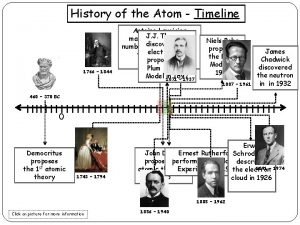
John Dalton l English scientist l 1766 1844
















- Slides: 22

John Dalton l. English scientist l 1766 -1844 l. Dalton’s atomic theory

Dalton’s Theory: l 1. All elements are composed of tiny indivisible particles l 2. Atoms of the same element are identical. – The atoms of any one element are different from those of any other element.

Dalton’s Theory cont. l 3. Different elements can physically or chemically combine to form compounds l 4. Chemical reactions occur when elements are separated, joined or rearranged. – We cannot change an atom of one element into an atom of another element by chemical means

The atom: l. The atom composed of the following particles: – Electrons – Protons – Neutrons

Electrons: l. Negatively charged l. J. J. Thomson discovered electrons with cathode ray tube l. Found in “electron cloud” surrounding nucleus of atom

Protons: l. Positively charged sub atomic particles

Neutrons: l. Neutrally charged subatomic particles l. Have a mass nearly equal to protons

Distinguishing between the atoms l. Atomic number = the number of protons l. Mass number = the total number of protons + neutrons l. Number of neutrons = the mass number – number of protons

Table 5. 2 l. Table 5. 2 is a list of the first ten elements and some relative information l. Properties such as atomic number and mass number are identifying features of an element

Examples: l. Practice problems pg. 115 and 116 l. Before we answer these questions lets look at the periodic table of elements.

PCA: l. Pg 114 l. Chem. Math l. Practice problems A-N

Isotopes: l. What is an Isotope? – Hint: think back to the lab with the fictitious element vegium! l. Isotope = same number of protons different number of neutrons – Give an example: l. Carbon 14 (Carbon 12)

Hydrogen Isotopes: l. Hydrogen has three isotopes l. Most common- Hydrogen – 1 proton – No neutrons l. Second isotope = Hydrogen – 2 – 1 proton and 1 neutron – Also called deuterium

Hydrogen Isotopes: l. Third isotope = Hydrogen-3 – 1 proton – 2 neutrons – Also called tritium

Practice Problems: l. Try 117 the practice problems on pg.

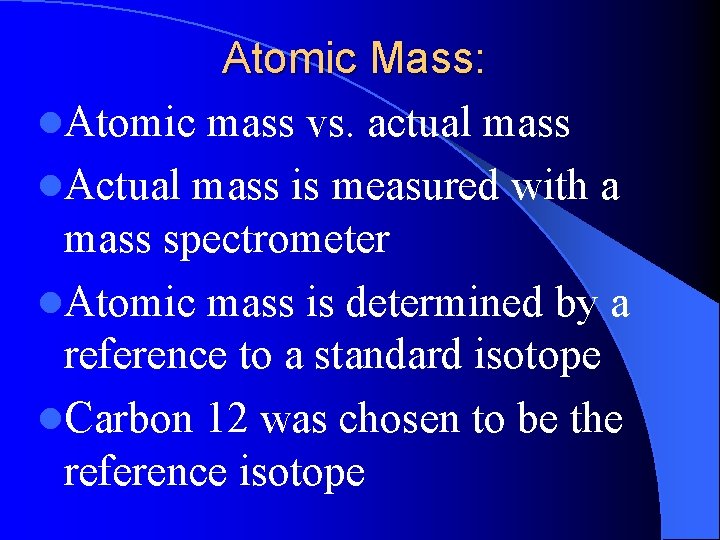
Atomic Mass: l. Atomic mass vs. actual mass l. Actual mass is measured with a mass spectrometer l. Atomic mass is determined by a reference to a standard isotope l. Carbon 12 was chosen to be the reference isotope

Atomic Mass: l. Carbon was assigned 12 amu’s – Or atomic mass units – amu’s are defined as one – twelfth the mass of carbon – It was assigned this number because Carbon has 6 protons and 6 neutrons, of which almost all of the mass of the element is composed


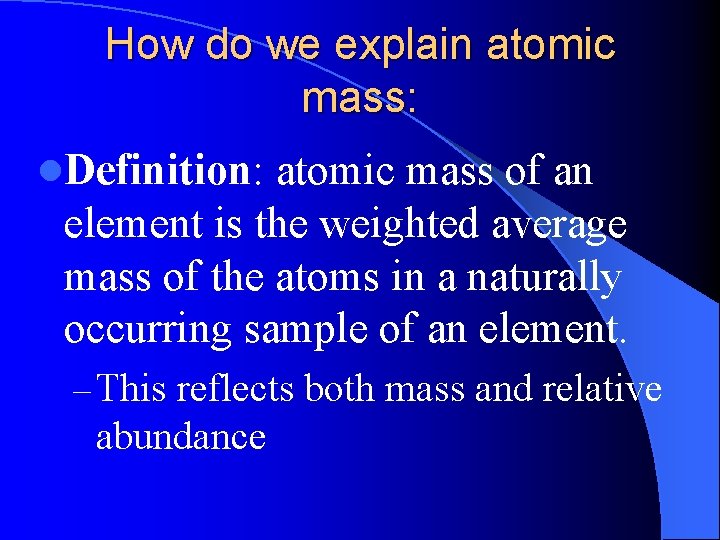
How do we explain atomic mass: l. Definition: atomic mass of an element is the weighted average mass of the atoms in a naturally occurring sample of an element. – This reflects both mass and relative abundance

Lets try again: l. Naturally, elements occur as a mixture of two or more isotopes. l. Each isotope having a fixed mass and percent abundance l. The amu of an element is usually very close to the atomic mass number of the most frequently occurring isotope

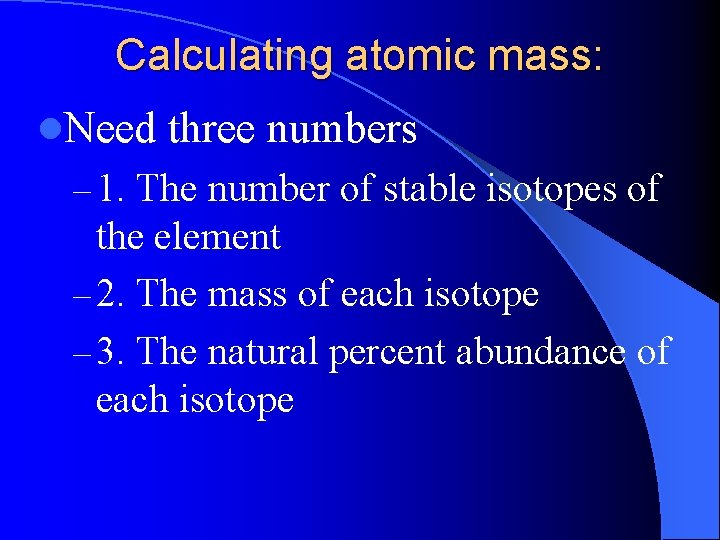
Calculating atomic mass: l. Need three numbers – 1. The number of stable isotopes of the element – 2. The mass of each isotope – 3. The natural percent abundance of each isotope

Sample Problems: pg 121 l. Review the sample problems then attempt the practice problems on page 121.
 Thomas robert malthus (1766-1834)
Thomas robert malthus (1766-1834) Prantsuse poeet 1844-1896
Prantsuse poeet 1844-1896 1844 room buckingham palace
1844 room buckingham palace The age of jackson 1824-1844
The age of jackson 1824-1844 Prantsuse poeet 1844-1896
Prantsuse poeet 1844-1896 1844 1910
1844 1910 The age of jackson 1824-1844
The age of jackson 1824-1844 1844 eerste telegraaflijn
1844 eerste telegraaflijn Mensagem dos 3 anjos
Mensagem dos 3 anjos Jointstock
Jointstock Famous english scientists
Famous english scientists Who is the english scientist that discovered cell in 1665
Who is the english scientist that discovered cell in 1665 Bohr
Bohr John dalton 1808
John dalton 1808 John dalton billiard ball model
John dalton billiard ball model John dalton facebook
John dalton facebook John dalton atomic theory
John dalton atomic theory Atomic model timeline
Atomic model timeline Bunyi teori dalton
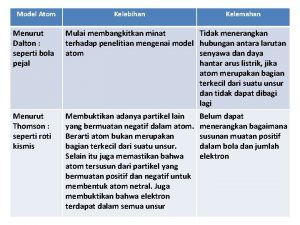
Bunyi teori dalton Kelebihan atom john dalton
Kelebihan atom john dalton History of the atom john dalton
History of the atom john dalton Plum pudding model
Plum pudding model Jj thomson
Jj thomson