History of the Atom Timeline 1766 1844 Antoine





![Atomic Mass Calculations Formula Atomic Mass= Σ[(Percent Abundance) x (Mass of Isotope)] Σ = Atomic Mass Calculations Formula Atomic Mass= Σ[(Percent Abundance) x (Mass of Isotope)] Σ =](https://slidetodoc.com/presentation_image_h2/38c43ea341733c9d1003ba84d2a81116/image-25.jpg)
![Formula Atomic Mass= Σ[(Percent Abundance) x (Mass of Isotope)] Σ = take the Formula Atomic Mass= Σ[(Percent Abundance) x (Mass of Isotope)] Σ = take the](https://slidetodoc.com/presentation_image_h2/38c43ea341733c9d1003ba84d2a81116/image-26.jpg)

- Slides: 34

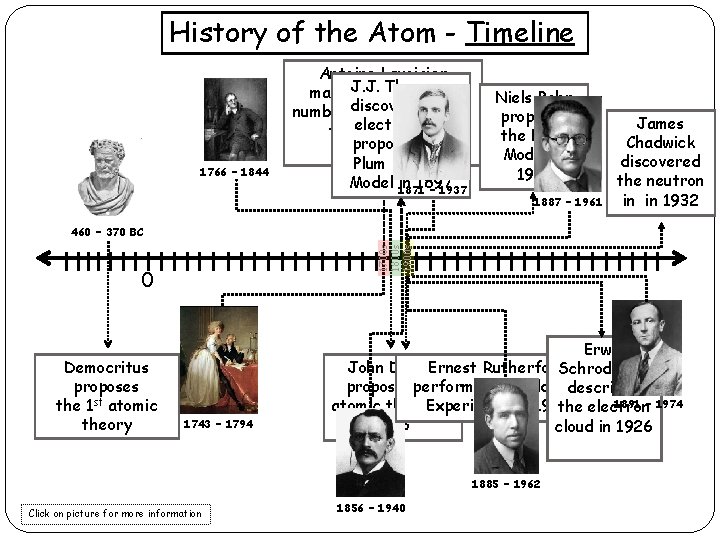
History of the Atom - Timeline 1766 – 1844 Antoine Lavoisier Thomson makes. J. J. a substantial the number discovers of contributions electron and to the field of proposes the Chemistry Plum Pudding Model 1871 in 1897 – 1937 Niels Bohr proposes the Bohr Model in 1913 1887 – 1961 James Chadwick discovered the neutron in in 1932 1700 s 1800 s 1900 s 460 – 370 BC 0 Democritus proposes the 1 st atomic theory 1743 – 1794 Erwin John Dalton Ernest Rutherford Schrodinger proposes performs his the Gold Foil describes 1891 – 1974 atomic theory Experiment in in 1909 the electron 1803 cloud in 1926 1885 – 1962 Click on picture for more information 1856 – 1940

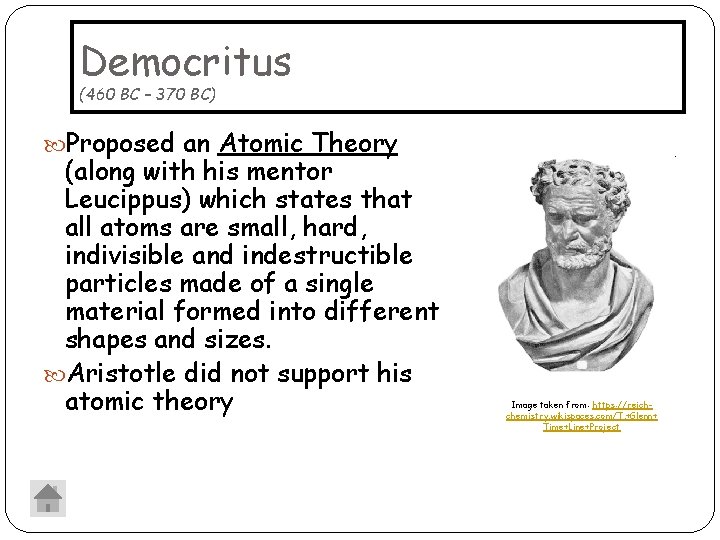
Democritus (460 BC – 370 BC) Proposed an Atomic Theory (along with his mentor Leucippus) which states that all atoms are small, hard, indivisible and indestructible particles made of a single material formed into different shapes and sizes. Aristotle did not support his atomic theory Image taken from: https: //reichchemistry. wikispaces. com/T. +Glenn+ Time+Line+Project

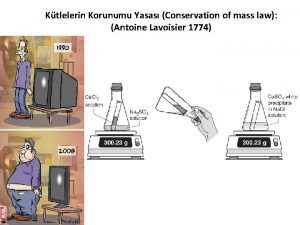
Antoine Lavoisier (1743 – 1794) Known as the “Father of Modern Image taken from: www. ldeo. columbia. edu/. . . /v 1001/geo time 2. html Chemistry” Was the first person to generate a list of thirty-three elements in his textbook Devised the metric system Was married to a 13 -year old Marie-Anne Pierette Paulze; she assisted him with much of his work Was a tax-collector that was consequently guillotined during the French Revolution Discovered/proposed that combustion occurs when oxygen combines with other elements Discovered/proposed the Law of Conservation of Mass (or Matter) which states, in a chemical reaction, matter is neither created nor destroyed

John Dalton (1766 – 1844) In 1803, proposed an Atomic Theory which states: o All substances are made of atoms; atoms are small particles that cannot be created, divided, or destroyed. o Atoms of the same element are exactly alike, and atoms of different elements are different o Atoms join with other atoms to make new substances Calculated the atomic weights of many various elements Was a teacher at a very young age Was color blind Image taken from: chemistry. about. com/. . . /John. Dalton. htm

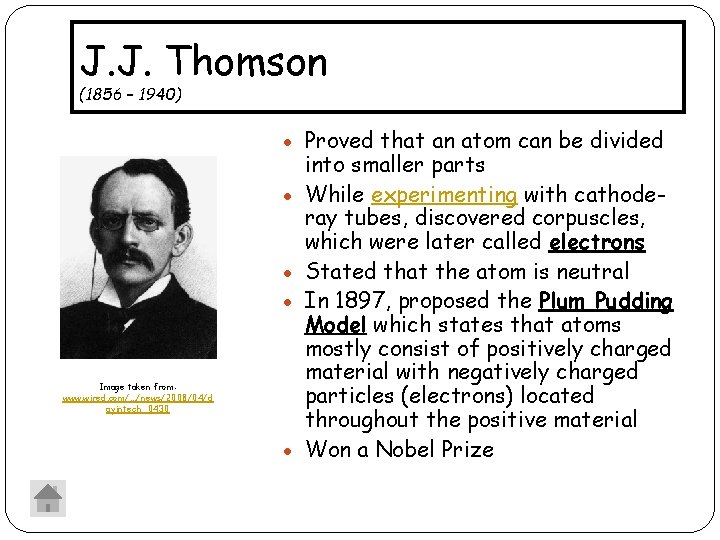
J. J. Thomson (1856 – 1940) Proved that an atom can be divided Image taken from: www. wired. com/. . . /news/2008/04/d ayintech_0430 into smaller parts While experimenting with cathoderay tubes, discovered corpuscles, which were later called electrons Stated that the atom is neutral In 1897, proposed the Plum Pudding Model which states that atoms mostly consist of positively charged material with negatively charged particles (electrons) located throughout the positive material Won a Nobel Prize

Ernest Rutherford (1871 – 1937) In 1909, performed the Gold Foil Experiment and suggested the following characteristics of the atom: o It consists of a small core, or nucleus, that contains most of the mass of the atom o This nucleus is made up of particles called protons, which have a positive charge o The protons are surrounded by negatively charged electrons, but most of the atom is actually empty space Did extensive work on radioactivity (alpha & beta particles, gamma rays/waves) and was referred to as the “Father of Nuclear Physics” Won a Nobel Prize Was a student of J. J. Thomson Was on the New Zealand $100 bill Image taken from: http: //www. scientificweb. com/en/Physics/Biographies/Er nest. Rutherford. html

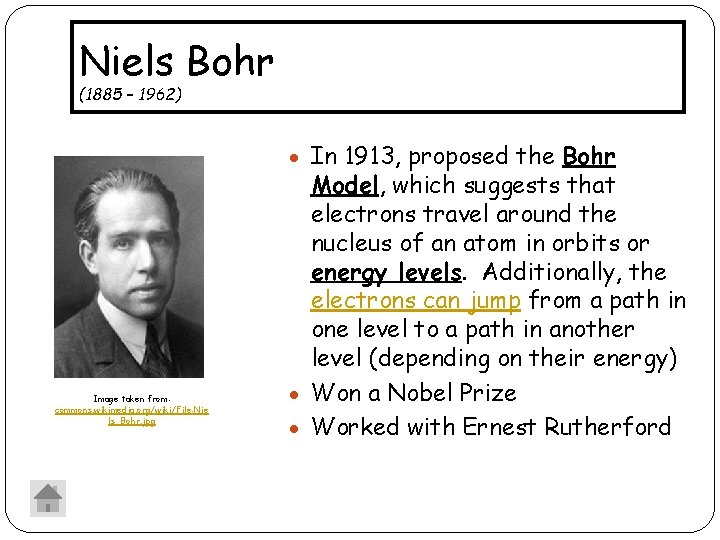
Niels Bohr (1885 – 1962) In 1913, proposed the Bohr Image taken from: commons. wikimedia. org/wiki/File: Nie ls_Bohr. jpg Model, which suggests that electrons travel around the nucleus of an atom in orbits or energy levels. Additionally, the electrons can jump from a path in one level to a path in another level (depending on their energy) Won a Nobel Prize Worked with Ernest Rutherford

Erwin Schrodinger (1887 -1961) In 1926, he further explained the nature of electrons in an atom by stating that the exact location of an electron cannot be stated; therefore, it is more accurate to view the electrons in regions called electron clouds; electron clouds are places where the electrons are likely to be found Did extensive work on the Wave formula Schrodinger equation Won a Nobel Prize Image taken from: nobelprize. org/. . . /1933/schrodinger -bio. html

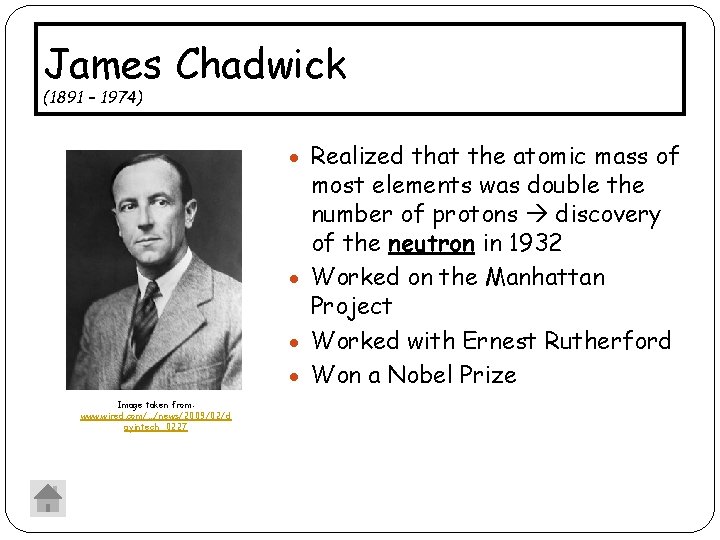
James Chadwick (1891 – 1974) Realized that the atomic mass of most elements was double the number of protons discovery of the neutron in 1932 Worked on the Manhattan Project Worked with Ernest Rutherford Won a Nobel Prize Image taken from: www. wired. com/. . . /news/2009/02/d ayintech_0227

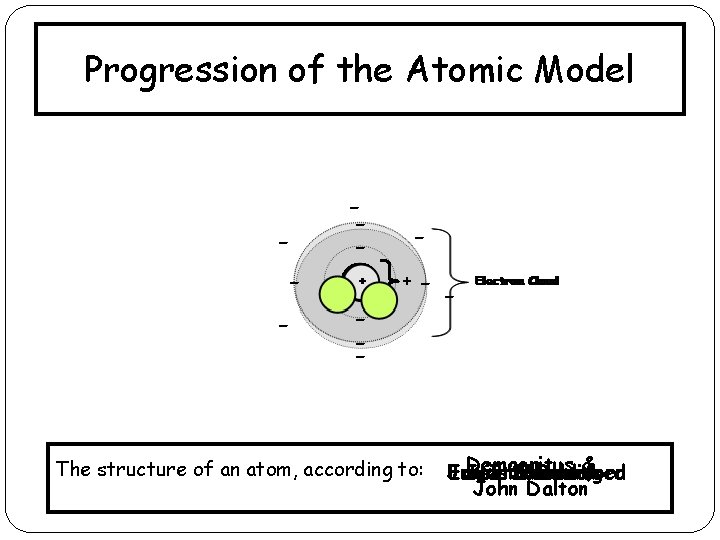
Progression of the Atomic Model - -- - + The structure of an atom, according to: Democritus James Ernest Erwin Neils Schrodinger Chadwick Rutherford Bohr& J. J. Thomson John Dalton

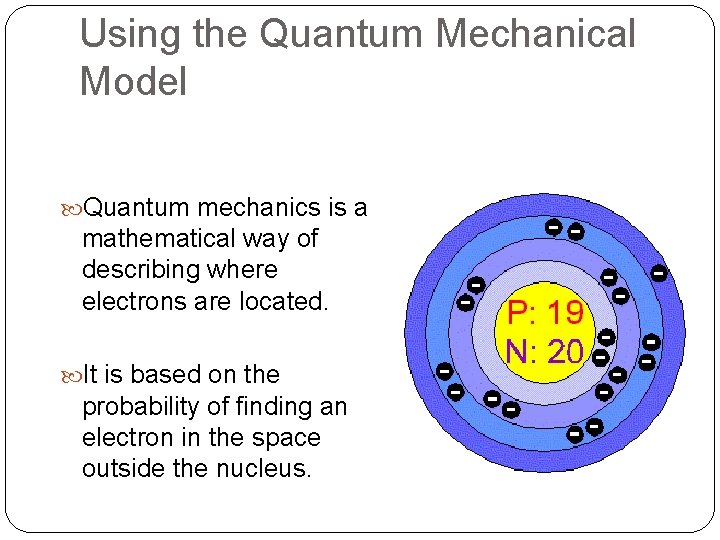
Quantum Mechanical Model In addition to knowing that there were energy levels in the atom, three scientists began to notice other things. . . Heisenberg – impossible to know the exact position and exact speed of an electron at the same time De Broglie – electrons have wave-like properties, as in they move in wave patterns Schroedinger – developed probability of finding each electron in a given location

Using the Quantum Mechanical Model Quantum mechanics is a mathematical way of describing where electrons are located. It is based on the probability of finding an electron in the space outside the nucleus.

Rules for Energy Levels 1. 2. 3. 4. Level 1 (closest to the nucleus) can hold a maximum of 2 e. Level 2 can hold a max of 8 e. Level 3 can hold a max of 18 e. Level 4 can hold a max of 32 e. You must fill one level before going on to draw the next level!

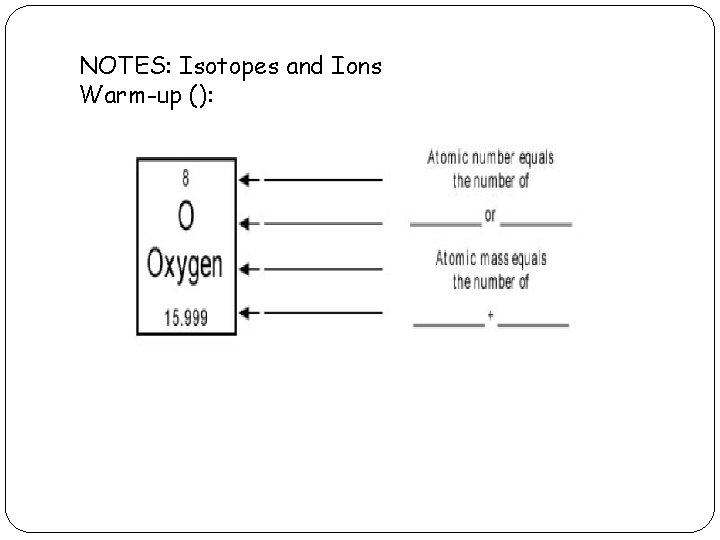
NOTES: Isotopes and Ions Warm-up ():

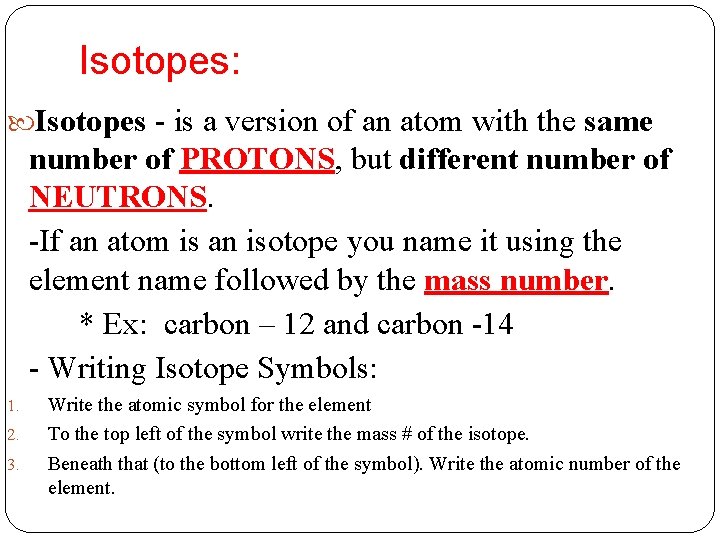
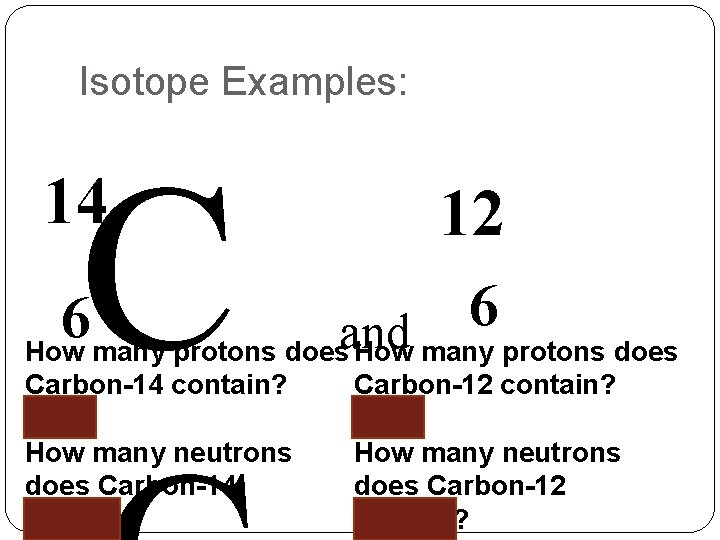
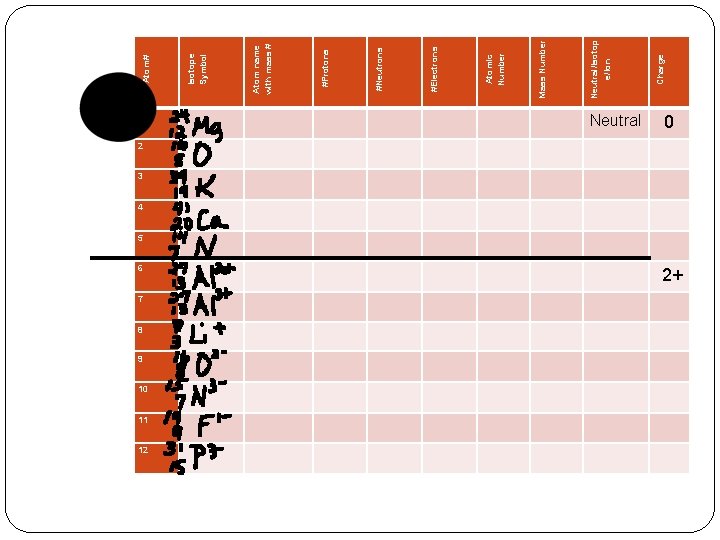
Isotopes: Isotopes - is a version of an atom with the same number of PROTONS, but different number of NEUTRONS. -If an atom is an isotope you name it using the element name followed by the mass number. * Ex: carbon – 12 and carbon -14 - Writing Isotope Symbols: 1. 2. 3. Write the atomic symbol for the element To the top left of the symbol write the mass # of the isotope. Beneath that (to the bottom left of the symbol). Write the atomic number of the element.

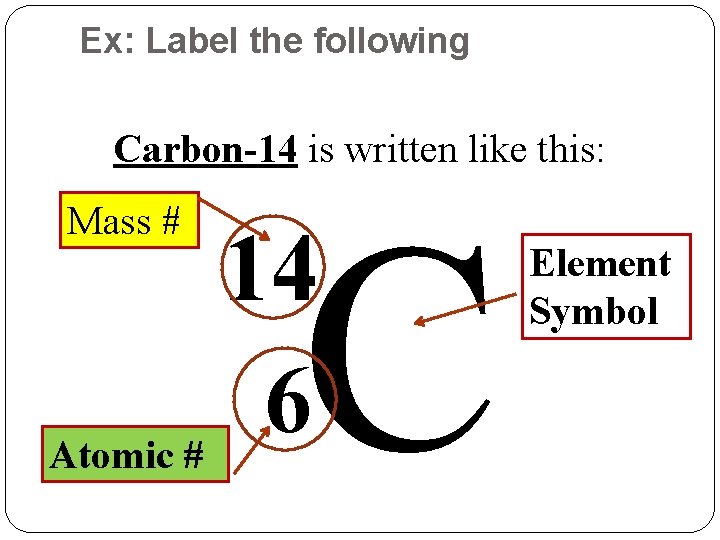
Ex: Label the following Carbon-14 is written like this: Mass # C 14 6 Atomic # Element Symbol

Isotope Examples: C 14 12 6 6 How many protons doesand How many protons does Carbon-14 contain? 6 How many neutrons does Carbon-14 contain? Carbon-12 contain? 6 How many neutrons does Carbon-12 contain?

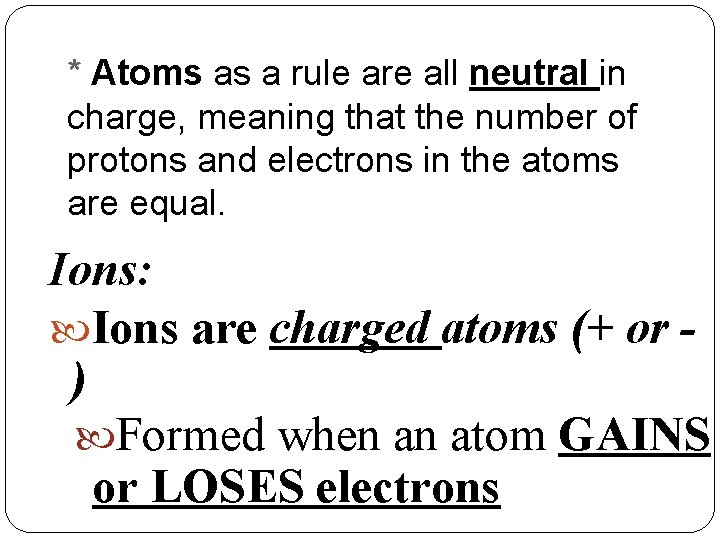
* Atoms as a rule are all neutral in charge, meaning that the number of protons and electrons in the atoms are equal. Ions: Ions are charged atoms (+ or ) Formed when an atom GAINS or LOSES electrons

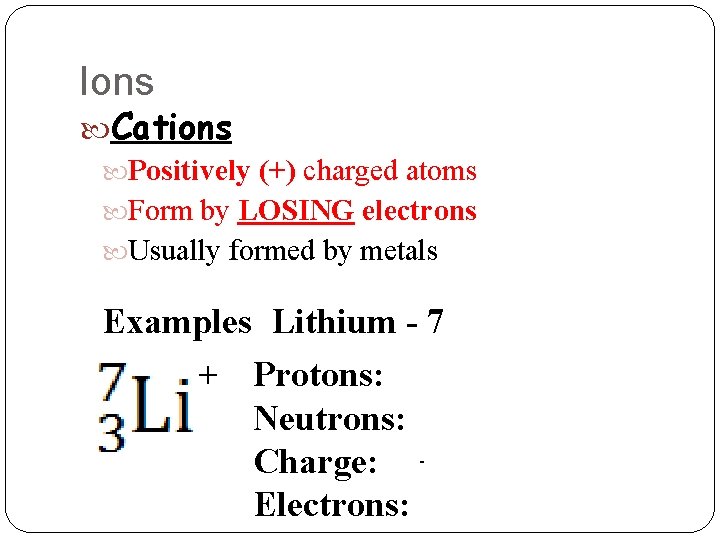
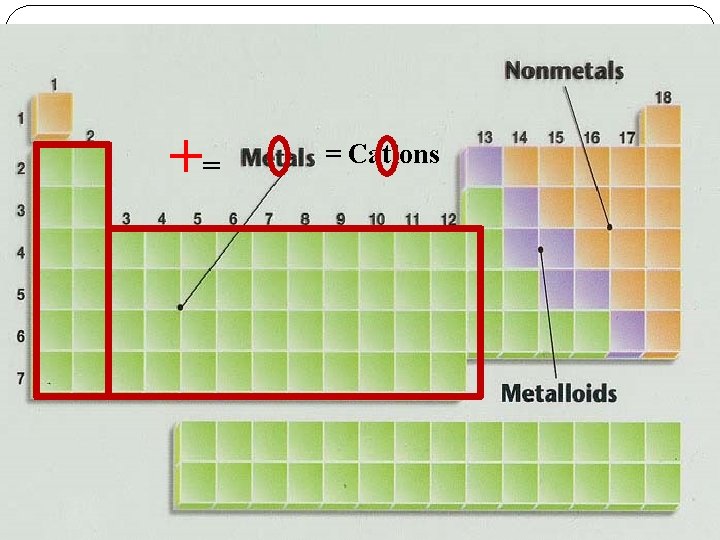
Ions Cations Positively (+) charged atoms Form by LOSING electrons Usually formed by metals Examples Lithium - 7 + Protons: 3 Neutrons: 4 Charge: 1+ Electrons: 2

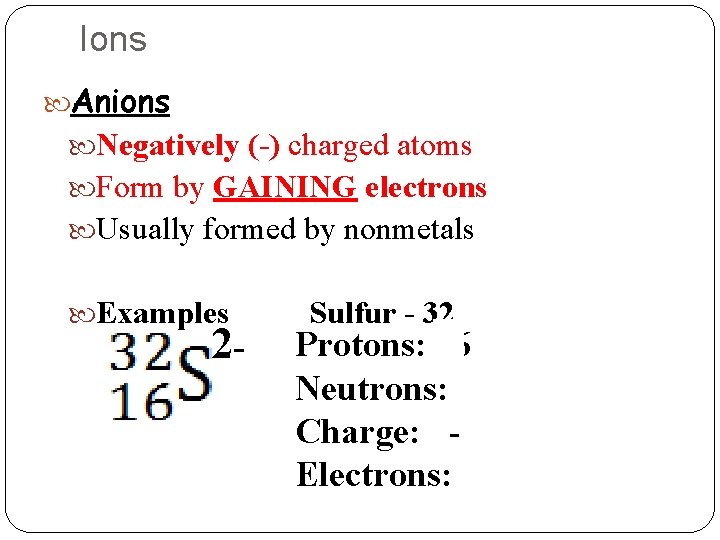
Ions Anions Negatively (-) charged atoms Form by GAINING electrons Usually formed by nonmetals Examples 2 - Sulfur - 32 Protons: 16 Neutrons: 16 Charge: 2 Electrons: 18

Homework Complete the table as directed on Complete the chart and answer the questions on Pg. 8 in group of 2 (no larger!). Homework:

1 6 7 8 9 10 11 12 Neutral 0 2 3 4 5 2+ Charge Neutral/Isotop e/Ion Mass Number Atomic Number #Electrons #Neutrons #Protons Atom name with mass # Isotope Symbol Atom#

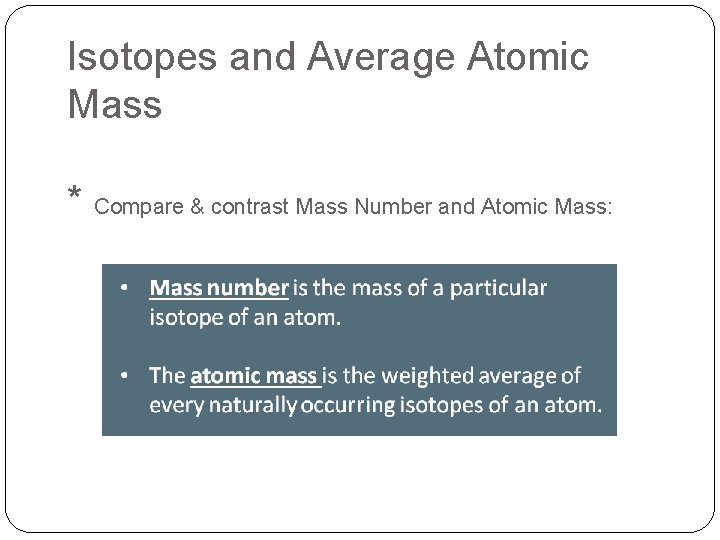
Isotopes and Average Atomic Mass * Compare & contrast Mass Number and Atomic Mass:

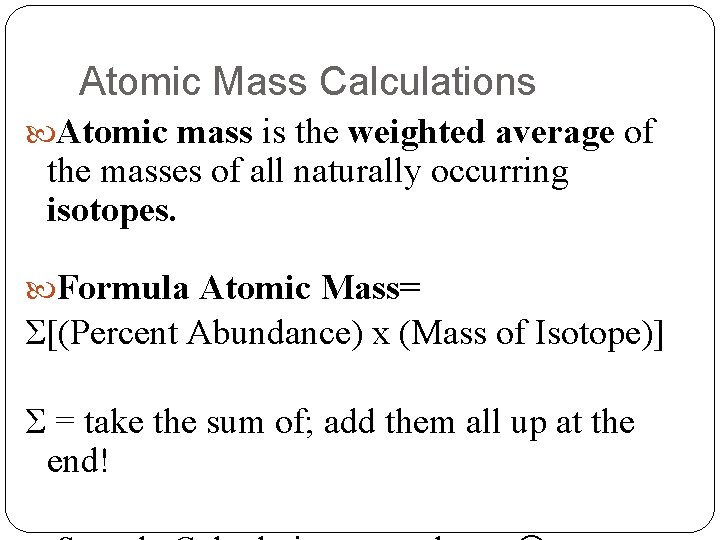
Atomic Mass Calculations Atomic mass is the weighted average of the masses of all naturally occurring isotopes. Formula Atomic Mass= Σ[(Percent Abundance) x (Mass of Isotope)] Σ = take the sum of; add them all up at the end!
![Atomic Mass Calculations Formula Atomic Mass ΣPercent Abundance x Mass of Isotope Σ Atomic Mass Calculations Formula Atomic Mass= Σ[(Percent Abundance) x (Mass of Isotope)] Σ =](https://slidetodoc.com/presentation_image_h2/38c43ea341733c9d1003ba84d2a81116/image-25.jpg)
Atomic Mass Calculations Formula Atomic Mass= Σ[(Percent Abundance) x (Mass of Isotope)] Σ = take the sum of; add them all up at the end! (. 7553 x 34. 969) + (. 2447 x 36. 966)= 35. 46 amu
![Formula Atomic Mass ΣPercent Abundance x Mass of Isotope Σ take the Formula Atomic Mass= Σ[(Percent Abundance) x (Mass of Isotope)] Σ = take the](https://slidetodoc.com/presentation_image_h2/38c43ea341733c9d1003ba84d2a81116/image-26.jpg)
Formula Atomic Mass= Σ[(Percent Abundance) x (Mass of Isotope)] Σ = take the sum of; add them all up at the end! (. 7553 x 34. 969) + (. 2447 x 36. 966)= 35. 46 amu Cl chlorine

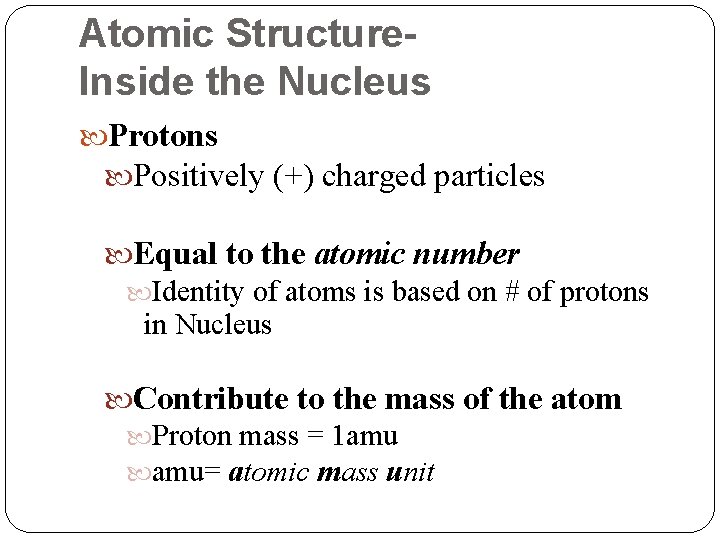
Atomic Structure. Inside the Nucleus Protons Positively (+) charged particles Equal to the atomic number Identity of atoms is based on # of protons in Nucleus Contribute to the mass of the atom Proton mass = 1 amu amu= atomic mass unit

Atomic Structure- Outside the Nucleus (Electron Cloud) Electrons Negatively (-) charged particles Responsible for the CHEMICAL PROPERTIES of the atom In a neutral atom (no charge), protons=electrons Very little mass ~0 amu

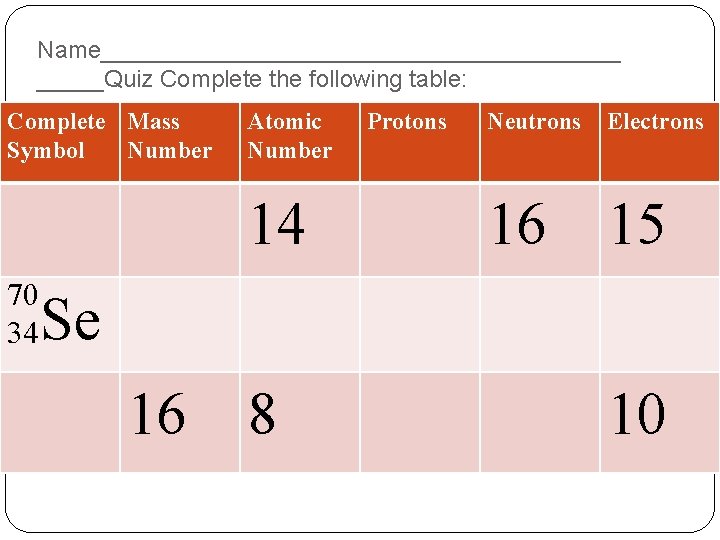
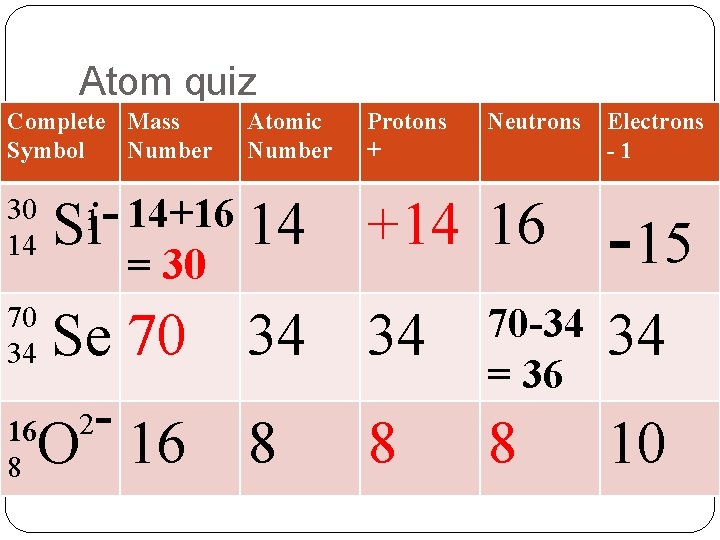
Name____________________Quiz Complete the following table: Complete Mass Symbol Number Atomic Number 14 70 34 Protons Neutrons Electrons 16 15 Se 16 8 10

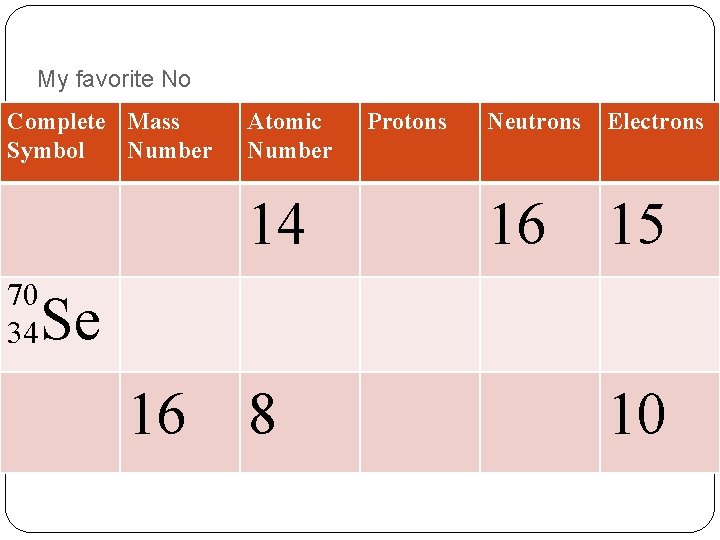
My favorite No Complete Mass Symbol Number Atomic Number 14 70 34 Protons Neutrons Electrons 16 15 Se 16 8 10

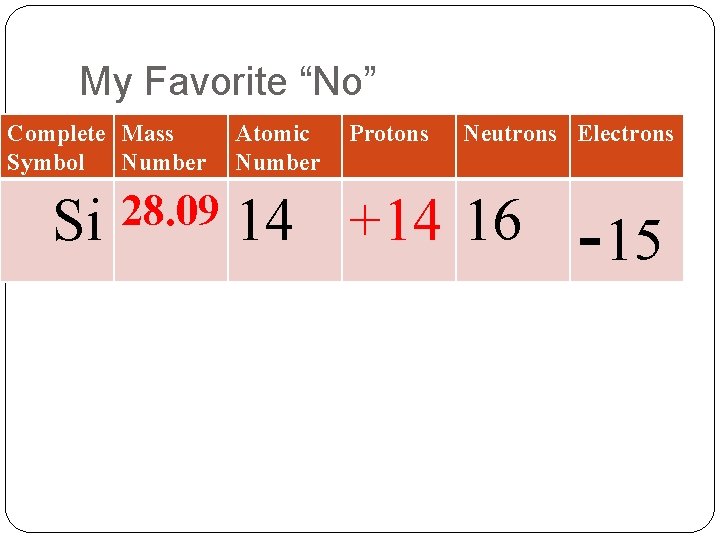
My Favorite “No” Complete Mass Symbol Number Si 28. 09 Atomic Number Protons Neutrons Electrons 14 +14 16 -15

Atom quiz Complete Mass Symbol Number 30 14 Atomic Number 14+16 Si 14 1 = 30 70 34 34 8 8 Se 70 16 2 O 16 Protons + Neutrons +14 16 Electrons -1 -15 34 70 -34 = 36 34 8 8 10

+= = Cations

 Thomas robert malthus (1766-1834)
Thomas robert malthus (1766-1834) Antoine lavoisier atom model
Antoine lavoisier atom model Prantsuse poeet 1844-1896
Prantsuse poeet 1844-1896 1844 room buckingham palace
1844 room buckingham palace Guided reading & analysis: the age of jackson, 1824-1844
Guided reading & analysis: the age of jackson, 1824-1844 Prantsuse poeet 1844-1896
Prantsuse poeet 1844-1896 1844 1910
1844 1910 The age of jackson 1824-1844
The age of jackson 1824-1844 1844 eerste telegraaflijn
1844 eerste telegraaflijn Anjo 1844
Anjo 1844 Jointstock company
Jointstock company The structure of the atom section 2 defining the atom
The structure of the atom section 2 defining the atom Kelemahan teori thomson
Kelemahan teori thomson History of atomic theory timeline
History of atomic theory timeline Atomic history webquest
Atomic history webquest History of atom models
History of atom models Democritus atomic model
Democritus atomic model History of the atom john dalton
History of the atom john dalton Democritus atom modeli
Democritus atom modeli John dalton billiard ball model
John dalton billiard ball model Bohr model vs quantum model venn diagram
Bohr model vs quantum model venn diagram Louis vuitton history timeline
Louis vuitton history timeline New mexico timeline
New mexico timeline How was hawaii formed
How was hawaii formed Crayola history timeline
Crayola history timeline History of calculus timeline
History of calculus timeline Communication history timeline
Communication history timeline Georgia history timeline 1877-1919
Georgia history timeline 1877-1919 Timeline of telescope
Timeline of telescope Armenian history timeline
Armenian history timeline M&s
M&s Film history timeline
Film history timeline History of western typography
History of western typography History of photojournalism timeline
History of photojournalism timeline Digital mechanical calculator
Digital mechanical calculator