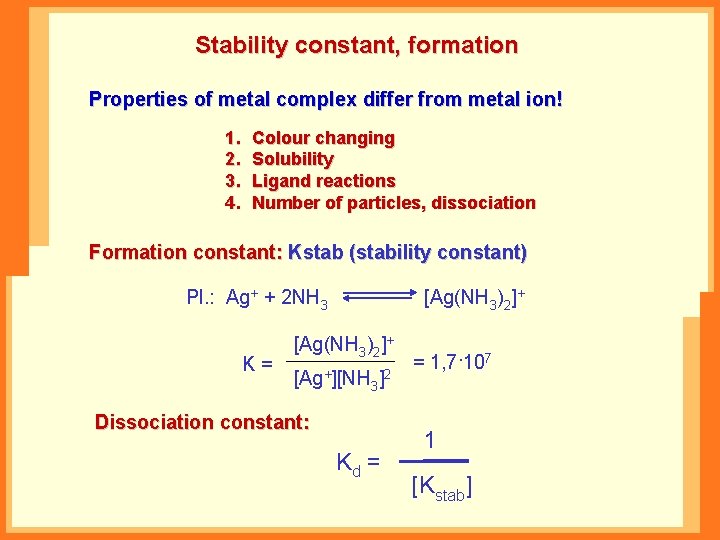
Stability constant, formation Properties of metal complex differ from metal ion! 1. 2. 3. 4. Colour changing Solubility Ligand reactions Number of particles, dissociation Formation constant: Kstab (stability constant) Pl. : Ag+ + 2 NH 3 K= [Ag(NH 3)2]+ [Ag+][NH 3]2 Dissociation constant: Kd = = 1, 7·107 1 [Kstab]

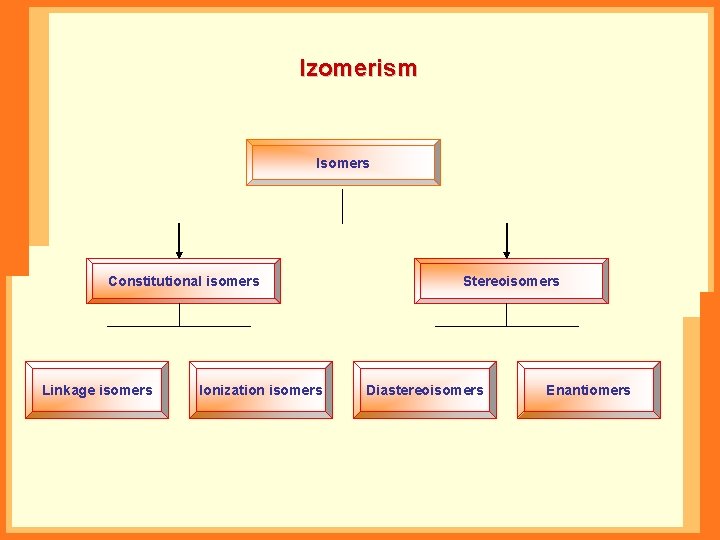
Izomerism Isomers Constitutional isomers Linkage isomers Ionization isomers Stereoisomers Diastereoisomers Enantiomers
![Cr Cl 36 H 2 O CrH 2 O6Cl 3 CrH 2 O4 Cl Cr. Cl 3·6 H 2 O [Cr(H 2 O)6]Cl 3 [Cr(H 2 O)4 Cl](https://slidetodoc.com/presentation_image_h2/9683a4af1273358f85277427c531d824/image-17.jpg)
Cr. Cl 3·6 H 2 O [Cr(H 2 O)6]Cl 3 [Cr(H 2 O)4 Cl 2]Cl·2 H 2 O [Cr(H 2 O)5 Cl]Cl 2·H 2 O
![cis trans NH 3 Cl Co NH 3 Cl NH 3 dichlorotetraamminecobaltIII ion cis trans NH 3 Cl Co NH 3 Cl NH 3 [dichloro-tetraammine-cobalt(III)] ion](https://slidetodoc.com/presentation_image_h2/9683a4af1273358f85277427c531d824/image-18.jpg)
cis trans NH 3 Cl Co NH 3 Cl NH 3 [dichloro-tetraammine-cobalt(III)] ion
![Cl Cl Cl en Cl Co Co en en dichlorobisetylenediamminecobaltIII ion en etylenediammine Cl Cl Cl en Cl Co Co en en [dichloro-bis(etylenediammine)cobalt(III)] ion en = etylenediammine](https://slidetodoc.com/presentation_image_h2/9683a4af1273358f85277427c531d824/image-19.jpg)
Cl Cl Cl en Cl Co Co en en [dichloro-bis(etylenediammine)cobalt(III)] ion en = etylenediammine en

Bonding in complexes: • Valence Bond Theory • Crystal Field Theory