TRANSITIONS IN COMPLEXES TRANSITIONS IN COMPLEXES Transitions in








- Slides: 10

TRANSITIONS IN COMPLEXES

TRANSITIONS IN COMPLEXES Transitions in complexes can be grouped into three categories; intraligand transitions, ligand field transitions and charge transfer transitions.

INTRALIGAND TRANSITIONS These are transitions that depend on the nature of the chromophores (group of atoms capable of absorbing light energy) present in the ligands. These chromophores include organic functional groups such as alkenes, aromatic hydrocabons, heterocyclic compounds, amines, alcohols and ethers.

n→σ* transition: - In saturated molecules that containing heteroatoms with nonbonding lone pairs of electrons, the transition of the lowest energy will be n→σ*. This is possible because both the bonding and nonbonding orbitals are filled with electron and the highest occupied molecular orbital (HOMO) is the nonbonding orbital n while the lowest unoccupied molecular orbital (LUMO) is antibonding σ*. Examples of ligands with this transition are; amines, alcohols, ammonia, water and ethers.

n→π* transition: - this is possible in ligands with both nonbonding and π bonding molecular orbitals. In such a ligand, the HOMO will be n, LUMO will be π* and transition with the lowest energy will be n→π*. Examples of such ligands are aldehydes (R-CHO), ketones (R- CO-R), esters (RCOOR), amides (RCONH 2) and carboxylic acids (RCOOH).

π →π* transition: - ligands containing double bonds or triple bonds without any heteroatom will have transition of lowest possible energy to be π →π*. Examples are olefins, conjugated dienes and aromatic hydrocarbons.

Unsaturated heterocyclic compounds (such as 1, 10 -phenanthroline, 2, 2’bipyridine and pyridine, multiple transitions) have different transitions including; π →π*, n→π* and n→σ*. The ligand transitions are often in the ultra violet range of the spectrum but with extended conjugations, some transitions in the visible regions will be inevitable.

CHARGE TRANFER TRANSITIONS Electron can be excited from a metal orbital to ligand orbital in a transition complex. This is made possible when, the metal has electron in t 2 g orbitals (which are nonbonding orbitals) and the ligand has suitable empty orbitals (π*).

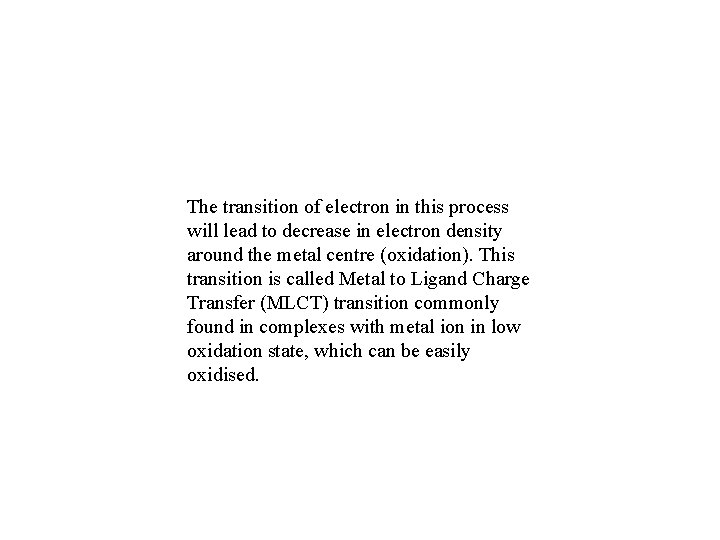
The transition of electron in this process will lead to decrease in electron density around the metal centre (oxidation). This transition is called Metal to Ligand Charge Transfer (MLCT) transition commonly found in complexes with metal ion in low oxidation state, which can be easily oxidised.

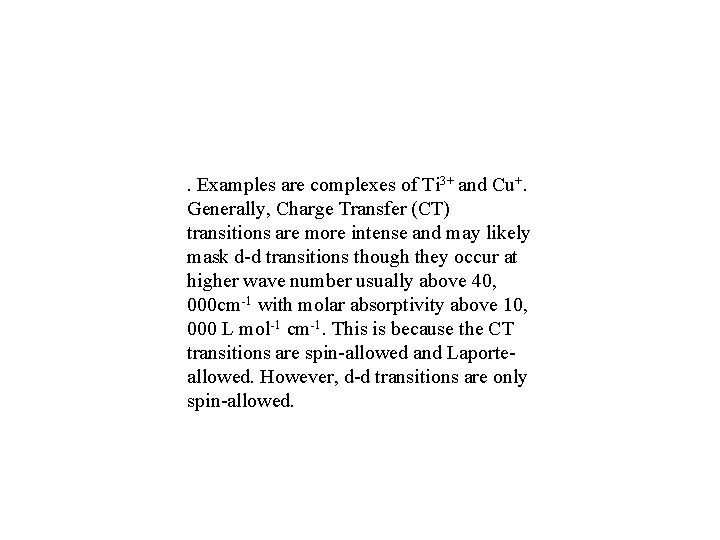
. Examples are complexes of Ti 3+ and Cu+. Generally, Charge Transfer (CT) transitions are more intense and may likely mask d-d transitions though they occur at higher wave number usually above 40, 000 cm-1 with molar absorptivity above 10, 000 L mol-1 cm-1. This is because the CT transitions are spin-allowed and Laporteallowed. However, d-d transitions are only spin-allowed.