Models of an Atom John Dalton 1766 1844








- Slides: 17

Models of an Atom

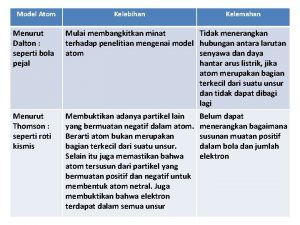
John Dalton (1766 – 1844) Experiment/Observations: • He studied partial pressures of the atmosphere • He noticed that the ratios in which elements combine remain in fixed, simple proportions.

Theory All matter is made up of individual particles called atoms, which cannot be divided: The four postulates: • All elements are made of atoms • All atoms of the same element have the same mass, and atoms of different elements have different masses • Compounds contain atoms of more than one element • In a particular compound, atoms of different elements always combine in the same way

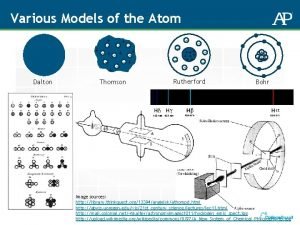
Billiard Ball Model

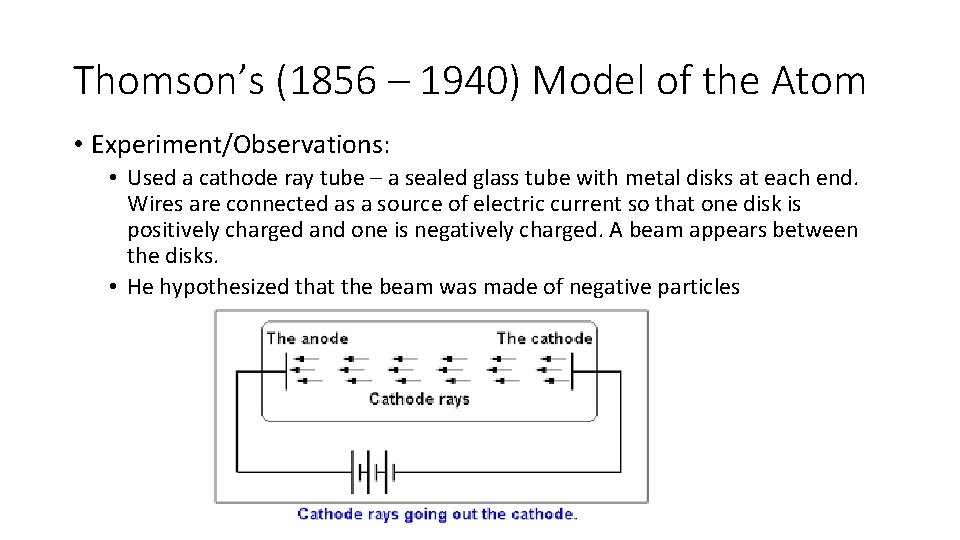
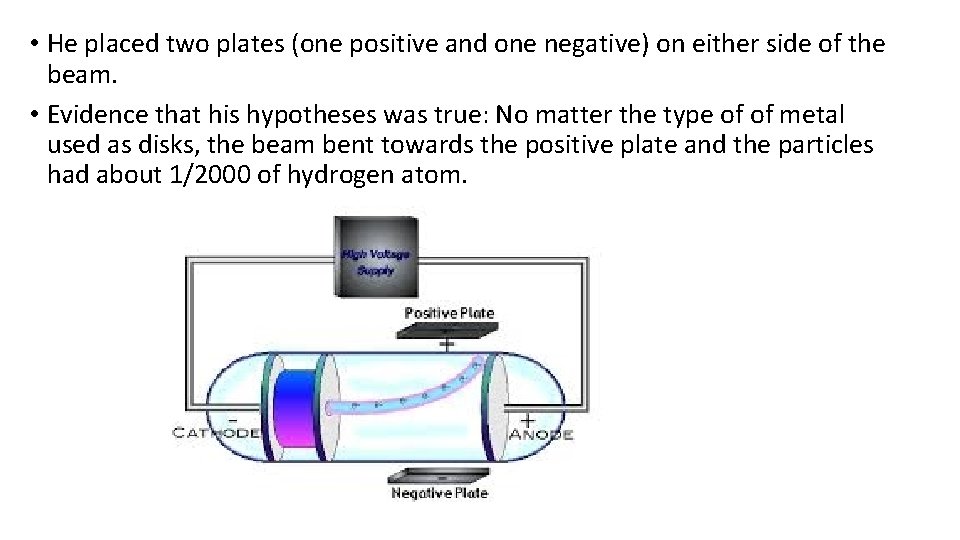
Thomson’s (1856 – 1940) Model of the Atom • Experiment/Observations: • Used a cathode ray tube – a sealed glass tube with metal disks at each end. Wires are connected as a source of electric current so that one disk is positively charged and one is negatively charged. A beam appears between the disks. • He hypothesized that the beam was made of negative particles

• He placed two plates (one positive and one negative) on either side of the beam. • Evidence that his hypotheses was true: No matter the type of of metal used as disks, the beam bent towards the positive plate and the particles had about 1/2000 of hydrogen atom.

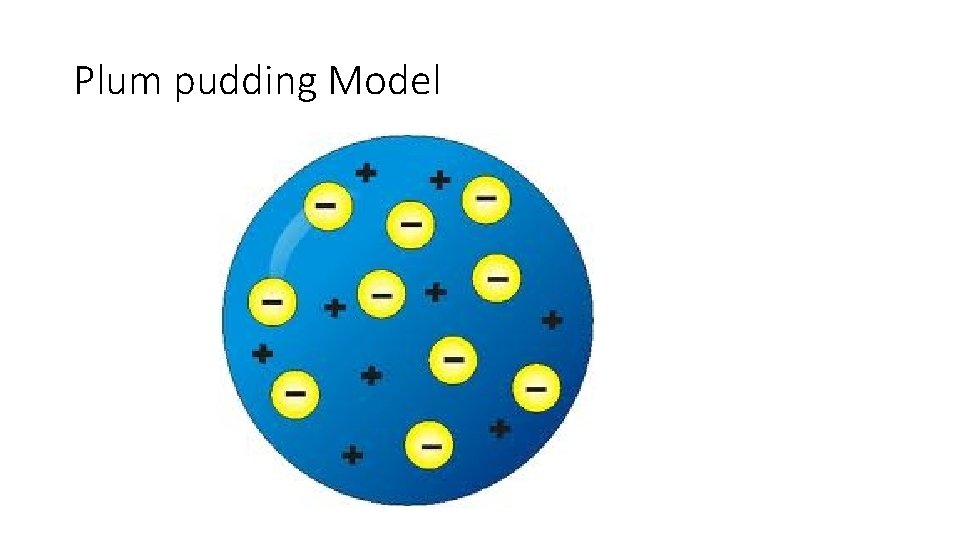
Theory • Atoms are made of subatomic particles – the plum pudding model.

Plum pudding Model

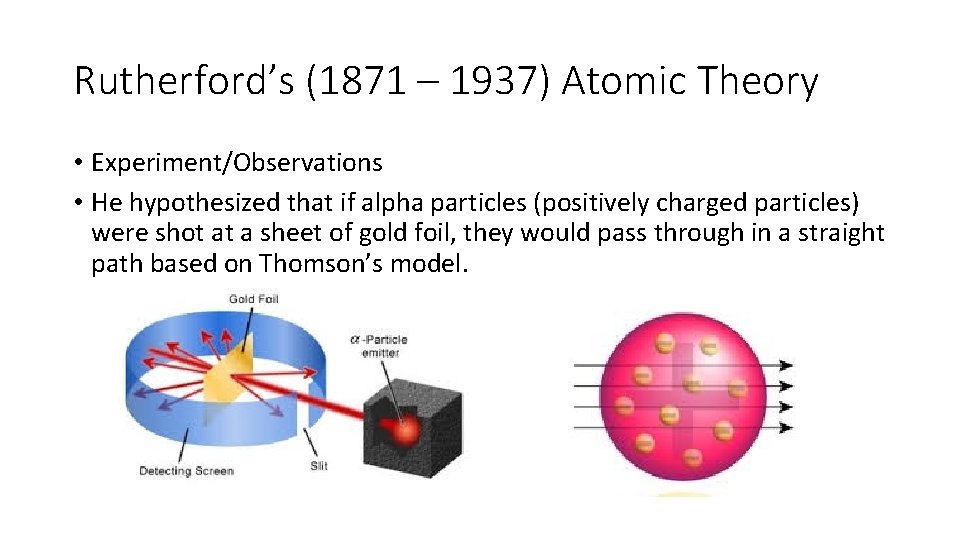
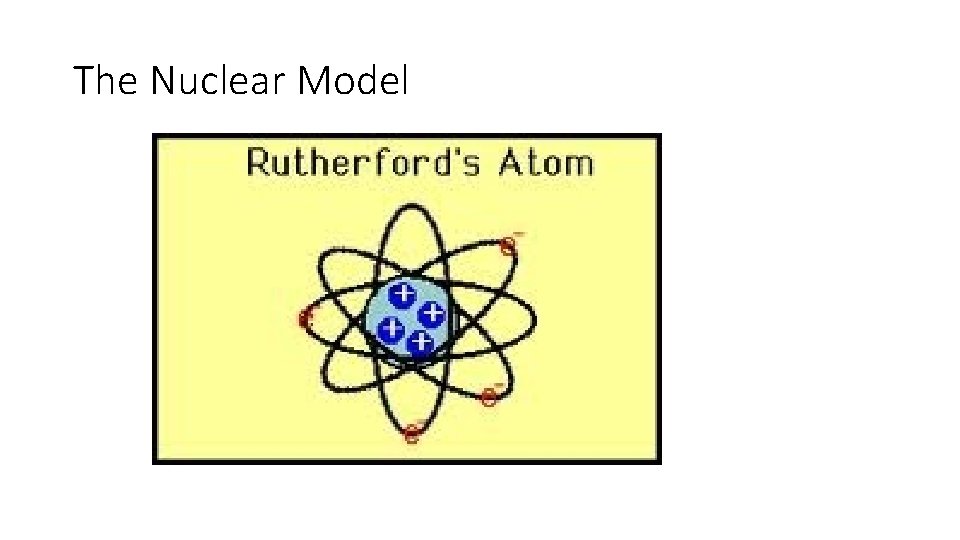
Rutherford’s (1871 – 1937) Atomic Theory • Experiment/Observations • He hypothesized that if alpha particles (positively charged particles) were shot at a sheet of gold foil, they would pass through in a straight path based on Thomson’s model.

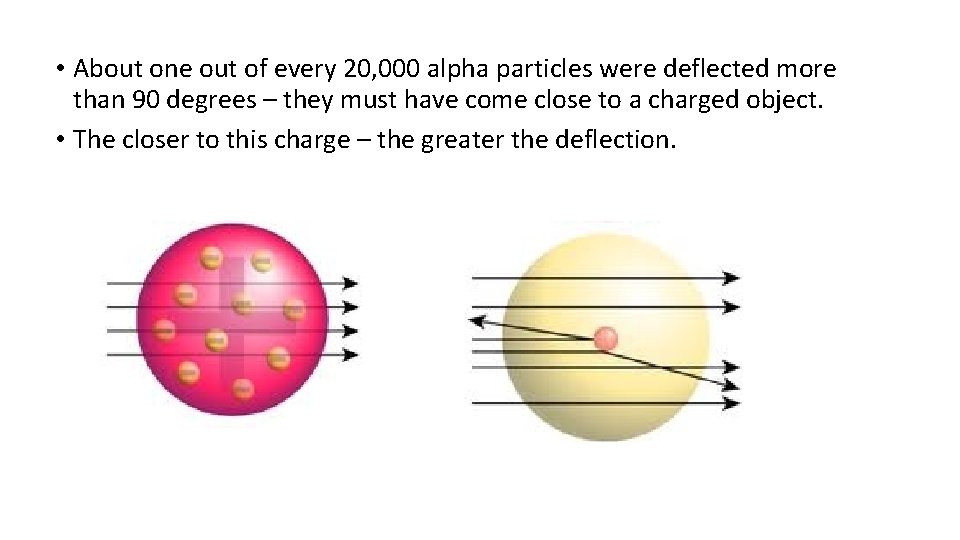
• About one out of every 20, 000 alpha particles were deflected more than 90 degrees – they must have come close to a charged object. • The closer to this charge – the greater the deflection.

Theory • The positive charge of an atom is concentrated in it’s nucleus – the nuclear model.

The Nuclear Model

Neils Bohr (1885 – 1962) • Experiment/Observations: • In Rutherford’s model, the electrons would be constantly emitting energy according to classical physics, and the atom would be very unstable. • To fix this, Bohr theorized that electrons must have a fixed speed, and fixed energy. • Using Planck’s constant for light emissions, Bohr obtained the accurate formulas for energy levels of hydrogen atom.

Theory • Electrons orbit the nucleus at fixed spends and fixed energy. • Electrons may change energy levels by gaining or losing energy.

Bohr Model

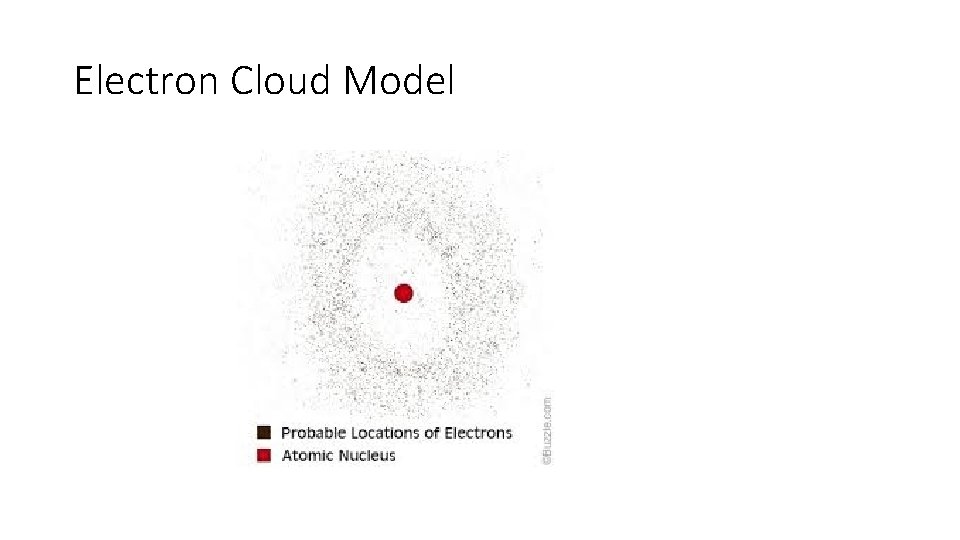
Current – Electron Cloud Model • Schrodinger developed probability function of where an electron may be found – we cannot say with certainty where an electron actually is at any point.

Electron Cloud Model
 Thomas robert malthus (1766-1834)
Thomas robert malthus (1766-1834) Bunyi teori atom john dalton
Bunyi teori atom john dalton Kelebihan atom dalton
Kelebihan atom dalton History of the atom john dalton
History of the atom john dalton John dalton atom modeli
John dalton atom modeli Prantsuse poeet 1844-1896
Prantsuse poeet 1844-1896 1844 room buckingham palace
1844 room buckingham palace The age of jackson 1824-1844
The age of jackson 1824-1844 Prantsuse poeet 1844-1896
Prantsuse poeet 1844-1896 Henri julien rousseau
Henri julien rousseau The age of jackson 1824-1844
The age of jackson 1824-1844 1844 eerste telegraaflijn
1844 eerste telegraaflijn A mensagem dos 3 anjos
A mensagem dos 3 anjos Importance of joint stock company
Importance of joint stock company Unit 4 atomic structure
Unit 4 atomic structure Dalton ve thomson atom modeli
Dalton ve thomson atom modeli Model atom dalton thomson rutherford bohr
Model atom dalton thomson rutherford bohr Dalton atom modeli maketi
Dalton atom modeli maketi