Infant Feeding Human Milk and Formula Infant Feeding















































































- Slides: 95

Infant Feeding: Human Milk and Formula

Infant Feeding: History and Perspective • Human Milk Substitutes • Science, Medicine and Industry

Feeding the Infant • Considerations – Infant (needs, tolerance, acceptance, safety) – Family preferences – Cost and availability – Prevention, health, development, and programming

Feeding the Infant • Choices: – Human Milk – Standard Infant Formula (Cow, Soy) – Hypoallergenic (hydrolysates vs. amino acid based – Other specialty formulas – Beikost/solids/table foods

History Human milk substitutes Health and welfare Programs Formula Industry

Human Milk Substitutes • Early evidence of artificial feeding • Majority of infants received breast milk – Maternal BF – Wet nurses • Wealthy women • Orphans, abandoned, “illegitimate” • Prematurity or congenital deformities

Wet Nurses • Work demands, societal needs, vanity, health requirements, social diversion • Proper selection: Questionable character-- Infant would suck in her vices • Wet Nurse Industry: emerging infant mortality/abuse • Impact of industrial revolution: Wet nurses made better money in factories

Human Milk Substitutes: Infant Mortality • Artificial feeding in first weeks of life associated with 100% mortality • 19 th century infant mortality with “hand feeding” was 88% • Foundlings: 80% • In Dublin Foundling hospital 1775 -96: 99. 6%

Science, Medicine, and Industry Growth of child Health and welfare in early 20 th century

Science, Medicine, and Industry • Infant Morbidity and Mortality • Recognition of association with human milk substitutes, and infection • Industrial development – Storage – Safety – Food industry

Human Milk Substitutes • 1920 -1950’s: evaporated or fresh cow’s milk, water and added CHO (prepared at home) • 1950’s to present commercially prepared infant formulas have replaced home recipes

Infant Formulas - History • • Cow’s milk is high in protein, low in CHO, results in large initial curd formation in gut if not heated before feeding Early Formulas – – from 1920 -1950 majority of non-breastfed infants received evaporated milk formulas boiled or evaporated milk solved curd formation problems cho provided by corn syrup or other cho to decrease relative protein kcals

Historical timeline • 1900 – Pasteurization of milk in US – Association between bacteria and diarrhea • 1912 – U. S Children’s Bureau – Public Health and Pediatricians efforts to improve infant/child health and decrease mortality • 1920 – Intro evaporated milk – Cod liver oil prevents rickets – Curd tension of milk altered – Increased availability of refrigeration – Vitamin C isolated – Vitamin D prepared in pure form – Improved sanitation

Historical timeline • 1940 – Homogenized milk widely marketed • 1960 – Further advances in technology and packaging – Commercially prepared infant formula becoming increasingly popular

Interesting Milestones in Infant Nutrition • 1784: Underwood recommends cows milk as alternative to breast feeding • 1800: glass feeding bottles • 1838: Simon determines protein CM>BM • 1845: Pratt patents rubber nipple • 1856: Borden patents condensed milk • 1883: Meyenberg patents evaporated goats milk • 1885: Meigs analyses human milk

Interesting Milestones in Infant Nutrition 1911: MJ introduces Dextri-maltose 1915: SMA 1920: Franklyn (Similac) 1929: MJ markets Sobee, hypoallergenic 1930 -60: Concentrated liquid, hydrolysed, elemental, and ready to feed formulas introduced • What now? • • •

Interesting Milestones in Infant Nutrition 1911: MJ introduces Dextri-maltose 1915: SMA 1920: Franklyn (Similac) 1929: MJ markets Sobee, hypoallergenic 1930 -60: Concentrated liquid, hydrolysed, elemental, and ready to feed formulas introduced • What now? • • •

Infant Formula - History, cont. • • • 50 s and 60 s commercial formulas replaced home preparation 1959: iron fortification introduced, but in 1971 only 25% of infants were fed Fe fortified formula Cow’s milk feedings started in middle of first year between 1950 -1970 s. In 1970 almost 70% of infants were receiving cow’s milk.

• “No two hemispheres of any learned professor’s brain are equal to two healthy mammary glands in the production of a satisfactory food for infants” - Oliver Wendell Holmes

Human Milk • Complements infant Immaturity • Promotes maturation – Epithelial growth factors and hormones – Digestive enzymes - lipases and amylase

Characteristics and Advantages of Human Milk • Low renal solute load • Immunologic, growth and trophic factors – Decrease illness, infection, allergy • • Improved digestion and absorption Nutrient Composition: CHO, Protein, Fatty Acid, etc Cost Other

Human Milk • Colostrum – Higher concentration of protein and antibodies – Transitions around days 3 -5 – Mature by day 10

Human Milk • Nutrient composition of human milk is remarkable for its variability, as the content of some of the nutrients change during lactation, throughout the day, or differ among women, while the content of some nutrients remain relatively constant throughout lactation.

Role of Human Milk Components in GI Development: Current Knowledge and Future Needs: Donovan J Pediatr 2006: 149: S 49 -S 61 “ existing clinical and epidemiological studies support a developmental advantage for breastfeeding. However, our understanding of the mechanisms by which HM components exert their actions within the human infant are limited by the large number of bioactive compounds in milk and the complexity of the potential interactions among the components and with the developing intestine”

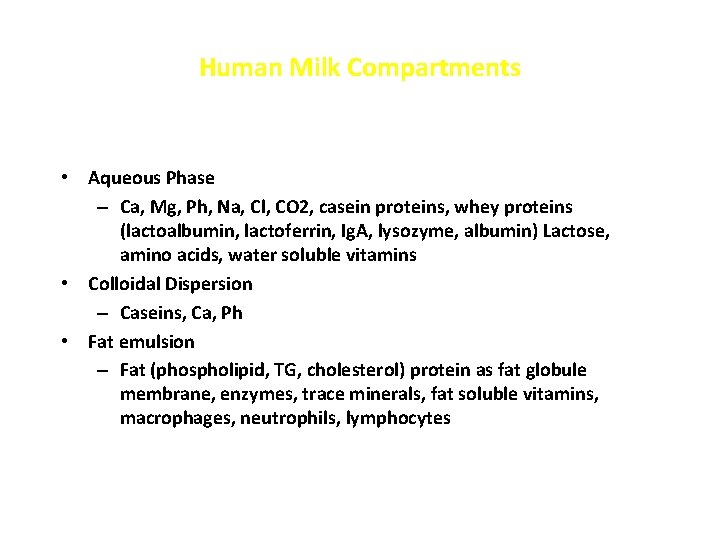
Human Milk Compartments • Aqueous Phase – Ca, Mg, Ph, Na, Cl, CO 2, casein proteins, whey proteins (lactoalbumin, lactoferrin, Ig. A, lysozyme, albumin) Lactose, amino acids, water soluble vitamins • Colloidal Dispersion – Caseins, Ca, Ph • Fat emulsion – Fat (phospholipid, TG, cholesterol) protein as fat globule membrane, enzymes, trace minerals, fat soluble vitamins, macrophages, neutrophils, lymphocytes

Milk Synthesis • Mammary gland contains stem cells and highly differentiated secretory alveolar cells at the terminal ducts. Stimulated by insulin and HGH synergized by prolactin, these cells are active in milk synthesis and secretion

Milk synthesis and secretion • Exocytosis (protein, lactose, Ca/Ph, citrate) • Fat synthesis (TG synthesized in cytoplasm and smooth endoplasmic reticulum + precursors imported from maternal circulation): alveolar cells synthesize SCFA • Secretion of ions and water • Immunoglobins transferred from extracellular spaces

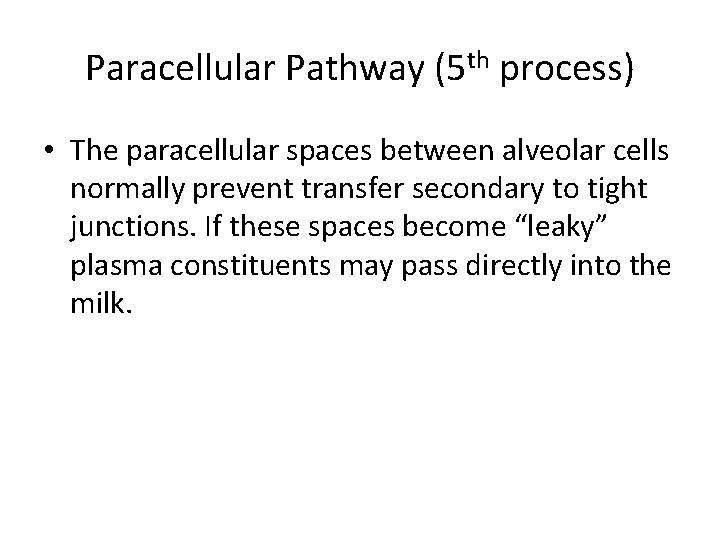
Paracellular Pathway (5 th process) • The paracellular spaces between alveolar cells normally prevent transfer secondary to tight junctions. If these spaces become “leaky” plasma constituents may pass directly into the milk.

Milk Synthesis and secretion • Under neuroendocrine control that varies with timing and stage of lactation – Prolactin – Lactogens – Estrogen – Thyroxine – Growth hormone – ACTH – other – Stimulus: infant suckling

Milk synthesis • Protein: vast majority of proteins present in human milk are specific to mammary secretions and not identified in any quantity elsewhere in nature: – Immunoglobins transferred from plasma in early stages of lactation – De novo protein synthesis by mammary gland

Diet, milk production, and milk composition • There is a great variation in milk composition during a feed, from feed to feed, and even between breasts. • The impact of dietary variation and milk composition is unclear. Overall milk composition remains relatively unaffected by diet variations although there are reports to the contrary: – DHA and ARA supplementation, vegan diet, drugs and environmental contaminants, …. .

Breast milk composition and Diet • DHA levels of breast milk vary with diet. Increased amounts of DHA have been found in the breast milk of mothers consuming fish or fish oil, and with supplementation. • Water soluble vitamins may vary with diet. Diets inadequate in B 12 or thiamin have been associated with case reports of deficiency in infants. High intakes of Vitamin C, however, does not appear to change the content of breast milk. • Supplementation of fat soluble vitamins do not appear to alter the content of breast milk • Iron supplementation does not appear to alter the iron content of breast milk

Influence of diet on milk composition • Protein-energy malnutrition impacts milk volume. Composition remains relatively unaffected • Water soluble vitamins move readily from serum to milk thus dietary fluctuations are more apparent – B 12 vegan, case report of beri…. . • Fat soluble vitamin content not improved with supplementation • Fatty acid composition (DHA and ARA) altered by maternal diet and supplementation

Science and Lactation: Frank Hytten • “ In general, it is probable that the breast has a high priority for nutrients and that moderate maternal under nutrition will have little effect on milk production. But severe malnutrition, which rarely exists without associated illhealth and other adverse circumstances, may reduce milk yield”

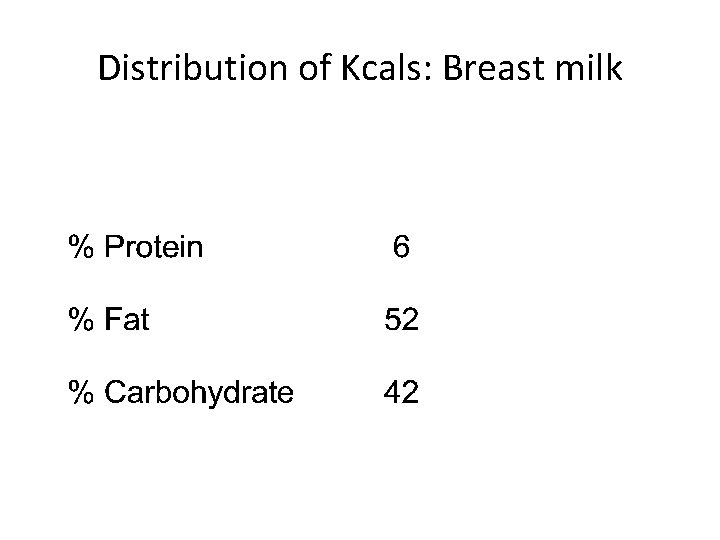
Distribution of Kcals: Breast milk

Protein: Predominant protein of human milk is whey. Casein/whey ratio is between 40: 60 and 30: 70 – Casein: proteins of the curd (low solubility at p. H 4. 6) – Whey: soluble proteins (remain soluble at p. H 4. 6) Lactalbumin Lactoferrin Secretory Ig. A Lactoglobulin

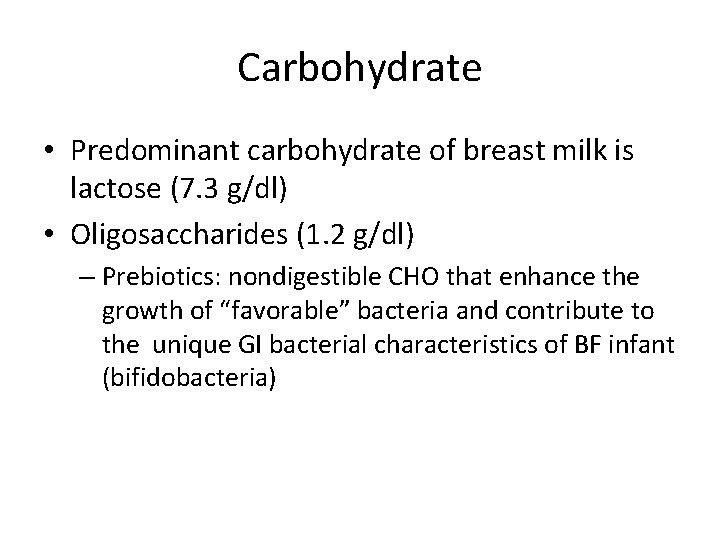
Carbohydrate • Predominant carbohydrate of breast milk is lactose (7. 3 g/dl) • Oligosaccharides (1. 2 g/dl) – Prebiotics: nondigestible CHO that enhance the growth of “favorable” bacteria and contribute to the unique GI bacterial characteristics of BF infant (bifidobacteria)

Fat • 2. 5 - 4. 5% Fat (provides approx 50% of calories) • Contained in membrane enclosed milk fat globules – Core: TG (98 -99%of total milk fat) – Membrane: phospholipids, cholesterol, protein • DHA/ARA: wide variations

DHA/ARA concentration variation in human milk • DHA: 0. 1 -1. 4% • ARA: 0. 31 - 0. 71% – DHA lowest in populations with high meat intake and highest in populations with high fish intake

Breast milk and establishment of core microbiome • Definition: Full collection of microbes that naturally exist within the body. • Alterations or disruptions in core microbiome associated with chronic illness: Crohns disease, increased susceptibility to infection, allergy, NEC, etc

Microbiome • Beneficial effect for the host: – Nutrient metabolism – Tissue development – Resistance to colonization with pathogens – Maintenance of intestinal homeostasis – Immunological activation and protection of GI integrity

Human milk and microbiome • Core microbiome established soon after birth • Core microbiome of breastfeeding infant similar to core microbiome of lactating mother • Components of breast milk supporting establishment of microbiome – Prebiotics, probiotics

AAP Policy Statement: Breastfeeding and the use of human milk • AAP statement includes 15 recommendations on Breastfeeding healthy term infants including: – Establish peripartum policies and practices supporting breastfeeding – Place infant skin to skin after delivery until first feeding is accomplished

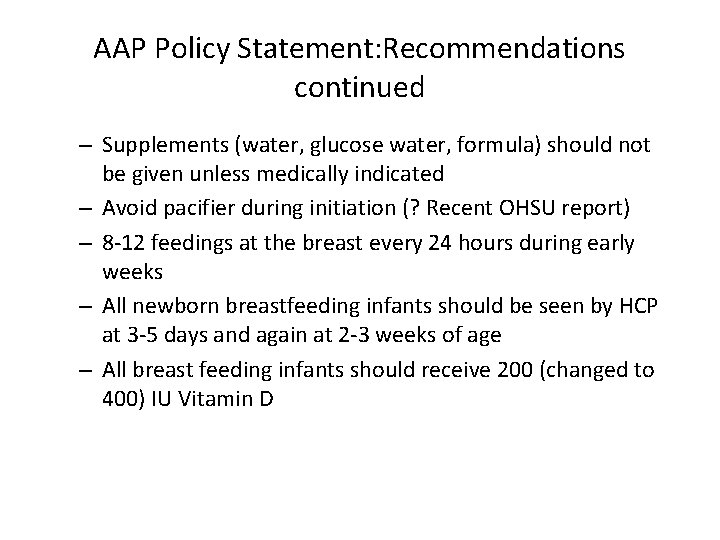
AAP Policy Statement: Recommendations continued – Supplements (water, glucose water, formula) should not be given unless medically indicated – Avoid pacifier during initiation (? Recent OHSU report) – 8 -12 feedings at the breast every 24 hours during early weeks – All newborn breastfeeding infants should be seen by HCP at 3 -5 days and again at 2 -3 weeks of age – All breast feeding infants should receive 200 (changed to 400) IU Vitamin D

AAP Policy Statement: Breastfeeding and the use of human milk: Pediatrics 115 #2 2005 • Human milk is species specific and uniquely superior for infant feeding • Exclusive breastfeeding is the reference or normative model against which all alternative methods must be measured in regards to growth, development and health • Research provides strong evidence that human milk feeding decreases the incidence and/or severity of a number of infectious diseases (meningitis, Otitis media, UTIs, Respiratory tract infections, NEC, diarrhea)

AAP Policy Statement: Breastfeeding and the use of human milk: • Contraindications to breastfeeding – Galactosemia – Maternal use/exposure to certain radioactive or chemotherapeutic agents – Maternal abuse of “street drugs” – Active HSV lesions of breast – Maternal HIV (in USA)

AAP Policy Statement: Breastfeeding and the use of human milk: • Some studies suggest decreased incidence of SIDS, diabetes (type 1 and 2), leukemia, obesity, hypercholesterolemia, and allergy (asthma and atopy) • Breastfeeding has been associated with slightly enhanced performance on tests of cognitive development.

Newborn Visit: Breastfeeding • Maternal care – rest – fluids – relieving breast engorgement – caring for nipples – eating properly • Follow-up support from the health professional by telephone, home visit, nurse visit, or early office visit.

Cautionary Tales • Cooper et al. Pediatrics 1995. Increased incidence of severe breastfeeding malnutrition and hypernatremia in a metropolitan area. • Rolf et al. ACTA Paediatrica 2009. A nationwide study on hospital admissions due to dehydration in exclusively breastfed infants in the Netherlands: its incidence, clinical characteristics, treatment and outcome • Lozoff et al. J Pediatrics 2009 Higher Infant Blood Levels with Longer Duration of Breastfeeding

Cooper. • 5 breastfed infants admitted to Children’s hospital in Cincinnati over 5 months period for breastfeeding malnutrition and dehydration – Age of admission: 5 -14 days – Weight loss at admission 23%, range 14 -32% – Serum Na: 186 mmol/L, range 161 -214 (136 -143 wnl) – mothers were between the ages of 28 and 38, had prepared for breastfeeding – 3 had inverted nipples and reported latch-on problems before discharge – 3 families had contact with health care providers before readmission including calls to PCP and home visit by PHN

Rolf • Survey to determine incidence and characteristics of hospital admission due to dehydration • Dutch Paediatric Surveillance Unit 2003 -2005 of all hospital admissions during 1 st 3 months in fully breast fed infants • 250 reported cases. • N= 158 (excluded cases with incomplete information or coexisting medical conditions accounting for hospitalization

Lozoff • Our findings support the conclusions… “that this phenomenon constitutes a potential public health problem in areas where environmental lead exposure is continuing as well as where environmental lead exposure has recently declined”… Our findings do not detract from the many known benefits of breastfeeding. Rather, they suggest that monitoring lead concentrations in breastfed infants should be considered….

Formula Composition • Breast Milk as “gold standard” – Attempt to duplicate composition of breastmilk – ? Bioactivity, relationship, function of all factors present in breast milk – ? Measure outcome: growth, composition, functional indices

Formula Categories • Standard – Cows milk base – Soy base • Elemental – Hydrolysates – Amino acid pased • Other Specialty Products – – Metabolics PM 60/40 Low fat/MCT Premature feeding products

Formula Brands • Ross – Similac/Isomil/Alimentum • Mead Johnson – Enfamil/Prosobee/Enfacare • Nestle – Good Start • Wyeth – Generic in USA; Gold Brands; SMA • SHS – Neo. Cate, Duo. Cal

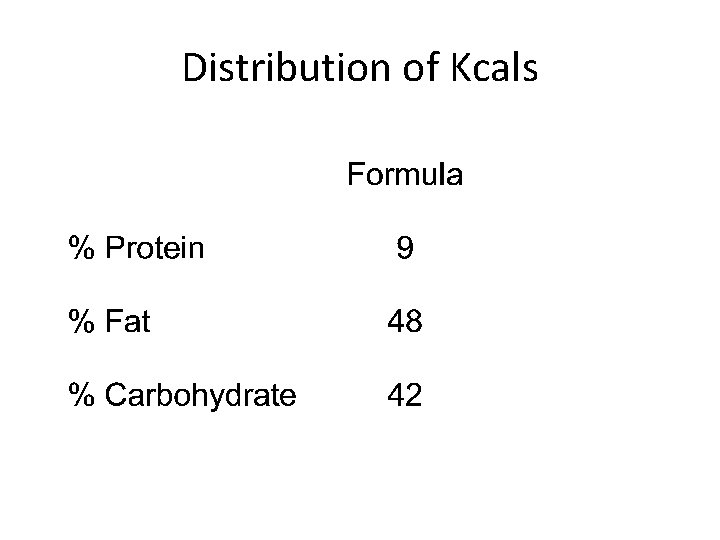
Distribution of Kcals

Vitamin and Mineral content • NAS/FDA • Meet levels at typical volumes ingested by infants (@ 24 -32 ounces) – i. e. RDA/DRI

Cow’s Milk Based Formula • Commercial formula designed to approximate nutrients provided in human milk • Some nutrients added at higher levels due to less complete digestion and absorption

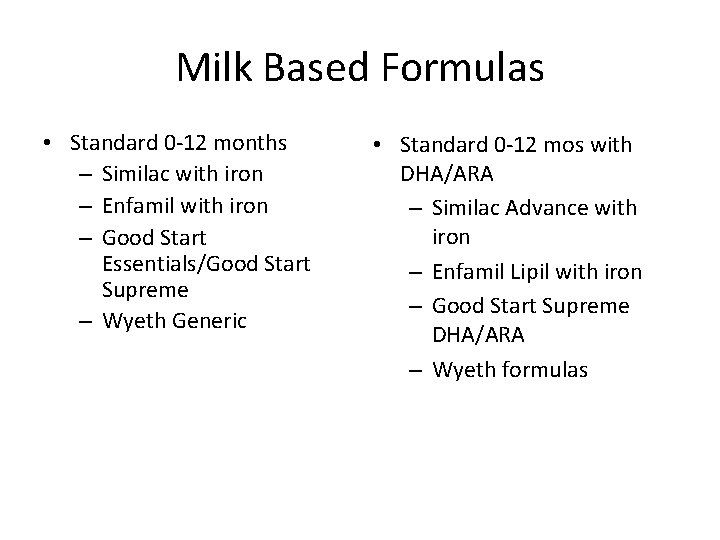
Milk Based Formulas • Standard 0 -12 months – Similac with iron – Enfamil with iron – Good Start Essentials/Good Start Supreme – Wyeth Generic • Standard 0 -12 mos with DHA/ARA – Similac Advance with iron – Enfamil Lipil with iron – Good Start Supreme DHA/ARA – Wyeth formulas

Protein • Blend of whey and casein proteins • 8. 2 -9. 6% total calories – whey proteins of human and cow’s milk are different and have different amino acid profiles. • Major whey proteins of human milk are lactalbumin (high levels of essential aa) , immunoglobulins, and lactoferrin( enhances iron transportation) • Cow’s milk has low levels of these proteins and high levels of b lactoglobulin

Cow’s Milk Based Formula: Fat & CHO • Fat: butterfat of cow’s milk is replaced with vegetable fat sources to make the fatty acid profile of cow’s milk formulas more like those of human milk and to increase the proportion of essential fatty acids • CHO: Lactose is the major carbohydrate in most cows’ milk based formulas. • Meets needs of healthy infants

Milk Based Pre and Probiotic Supplemented • Marketed to promote digestive health and support healthy immune fx • Probiotic – Bifidus BL • Gerber Good start Protect Plus – Lactobacillus rhamosus • Nutramigen Lipil with Enflora • Prebiotic – Galactooligosaccarides (GOS) – Similac Advance Early Shield (Triple Shield), Enfamil Premium, Generic Brands

Infant Formulas: AAP • Cow’s milk based formula is recommended for the first 12 months if breast milk is not available

Soy Formulas • • • First developed in 1930 s with soy flour Early formulas produced diarrhea and excessive gas Now use soy protein isolate with added methionine

Soy Formulas • Isomil/Isomil DF /Isomil Advance 2 • Prosobee/Prosobee Lipil/Next Step Prosobee • Good Start Essentials Soy/Good Start 2 Essentials Soy • Wyeth All iron fortified

Soy Formulas • Protein: soy protein isolate with added methionine • Fat: vegetables oils • CHO: usually corn based products

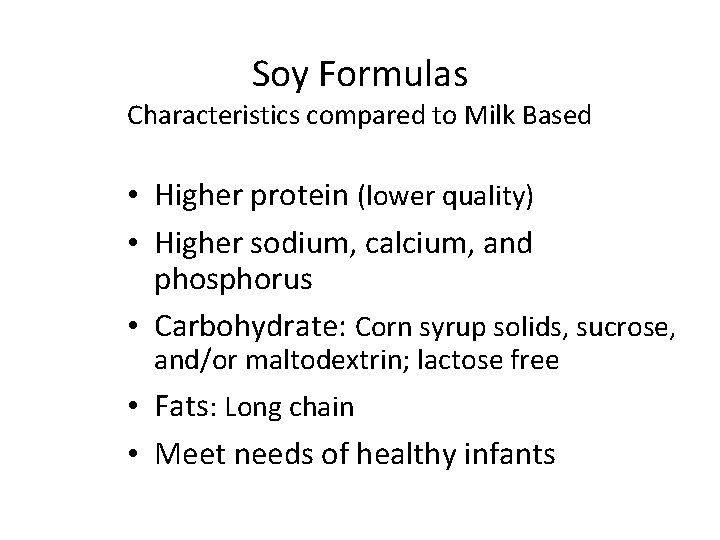
Soy Formulas Characteristics compared to Milk Based • Higher protein (lower quality) • Higher sodium, calcium, and phosphorus • Carbohydrate: Corn syrup solids, sucrose, and/or maltodextrin; lactose free • Fats: Long chain • Meet needs of healthy infants

American Academy of Pediatrics Committee on Nutrition. Soy Proteinbased Formulas: Recommendations for Use in Infant Feeding. Pediatrics 1998; 101: 148 -153. • Soy formulas given to 25% of infants but needed by very few • Offers no advantage over cow milk protein based formula as a supplement for breastfed infants • Provides appropriate nutrition for normal growth and development • Indicated primarily in the case of vegetarian families and for the very small number of infants with galactosemia and hereditary lactase deficiency

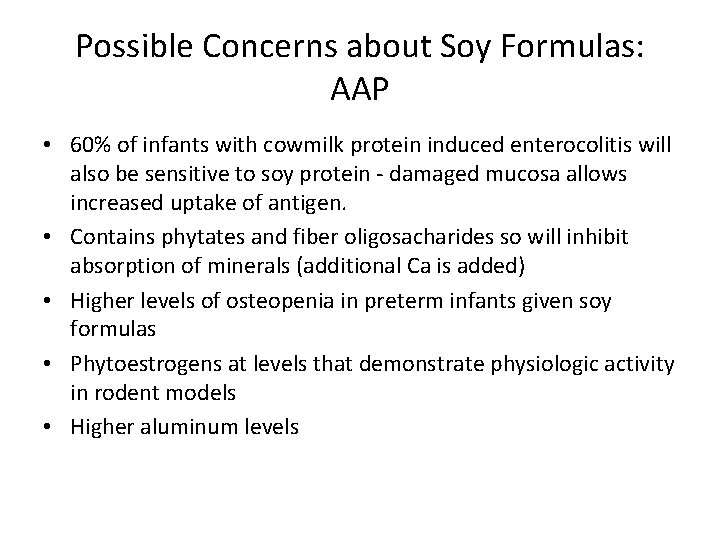
Possible Concerns about Soy Formulas: AAP • 60% of infants with cowmilk protein induced enterocolitis will also be sensitive to soy protein - damaged mucosa allows increased uptake of antigen. • Contains phytates and fiber oligosacharides so will inhibit absorption of minerals (additional Ca is added) • Higher levels of osteopenia in preterm infants given soy formulas • Phytoestrogens at levels that demonstrate physiologic activity in rodent models • Higher aluminum levels

Health Consequences of Early Soy Consumption. Badger et al. J Nutr. 2002 • • US soy formulas made with soy protein isolate (SPI+) SPI+ has several phytochemicals, including isoflavones Isoflavones are referred to as phytoestrogens Phytoestrogens bind to estrogen receptors & act as estrogen agonists, antagonists, or selective estrogen receptor modulators depending on tissue, cell type, hormonal status, age, etc.

Should we be Concerned? - Badger et al. • No human data support toxicity of soyfoods • Soyfoods have a long history in Asia • Millions of American infants have been fed soy formula over the past 3 decades • Rat studies indicate a potential protective effect of soy in infancy for cancer

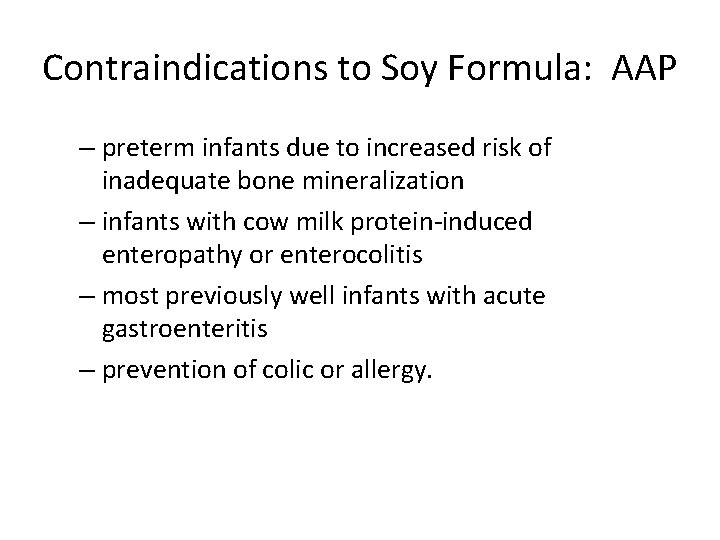
Contraindications to Soy Formula: AAP – preterm infants due to increased risk of inadequate bone mineralization – infants with cow milk protein-induced enteropathy or enterocolitis – most previously well infants with acute gastroenteritis – prevention of colic or allergy.

Elemental Formulas • Hydrolysates • Amino Acid Based

Protein Hydrolysate Formulas • Alimentum Advance • Pregestimil/Pregestimil Lipil • Nutramigen Lipil u u u Protein Casein hyrolysate + free AA’s Fat (Alimentum and Pregestimil) Medium chain + Long chain triglycerides; (Nutramigen) Long chain triglycerides Carbohydrate: Lactose free

Hydrolysate Formulas • Whey Hydrolysate Formula: Cow’s milk based formula in which the protein is provided as whey proteins that have been hydrolyzed to smaller protein fractions, primarily peptides. This formula may provoke an allergic response in infants with cow’s milk protein allergy. • Casein Hydrolysate Formula: Infant formula based on hydrolyzed casein protein, produced by partially breaking down the casein into smaller peptide fragments and amino acids. `

AAP Policy Statement Re: Hypoallergenic Infant Formulas (August, 2000) Recommendations

AAP Policy Statement Re: Hypoallergenic Infant Formulas (August, 2000) • Currently available, partially hydrolyzed formulas are not hypoallergenic.

2. Formula-fed infants with confirmed cow's milk allergy may benefit from the use of a hypoallergenic or soy formula as described for the breastfed infant.

Amino Acid Based

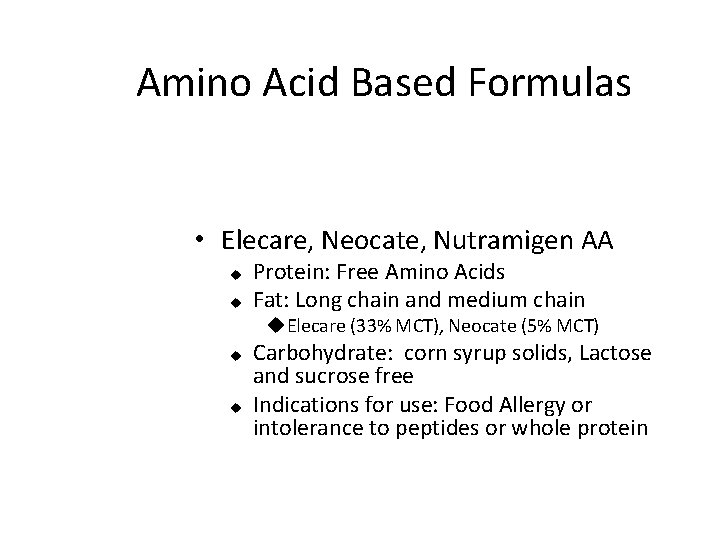
Amino Acid Based Formulas • Elecare, Neocate, Nutramigen AA u u Protein: Free Amino Acids Fat: Long chain and medium chain u. Elecare (33% MCT), Neocate (5% MCT) u u Carbohydrate: corn syrup solids, Lactose and sucrose free Indications for use: Food Allergy or intolerance to peptides or whole protein

Elemental Infant Formula • Neo. Cate (SHS) Protein: Free Amino Acids u Fat: Long chain u Carbohydrate: Lactose Free u Indications for use: Food Allergy or intolerance to peptides or whole protein u

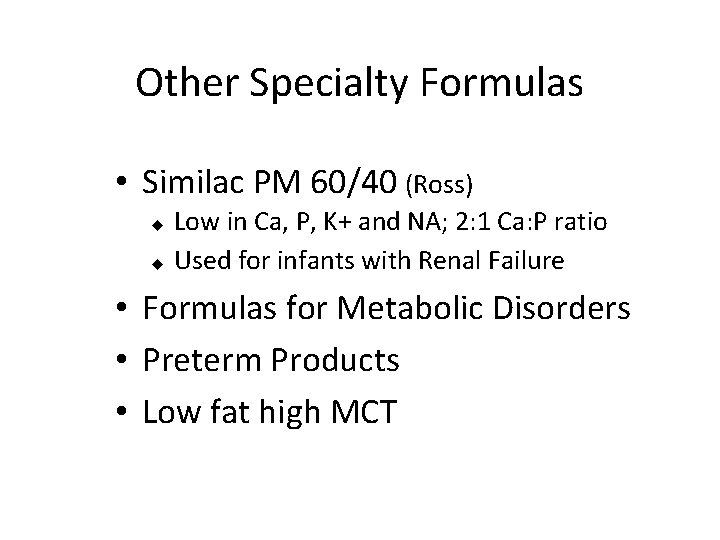
Other Specialty Formulas • Similac PM 60/40 (Ross) Low in Ca, P, K+ and NA; 2: 1 Ca: P ratio u Used for infants with Renal Failure u • Formulas for Metabolic Disorders • Preterm Products • Low fat high MCT

Indications • Cow’s milk based – Health term infant • Soy – Vegetarian – Galactosemia • Protein Hydrolysates – Protein intolerance/allergy – other • Preterm Formulas • Post-discharge Preterm formulas • Other Specialty Formulas – Specific medical, metabolic indications

Know What You Are Feeding n n n Caloric density, protein, fat and carbohydrate vitamin and mineral content. Osmolality: Renal Solute Load: Evaluate RSL in context of solute intake, fluid intake and output. n Evidence Based n Rationale n Cost and availability

Finding Up to Date Information • www. ross. com Similac products • www. meadjohnson. com Enfamil products • www. verybestbaby. com Nestle products • www. wyethnutritionals. com generic products – www. brightbeginnings. com lower cost formulas made by Wyeth • www. shsna. com/html/Hypoallergenic. htm Neocate formulas

Regulation of Infant Formula • FDA – Infant Formula Act • Manufacturers – Voluntary monitoring • AAP, National Academy of Sciences, other professional organizations – Guidelines for composition and intake: (e. g. DRI’s) – Guidelines for preparation and handling of formula/human milk in health care facilities

Regulation of Infant Formulas • Infant Formula Act: – Manufacturing regulations – Quality control • Non specific testing requirements, case by case basis, growth outcomes – Recall Proceedures – Nutrient content and labeling – Panel convened 1998 and 2002 (recommended revisions including exemptions)

Regulation of Infant Formulas • Infant Formula Act: The purpose of the infant formula act (1980) is to ensure the safety and nutrition of infant formulas – including minimum and in some cases maximum levels of specified nutrients. The act authorizes the FDA to establish appropriate regulations for 1) new formulas, 2) formulas entering the U. S. market, 3) major changes, revisions, or substitutions of macronutrients 4) formulas manufactured in new plants or processing lines, 5) addition of new constituents 6) use of new equipment or technology 7) packaging changes

Formula Regulation • • • Regulation is by the Infant Formula Act of 1980, under FDA authority Nutrient composition guidelines for 29 nutrients established by AAP Committee on Nutrition and adopted as regs by FDA Nutrient Requirements for Infant Formulas. Federal Register 36, 23553 -23556. 1985. 21 CFR Part 107.

Infant Formula Act • Institute of Medicine Food and Nutrition Board 3/2004 • “Although the federal regulatory processes for evaluating the safety of food ingredients have worked well for conventional substances, they were not designed to ensure the needs and vulnerabilities of infants and are insufficient to ensure the safety of new types of ingredients proposed for infant formulas

Infant Formula Act • “The current regulatory processed do not fully address the unique role of formula as a food source. Formula is the only infants’ food if they are not being breastfed. The processes used to regulate the safety of any new additions of formula should be tailored to these products distict role and the special needs and susceptibilities of infants”

Infant Formula Act • Key limitation: lack of explicit guideleines for determining when and what safety data is needed…. . (GRAS) • Clarification is crucial given the increasing number of bioactive peptides and enzymens generated from unconventional sources or new technologies

Infant Formula Act: Points for discussion • Addition of DHA and ARA to formulas • Addition of prebiotics to formula – Present in BM – GRAS – Vitamin/mineral content conforms to regulation – ? testing

Formula safety • FDA recall list 2005 -2006

Cows milk and goats milk • • Protein RSL Folic acid, iron, vitamin D pasteurization
 Difference between cow milk and human milk
Difference between cow milk and human milk Human milk vs cow milk
Human milk vs cow milk Brooke welker
Brooke welker Continuous feeding vs bolus feeding
Continuous feeding vs bolus feeding Sensory evaluation of milk and milk products
Sensory evaluation of milk and milk products Contamination of milk and milk products
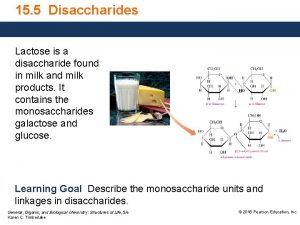
Contamination of milk and milk products Lactose is a disaccharide found in milk
Lactose is a disaccharide found in milk Milk for toddlers with milk allergynon dairy
Milk for toddlers with milk allergynon dairy Ecumene definition ap human geography
Ecumene definition ap human geography Casein in human milk
Casein in human milk Equations
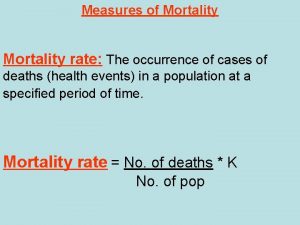
Equations Proportionate mortality rate formula
Proportionate mortality rate formula Gos infant formula
Gos infant formula Infant mortality rate formula
Infant mortality rate formula Infant mortality rate formula
Infant mortality rate formula Ngt feeding formula
Ngt feeding formula What is visitor pre registration in picme
What is visitor pre registration in picme Milk standardization formula
Milk standardization formula Keeping an infant safe and well section 7-3
Keeping an infant safe and well section 7-3 Kenmore park infant and nursery school
Kenmore park infant and nursery school Keeping an infant safe and well section 7-3
Keeping an infant safe and well section 7-3 Saguaro infant care and preschool
Saguaro infant care and preschool Sparhawk infant and nursery school
Sparhawk infant and nursery school Infant child and adolescent berk 8th edition chapter 1
Infant child and adolescent berk 8th edition chapter 1 Brigance self help scoring tool
Brigance self help scoring tool Jama 2017
Jama 2017 8.3 human needs
8.3 human needs Chapter 8 human needs and human development
Chapter 8 human needs and human development Human nouns
Human nouns Suspension and deposit feeding
Suspension and deposit feeding Chapter 27 nutritional therapy and assisted feeding
Chapter 27 nutritional therapy and assisted feeding Chapter 18 eating and feeding disorders
Chapter 18 eating and feeding disorders What is feeding relationship
What is feeding relationship Environment and feeding relationship
Environment and feeding relationship Oral placement therapy for speech clarity and feeding
Oral placement therapy for speech clarity and feeding Building vocabulary: diet categories and feeding mechanisms
Building vocabulary: diet categories and feeding mechanisms Lesson 11: feeding and digestion
Lesson 11: feeding and digestion Lesson 11 feeding and digestion
Lesson 11 feeding and digestion Hart plain infant school
Hart plain infant school Drdp essential view
Drdp essential view Infant-industry argument
Infant-industry argument Counter clockwise
Counter clockwise Infant compression to ventilation ratio
Infant compression to ventilation ratio What is 90 degree angle for injection
What is 90 degree angle for injection Pain scale with words
Pain scale with words Bilirubin chart
Bilirubin chart Baby reflexes chart
Baby reflexes chart Module 45 developmental issues
Module 45 developmental issues Juan soriano la niña muerta; the dead girl; dead infant
Juan soriano la niña muerta; the dead girl; dead infant Infant reflexes chart
Infant reflexes chart Infant blood pressure
Infant blood pressure Transport incubator
Transport incubator Where was botulism first discovered
Where was botulism first discovered Llf meaning cpr
Llf meaning cpr Convent of the holy infant jesus school
Convent of the holy infant jesus school Infant personality development
Infant personality development Weight gain in infant
Weight gain in infant Chest compression for infant 2 rescuer
Chest compression for infant 2 rescuer An infant's growth refers to changes in
An infant's growth refers to changes in High quality cpr child
High quality cpr child Femoral pulse in child
Femoral pulse in child Dormers wells infant school
Dormers wells infant school Woodfield infant school
Woodfield infant school Papoose infant spinal immobilizer
Papoose infant spinal immobilizer Infant/toddler sensory profile score sheet
Infant/toddler sensory profile score sheet Embrace infant warmer
Embrace infant warmer Social impulses foster infant language
Social impulses foster infant language Social impulses foster infant language
Social impulses foster infant language Phoenix infant academy
Phoenix infant academy Walter infant
Walter infant Wood street infant school
Wood street infant school Infant industry
Infant industry Infant
Infant Downs view infant school
Downs view infant school Baby shoes ernest hemingway
Baby shoes ernest hemingway Infant
Infant Infant age
Infant age Trauma chin lift
Trauma chin lift Cone of experience
Cone of experience Hhs
Hhs Crvs institute panel
Crvs institute panel What is sudden infant death syndrome
What is sudden infant death syndrome Keystone of cranium
Keystone of cranium Newborn reflexes
Newborn reflexes Pinewood infant school
Pinewood infant school Infant oral health care
Infant oral health care Catherine maguire infant mental health
Catherine maguire infant mental health Hip usg
Hip usg Corruption
Corruption Infant flow driver
Infant flow driver Infant industries apush
Infant industries apush Woolston infant school
Woolston infant school Infant motrin coupon
Infant motrin coupon The wringing method is used to do what to a sauce
The wringing method is used to do what to a sauce Judging and grading of milk
Judging and grading of milk Family guy is not appropriate to watch during school
Family guy is not appropriate to watch during school