ICH E5 Overview and Current Topics Mamoru Narukawa

















- Slides: 26

ICH E-5 Overview and Current Topics Mamoru Narukawa

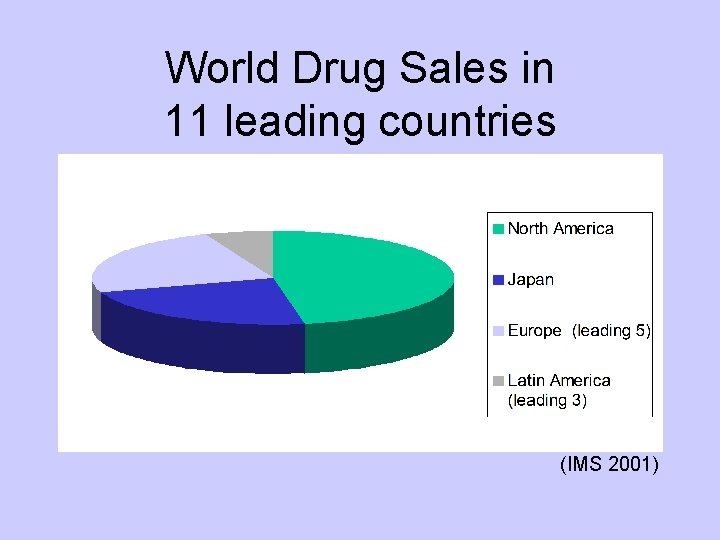
World Drug Sales in 11 leading countries (IMS 2001)

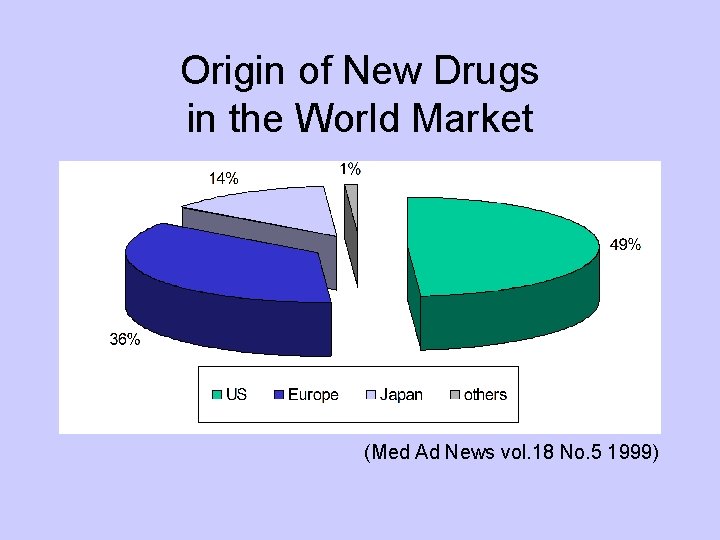
Origin of New Drugs in the World Market (Med Ad News vol. 18 No. 5 1999)

Reorganization of Pharmaceutical Industry Glaxo. Smith. Kline (1) Pfizer (2) Astra. Zeneca (4) Aventis (5) Bristol-Myers Squibb (6) Novartis (7) Johnson & Johnson (8) Pharmacia (9) Glaxo. Wellcome Smith. Kline Beecham Warner-Lambert Zeneca, Astra Hoechst, Rhone-Poulane Dupont Ciba-Geigy, Sandoz ALZA Pharmacia & Upjohn Monsanto (Economist 2001. 9. 11)

ICH • Europe (EU, EFPIA), Japan (MHLW, JPMA), and US (FDA, Ph. RMA) • Discuss scientific and technical aspects of requirements for pharmaceuticals • Eliminate unnecessary delay in the global development and availability of new drugs

ICH Topics – Efficacy (1) • E 1: The extent of population exposure to assess clinical safety • E 2 A: Definitions and standards for expedited reporting • E 2 B: Data elements for transmission of ADR reports • E 2 C: Periodic safety update reports • E 3: Structure and content of clinical study reports • E 4: Dose-response information to support drug registration

ICH Topics – Efficacy (2) • E 5: E 5 Ethnic factors in the acceptability of foreign clinical data • E 6: Good clinical practice • E 7: Clinical trials in special population – Geriatrics • E 8: General considerations • E 9: Statistical principles • E 10: Choice of control group • E 11: Clinical investigation in the pediatrics • E 12 A: Clinical trials on antihypertensives

History of ICH E-5 • 1992. 3 Discussion started • • Draft Guideline agreed Draft Guideline circulated Final Guideline agreed Guideline adopted by the MHW 1997. 3 1997. 5 1998. 2 1998. 8

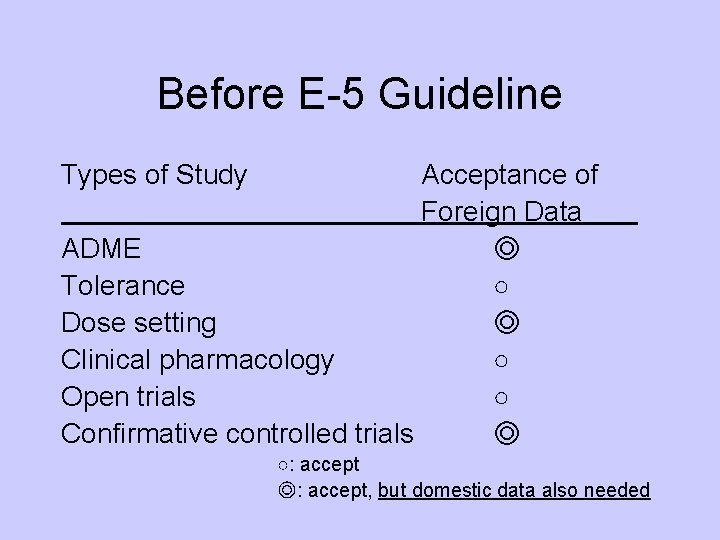
Before E-5 Guideline Types of Study Acceptance of Foreign Data ADME ◎ Tolerance ○ Dose setting ◎ Clinical pharmacology ○ Open trials ○ Confirmative controlled trials ◎ ○: accept ◎: accept, but domestic data also needed

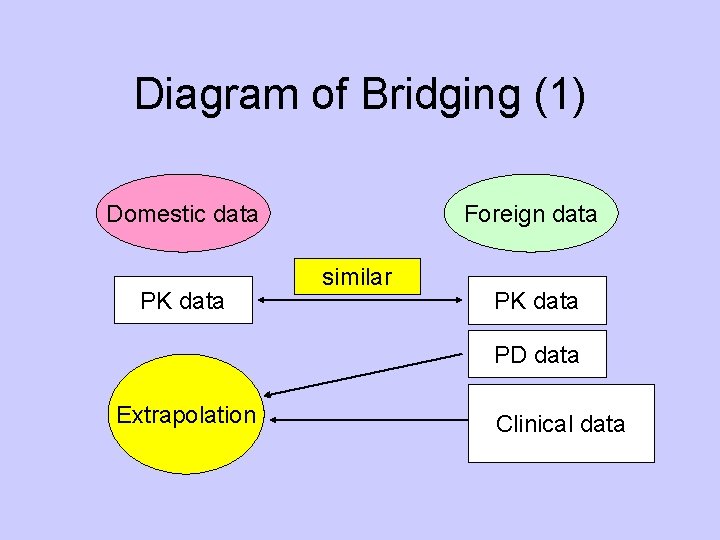
After E-5 Guideline • Necessity/Type of domestic clinical study (Bridging study) data is judged scientifically based on the E-5 Guideline

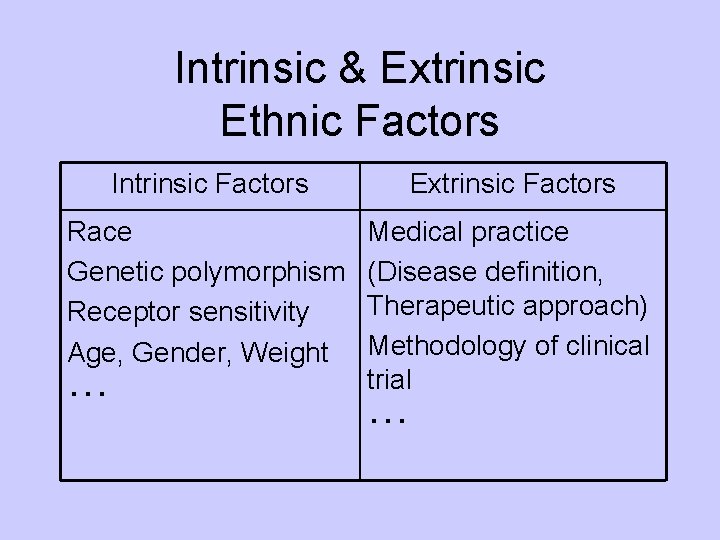
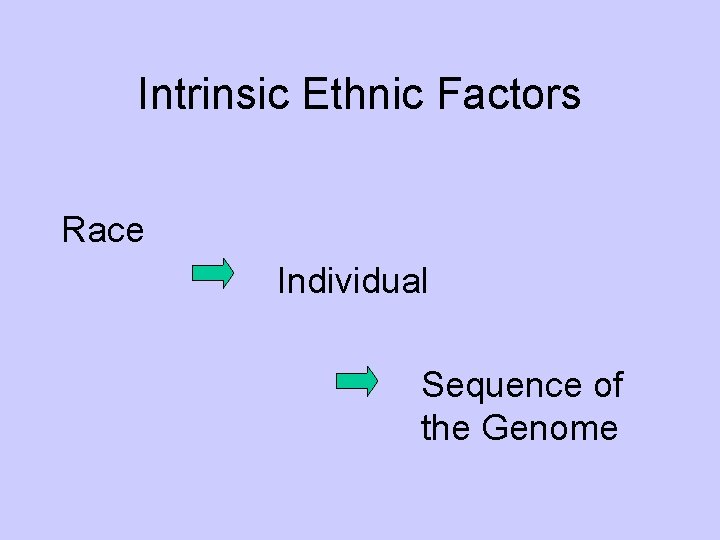
Intrinsic & Extrinsic Ethnic Factors Intrinsic Factors Extrinsic Factors Race Genetic polymorphism Receptor sensitivity Age, Gender, Weight ・・・ Medical practice (Disease definition, Therapeutic approach) Methodology of clinical trial ・・・

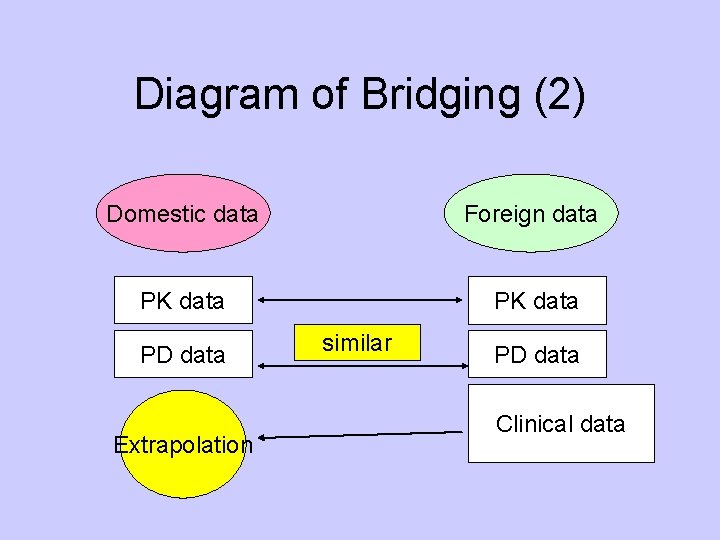
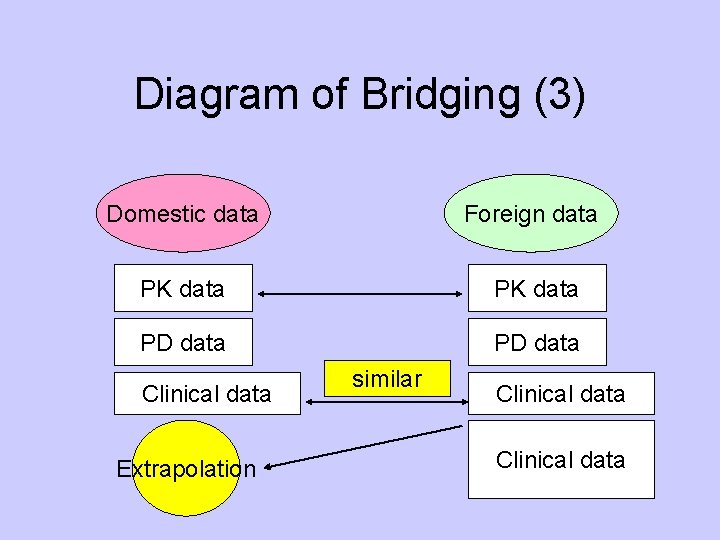
What is “Bridging Study” ? • A supplemental study performed in the new region to provide pharmacodynamic or clinical data on efficacy, safety, dosage and dose regimen in the new region that will allow extrapolation of the foreign clinical data to the new region. (E 5 Guideline)

Types of Bridging Study and Ethnic Factors • No Bridging Study (PK study) Intrinsic Factors • Study using pharmacological endpoint Intrinsic (+ Extrinsic) Factors • Study using clinical endpoint Intrinsic and Extrinsic Factors

Diagram of Bridging (1) Domestic data PK data Foreign data similar PK data PD data Extrapolation Clinical data

Diagram of Bridging (2) Domestic data Foreign data PK data PD data Extrapolation similar PD data Clinical data

Diagram of Bridging (3) Domestic data Foreign data PK data PD data Clinical data Extrapolation similar Clinical data

Recommended Bridging Study • Similar study design • Proper number of patients • Quality of data

Race “Census: US diversity on rise - The nation is much more diverse, and that diversity is much more complex. ” (USA TODAY: March 13, 2001) “Racial categorization may be only a surrogate marker for genetic or other factors. ” (NEJM: May 3, 2001)

Intrinsic Ethnic Factors Race Individual Sequence of the Genome

Extrinsic Ethnic Factors Medical Practice Clinical Trial Methodology ・・・ Region Harmonization of Medical Practice/ Clinical Trial Methodology

Can E-5 Guideline be a solution to the “drug-lag” in Japan?

Types of worldwide development of new drugs Types No. of Drugs Precede in Japan 14 (18. 4%) Simultaneously 16 (21. 1%) Precede in the US 39 (51. 3%) Precede in EU Total 7 ( 9. 2%) 76 (JETRO 2001)

International multi-center cooperative study • Use the same protocol • Progress simultaneously worldwide Stratification by Region/Race

Circumstances of Clinical Trial in Japan • Infrastructure of medical institutions has been improving (CRC, clinical research center, etc) • Information dissemination activities by sponsors/medical institutions to recruit patients began to take effect • Motivation of Investigators/Trial Staff ?

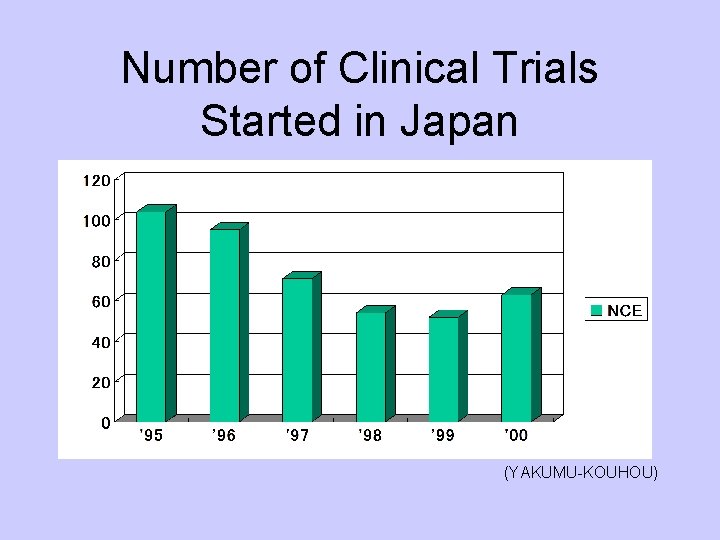
Number of Clinical Trials Started in Japan (YAKUMU-KOUHOU)

Future Perspectives • International Competitiveness of New Drug Development • Clinical Trials with – Quality – Speed