Coordination Chemistry Reactions of Metal Complexes NIKAM N


![Kinetics of interchange reactions For [Y] >> [ML 5 X] Fast equilibrium K 1 Kinetics of interchange reactions For [Y] >> [ML 5 X] Fast equilibrium K 1](https://slidetodoc.com/presentation_image_h/2aa9365d1e0f260a6a2815d5f1338309/image-6.jpg)
![Outer sphere mechanism [Fe(CN)6]4 - + [Ir. Cl 6]2[Co(NH 3)5 Cl]2+ + [Ru(NH 3)6]2+ Outer sphere mechanism [Fe(CN)6]4 - + [Ir. Cl 6]2[Co(NH 3)5 Cl]2+ + [Ru(NH 3)6]2+](https://slidetodoc.com/presentation_image_h/2aa9365d1e0f260a6a2815d5f1338309/image-19.jpg)
![Inner sphere mechanism [Co(NH 3)5 Cl)]2+ + [Çr(H 2 O)6]2+ [Co(NH 3)5 Cl)]2+: : Inner sphere mechanism [Co(NH 3)5 Cl)]2+ + [Çr(H 2 O)6]2+ [Co(NH 3)5 Cl)]2+: :](https://slidetodoc.com/presentation_image_h/2aa9365d1e0f260a6a2815d5f1338309/image-20.jpg)
- Slides: 21

Coordination Chemistry Reactions of Metal Complexes NIKAM N. D. DEPARTMENT OF CHEMISTRY

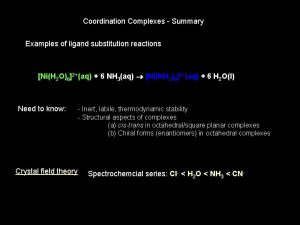
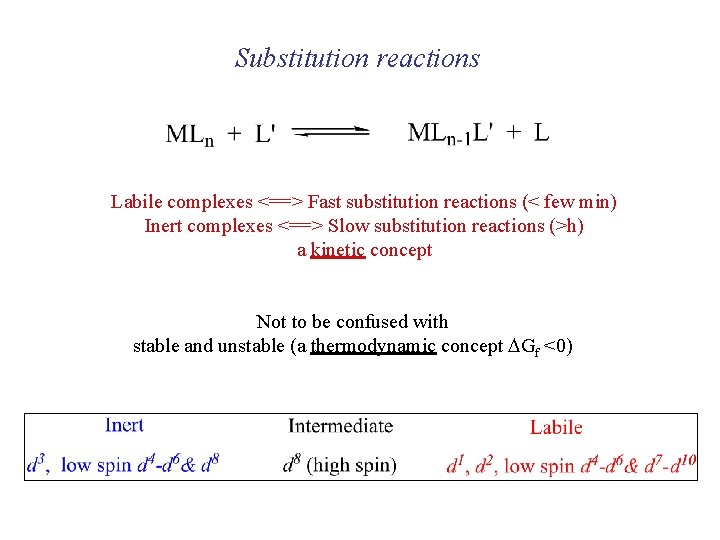
Substitution reactions Labile complexes <==> Fast substitution reactions (< few min) Inert complexes <==> Slow substitution reactions (>h) a kinetic concept Not to be confused with stable and unstable (a thermodynamic concept DGf <0)

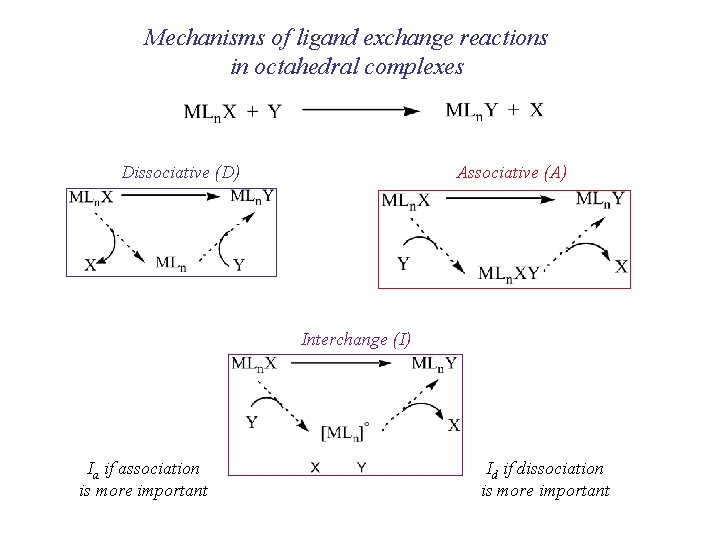
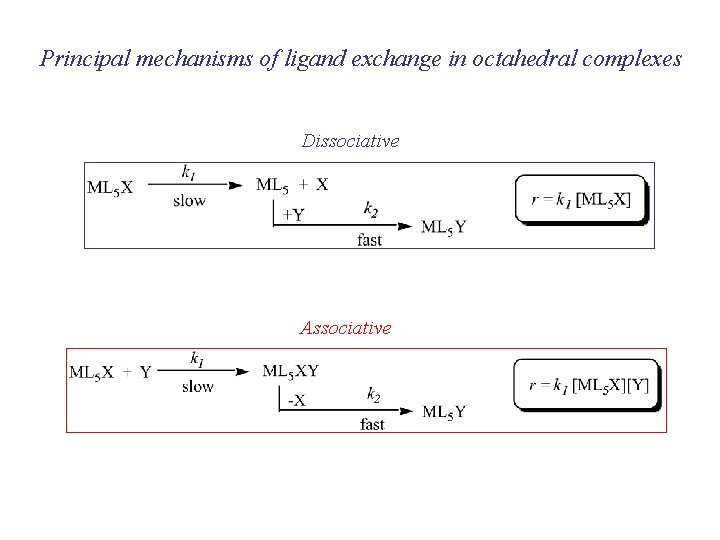
Mechanisms of ligand exchange reactions in octahedral complexes Dissociative (D) Associative (A) Interchange (I) Ia if association is more important Id if dissociation is more important


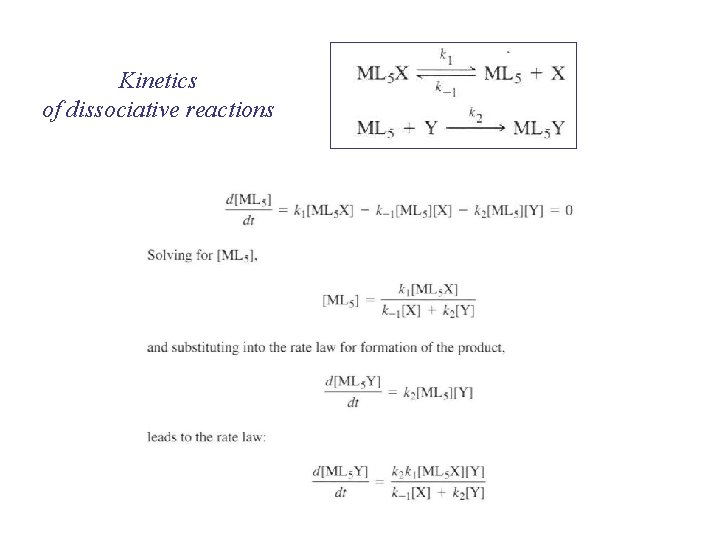
Kinetics of dissociative reactions
![Kinetics of interchange reactions For Y ML 5 X Fast equilibrium K 1 Kinetics of interchange reactions For [Y] >> [ML 5 X] Fast equilibrium K 1](https://slidetodoc.com/presentation_image_h/2aa9365d1e0f260a6a2815d5f1338309/image-6.jpg)
Kinetics of interchange reactions For [Y] >> [ML 5 X] Fast equilibrium K 1 = k 1/k-1 k 2 << k-1

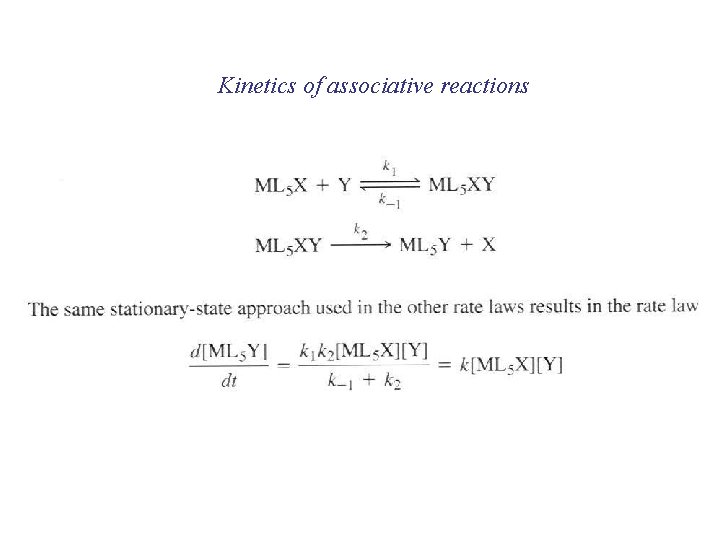
Kinetics of associative reactions

Principal mechanisms of ligand exchange in octahedral complexes Dissociative Associative

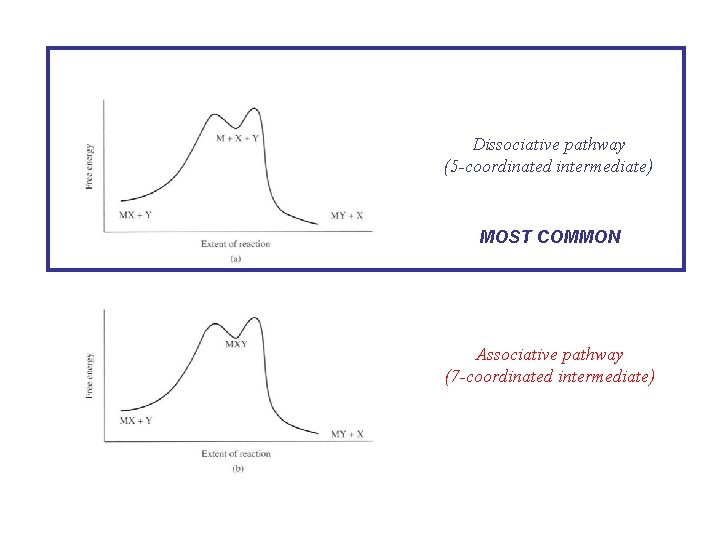
Dissociative pathway (5 -coordinated intermediate) MOST COMMON Associative pathway (7 -coordinated intermediate)

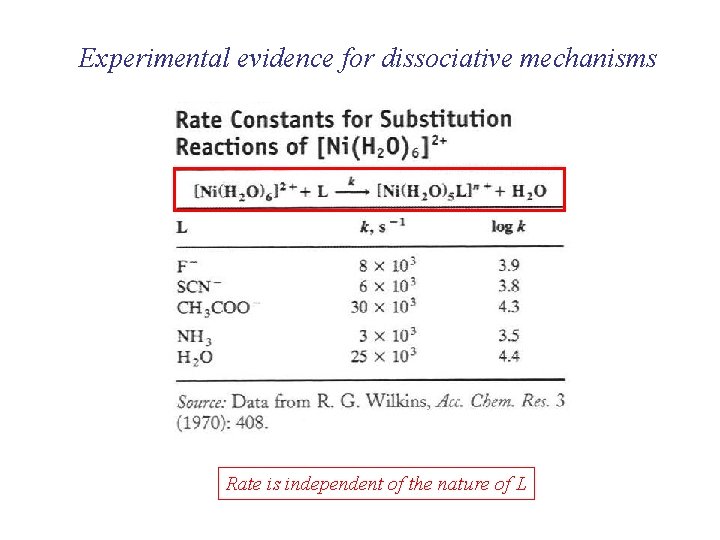
Experimental evidence for dissociative mechanisms Rate is independent of the nature of L

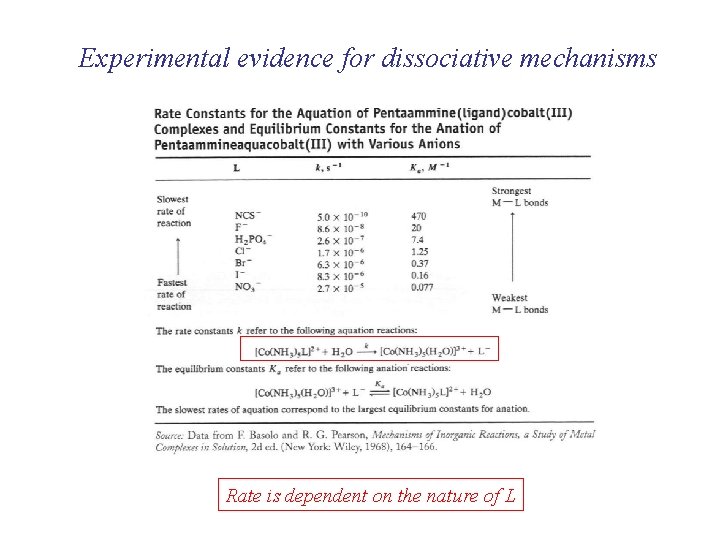
Experimental evidence for dissociative mechanisms Rate is dependent on the nature of L

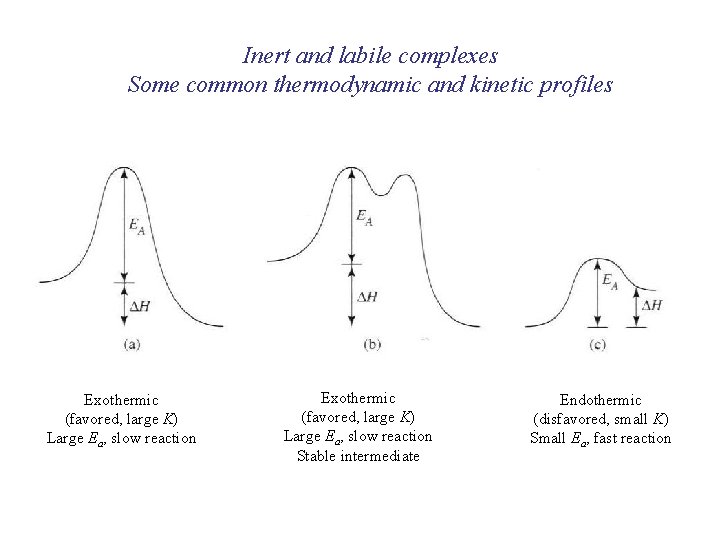
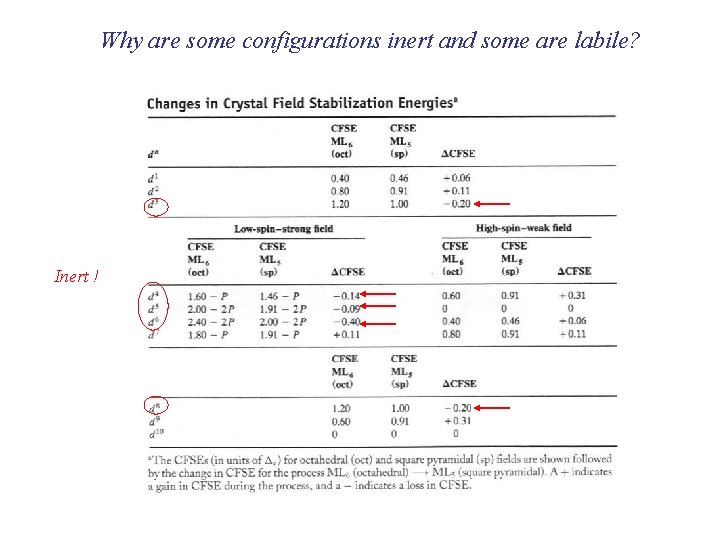
Inert and labile complexes Some common thermodynamic and kinetic profiles Exothermic (favored, large K) Large Ea, slow reaction Stable intermediate Endothermic (disfavored, small K) Small Ea, fast reaction

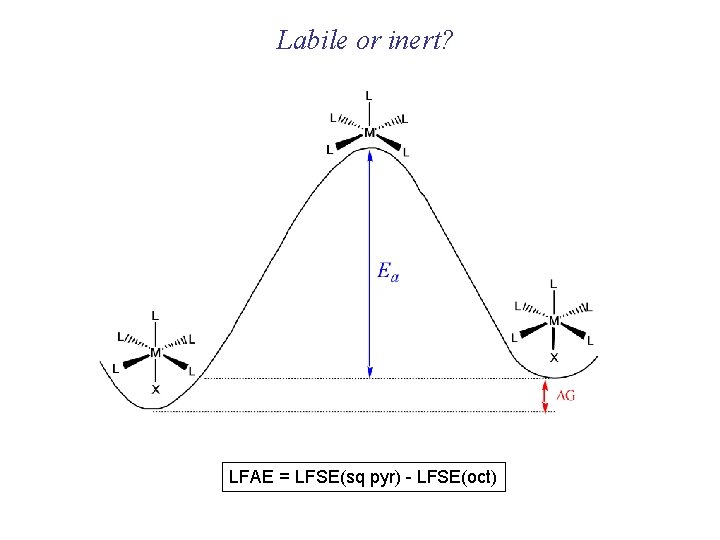
Labile or inert? LFAE = LFSE(sq pyr) - LFSE(oct)

Why are some configurations inert and some are labile? Inert !

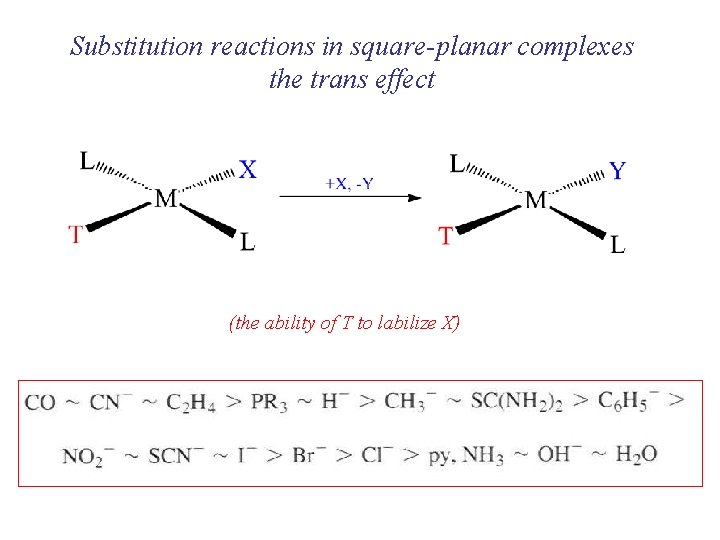
Substitution reactions in square-planar complexes the trans effect (the ability of T to labilize X)

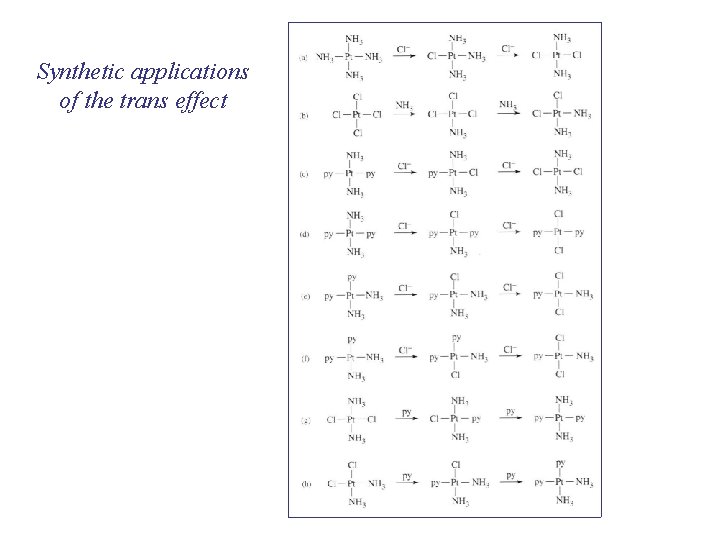
Synthetic applications of the trans effect

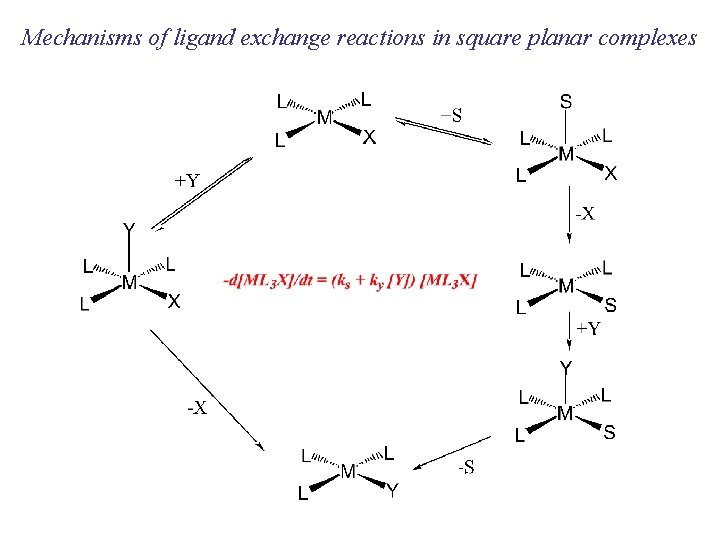
Mechanisms of ligand exchange reactions in square planar complexes

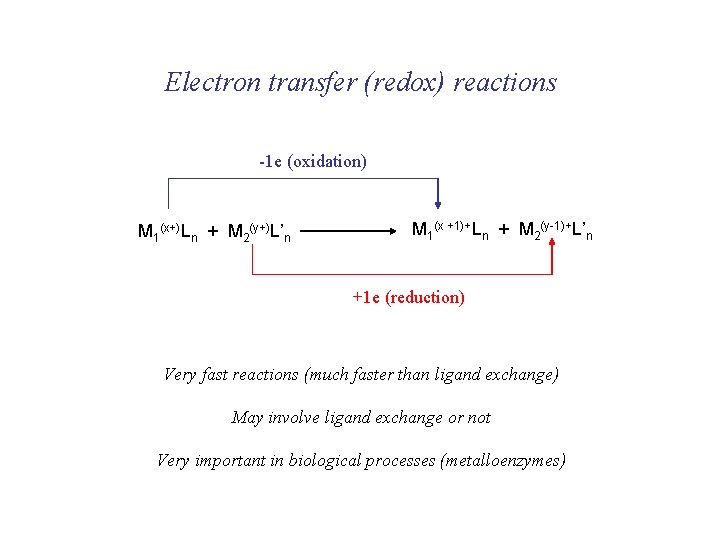
Electron transfer (redox) reactions -1 e (oxidation) M 1(x+)Ln + M 2(y+)L’n M 1(x +1)+Ln + M 2(y-1)+L’n +1 e (reduction) Very fast reactions (much faster than ligand exchange) May involve ligand exchange or not Very important in biological processes (metalloenzymes)
![Outer sphere mechanism FeCN64 Ir Cl 62CoNH 35 Cl2 RuNH 362 Outer sphere mechanism [Fe(CN)6]4 - + [Ir. Cl 6]2[Co(NH 3)5 Cl]2+ + [Ru(NH 3)6]2+](https://slidetodoc.com/presentation_image_h/2aa9365d1e0f260a6a2815d5f1338309/image-19.jpg)
Outer sphere mechanism [Fe(CN)6]4 - + [Ir. Cl 6]2[Co(NH 3)5 Cl]2+ + [Ru(NH 3)6]2+ Reactions ca. 100 times faster than ligand exchange (coordination spheres remain the same) r = k [A][B] Tunneling mechanism [Fe(CN)6]3 - + [Ir. Cl 6]3[Co(NH 3)5 Cl]+ + [Ru(NH 3)6]3+
![Inner sphere mechanism CoNH 35 Cl2 ÇrH 2 O62 CoNH 35 Cl2 Inner sphere mechanism [Co(NH 3)5 Cl)]2+ + [Çr(H 2 O)6]2+ [Co(NH 3)5 Cl)]2+: :](https://slidetodoc.com/presentation_image_h/2aa9365d1e0f260a6a2815d5f1338309/image-20.jpg)
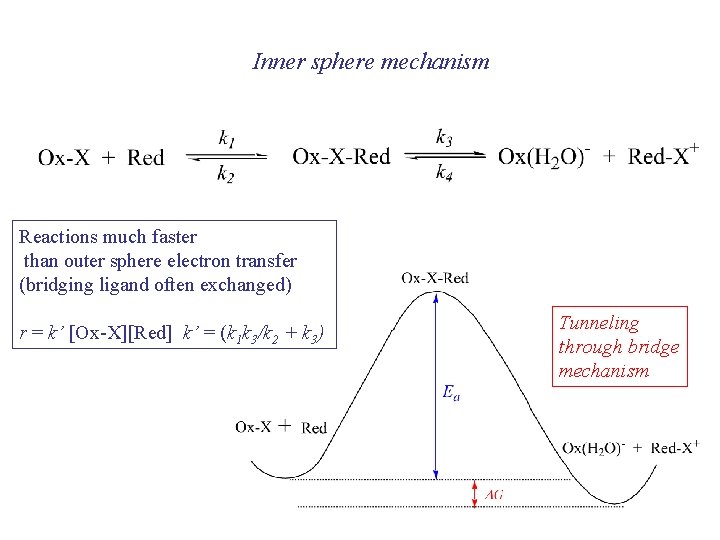
Inner sphere mechanism [Co(NH 3)5 Cl)]2+ + [Çr(H 2 O)6]2+ [Co(NH 3)5 Cl)]2+: : : [Çr(H 2 O)6]2+ [Co. III(NH 3)5(m-Cl)Çr. II(H 2 O)6]4+ [Co. II(NH 3)5(m-Cl)Çr. III(H 2 O)6]4+ [Co. II(NH 3)5(H 2 O)]2+ + [Çr. III(H 2 O)5 Cl]2+ [Ço(H 2 O)6]2+ + 5 NH 4+

Inner sphere mechanism Reactions much faster than outer sphere electron transfer (bridging ligand often exchanged) r = k’ [Ox-X][Red] k’ = (k 1 k 3/k 2 + k 3) Tunneling through bridge mechanism
 Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Racah parameter and nephelauxetic effect
Racah parameter and nephelauxetic effect In chromatography
In chromatography Complexes of the type m(aa)3 ±n show
Complexes of the type m(aa)3 ±n show Jean piaget 1896 a 1980
Jean piaget 1896 a 1980 Coordination of secondary circular reactions
Coordination of secondary circular reactions Redox reactions half reactions
Redox reactions half reactions Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Correlation diagram in coordination chemistry
Correlation diagram in coordination chemistry Coordination number chemistry
Coordination number chemistry Electronic spectra of coordination compounds
Electronic spectra of coordination compounds 5 types of reactions chemistry
5 types of reactions chemistry Chemistry in biology section 2 chemical reactions
Chemistry in biology section 2 chemical reactions Reaction types
Reaction types Chapter 6 section 1 atoms elements and compounds answer key
Chapter 6 section 1 atoms elements and compounds answer key Chemistry chapter 8 review chemical equations and reactions
Chemistry chapter 8 review chemical equations and reactions Types of reactions chemistry
Types of reactions chemistry Chemistry reactions
Chemistry reactions Type of reactions chemistry
Type of reactions chemistry Used of metals
Used of metals