GONADAL HORMONES ANDROGENS ESTROGEN PROGESTINS Gillian Tufts DNP













- Slides: 51

GONADAL HORMONES ANDROGENS ESTROGEN / PROGESTINS Gillian Tufts DNP, FNP-BC

Androgens

Case study S: 29 yr old Bui c/o enlarging breast tissue. He has not found any lumps, the tissue is not tender. He has noticed this over the past 6 months, maybe longer. He is otherwise well. He has experienced mild depression in the past. He has never fathered any children. He denies alcohol or drug (ie marijuana use) O: NAD Breast tissue: circular, rubbery/firm, ~ 3 cm mobile mass located under the areola, sym bilat Male GU: tanner 1 exam = no puberty hair, penis/testes (sm firm)/scrotal sac – consistent with exam of early childhood

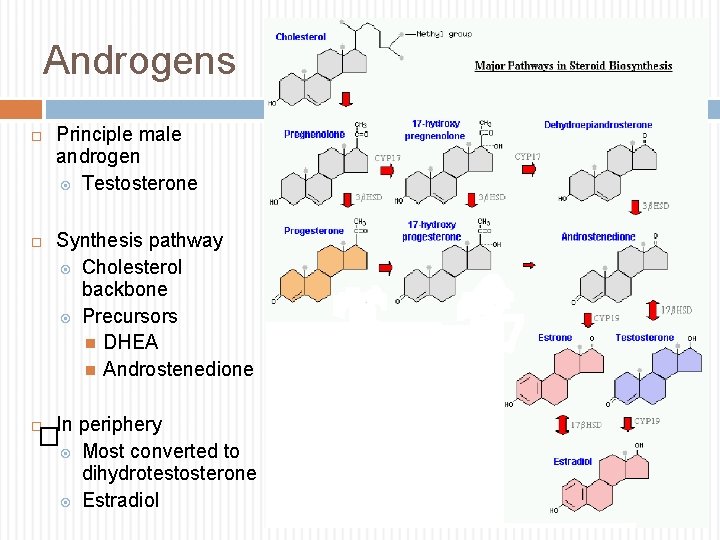
Androgens Principle male androgen Testosterone Synthesis pathway Cholesterol backbone Precursors DHEA Androstenedione In periphery � Most converted to dihydrotestosterone Estradiol

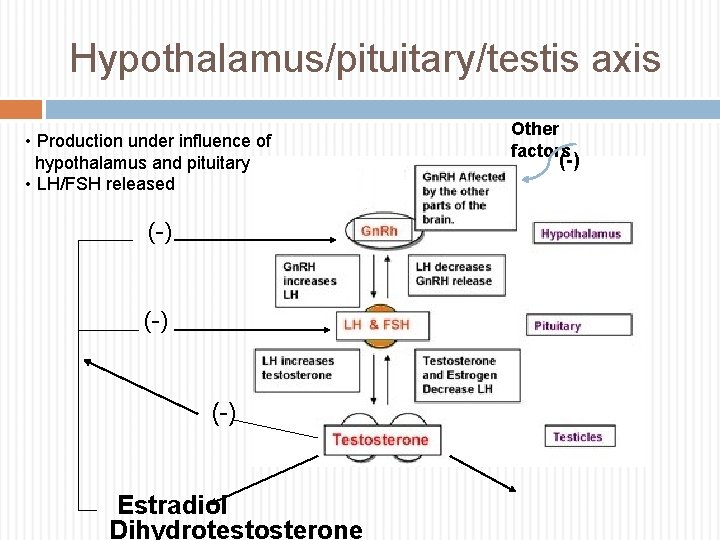
Hypothalamus/pituitary/testis axis • Production under influence of hypothalamus and pituitary • LH/FSH released (-) (-) Estradiol Dihydrotestosterone Other factors (-)

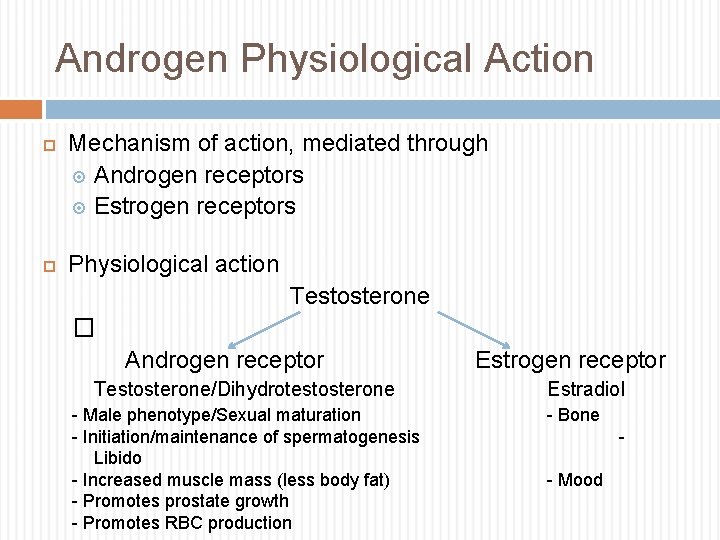
Androgen Physiological Action Mechanism of action, mediated through Androgen receptors Estrogen receptors Physiological action Testosterone � Androgen receptor Testosterone/Dihydrotestosterone - Male phenotype/Sexual maturation - Initiation/maintenance of spermatogenesis Libido - Increased muscle mass (less body fat) - Promotes prostate growth - Promotes RBC production Estrogen receptor Estradiol - Bone - Mood

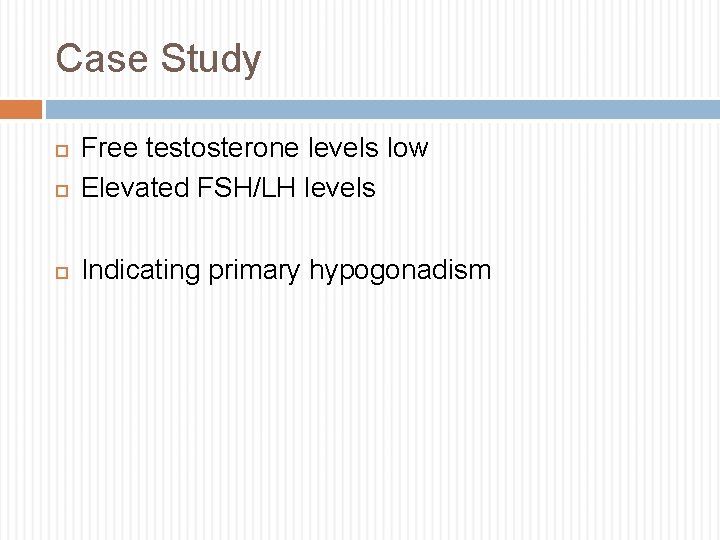
Case Study Free testosterone levels low Elevated FSH/LH levels Indicating primary hypogonadism

Testosterone use Hypogonadism: Failure of testes to produce physiologic levels of testosterone (androgen deficiency) Causes Genetic Chronic illness: DM, cirrhosis, chronic renal failure Chronic medication use: glucocorticoid or opioid use Idiopathic, including aging Signs/Symptoms Decreased sexual development / maintenance Decreased sperm production = infertility Loss of muscle mass and strength, decreased bone density, depression, decrease in libido

Testosterone use Testosterone treatment In those who exhibit symptoms of low T AND who have low testosterone*** Checked on 3 separate occasions, am samples Goal To replace testosterone, restoring serum levels Drugs Natural testosterone well absorbed but metabolized rapidly Synthetic testosterone preparations

Testosterone use Testosterone treatment contraindications: Breast or prostate cancer or palpable prostate nodule Severe lower urinary tract symptoms with International Prostate Symptom Score (IPSS) > 19 PSA > 4 ng/ml or 3 ng/ml in those with high risk for prostate cancer Hematocrit >50% http: //press. endocrine. org/doi/abs/10. 1210/jc. 2009 -2354 Untreated severe obstructive sleep apnea In men > 40 years Assess for prostate cancer before testosterone replacement Assess for possible sleep apnea

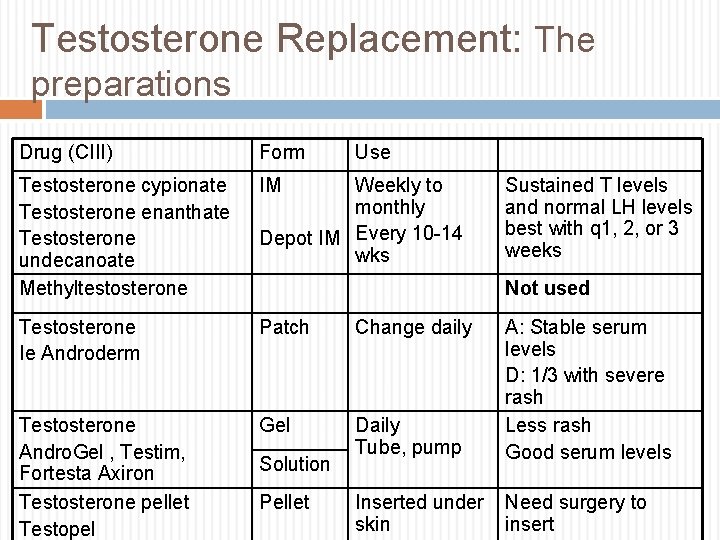
Testosterone Replacement: The preparations Drug (CIII) Form Use Testosterone cypionate Testosterone enanthate Testosterone undecanoate Methyltestosterone IM Testosterone Ie Androderm Patch Change daily Testosterone Andro. Gel , Testim, Fortesta Axiron Testosterone pellet Testopel Gel Daily Tube, pump A: Stable serum levels D: 1/3 with severe rash Less rash Good serum levels Inserted under skin Need surgery to insert Weekly to monthly Depot IM Every 10 -14 wks Sustained T levels and normal LH levels best with q 1, 2, or 3 weeks Not used Solution Pellet

Testosterone Replacement: The preparations Monitoring Symptoms Free testosterone level (in primary hypogonadism, consider checking LH) After 3 months of initiation / dose change About ½ way time for injections Peak ~ 6 -8 hrs after application for patches Gel, after 1 week of initiation Obtain or consider CBC, at 3 to 6 months, then annually DEXA scan Screen for: prostate, sleep apnea

Testosterone Replacement: Side effects Puberty elevated blood count, aggressive behavior Acne Gynecomastia Older men Prostate, refer to urology if Elevated blood count Increased serum PSA concentration >1. 4 ng/ml within any 12 -month AUA/IPSS of >19 Hematocrit >54%, stop therapy Worsening sleep apnea New depot form Pulmonary oil microembolism and anaphylaxis Now is restricted drug Only given in office setting with monitoring Patch: rash, up to 1/3 of men Can try applying steroid cream

Testosterone use: Black box warning Main concern is secondary exposure to testosterone Virilization has been reported in children Children should avoid contact with unwashed/unclothed application sites Healthcare providers should advise patients to strictly adhere to recommended instructions for use, ie Gel: potential of transfer with skin-skin contact Men should wear T-shirts when applied to trunk Wash hands after applying Allow to drug application to dry

Testosterone use: Adverse effects Recent concerns re adverse cardiovascular events Recent studies shown increased CV events in treatment group vs placebo, others not Possible explanations Testosterone causes salt and water retention Increases platelet thromboxane A 2 receptor density, platelet aggregation, promoting inflammatory cytokine production, possibly leading to coagulation, coronary plaque formation and ultimately, ACS Does not seem increase the risk of prostate cancer

Estrogen and Progesterone

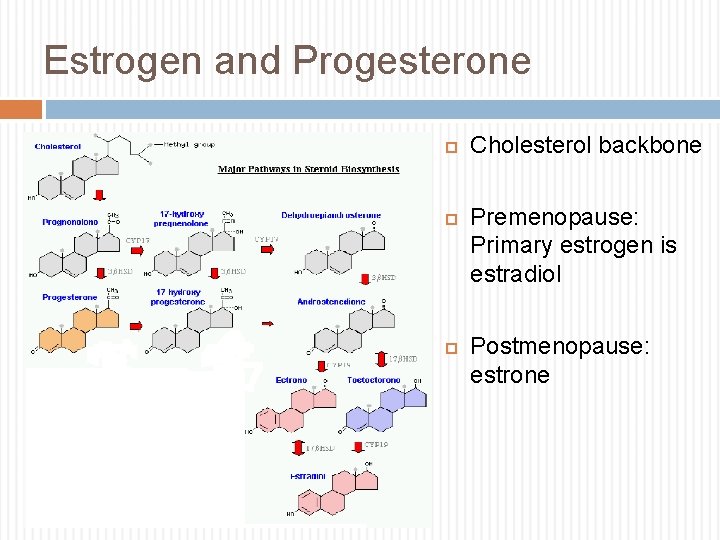
Estrogen and Progesterone Cholesterol backbone Premenopause: Primary estrogen is estradiol Postmenopause: estrone

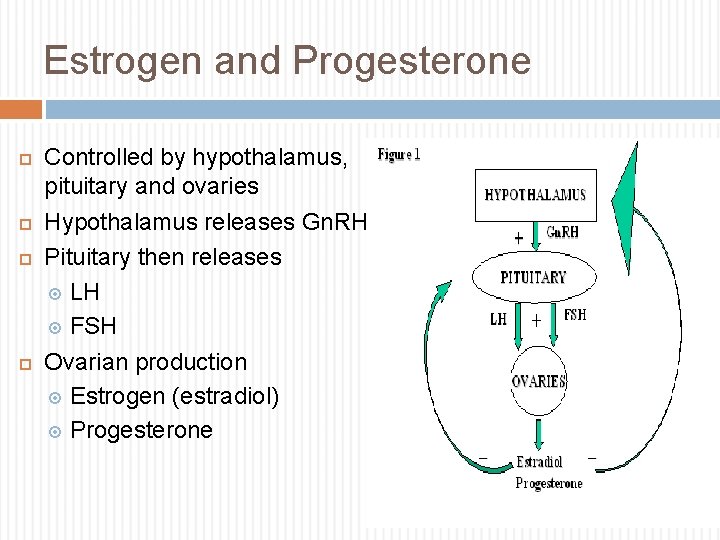
Estrogen and Progesterone Controlled by hypothalamus, pituitary and ovaries Hypothalamus releases Gn. RH Pituitary then releases LH FSH Ovarian production Estrogen (estradiol) Progesterone

Estrogen and Progesterone Estrogen mechanism of action Estrogen Receptor (ER) α and β Most located within nucleus ERα: uterus, ovarian cells, leydig cells, prostate, and lesser # at vascular endothelium, bone, breast, regions of brain, liver ERβ: prostate, testes, and ovary, and lesser # liver, pancreas, adipose tissue, bone Binds with estrogen receptors (ER) within nucleus Alteration in gene transcription occurs

Estrogen and Progesterone most important progestin in humans Synthesized from cholesterol in ovary, testis, and adrenal cortex Mechanism of action Binds with progesterone receptor (PR): PRα and PRβ Receptors present in variety of tissues: reproductive tract, breast tissue, CNS, metabolic effects Alteration in gene transcription occurs

Estrogen and Progesterone Physiologic effects In females Female phenotype and 2 nd sex characteristics Menstrual cycle: menarche, ovulation, endometrial proliferation Lipid, carbohydrate metabolism Bone cycle In males: Bone health, sperm formation, behavior Pharmacologic uses Contraception, control of menstrual cycle, menopausal hormone replacement, infertility, breast cancer

Estrogen and Progesterone Physiological effects of estrogen Proliferation of endometrium, ovulation, menses Carbohydrate and lipid metabolism LDL, HDL Cholesterol secretion gallstone production Clotting factors Slight increase in clotting factors Slight decrease in protein C and S Vascular effects nitric oxide, prostacyclin vasodilatation proliferation of vascular smooth muscle cells

Estrogen and Progesterone Physiological effects of estrogen con’t Bone growth and closure of epiphyseal Reduction number of osteoclasts Effects communication between osteoclasts and osteoblasts Appear to increase bone sensitivity to mechanical stress Physiological effects of progesterone Change/maturation in endometrium to secretory, menses Increase basal body temperature during ovulation Appears to promote fat deposition Depressive and hypnotic effects on brain

Estrogen Pharmacokinetics Absorption Lipid soluble = generally high absorption Estradiol with high first-pass effect ethinyl estradiol Distribution Highly protein bound, SHBG or albumin Metabolism Estradiol to estriol via CYP 3 A 4 Phase II reaction to sulfate and glucuronide conjugates Excretion Excreted in urine (50%) Enterohepatic recirculation (50%)

Progesterone Pharmacokinetics Absorption: high first-pass effect Distribution: highly protein bound Metabolism to sulfate and glucuronide conjugates Elimination in urine Half life varies Progesterone ~ 5 minutes ~ 7 hours norethindrone ~ 24 hours medroxyprogesterone acetate

Estrogen and Progestin Use (Pharmacologic) Agonist Oral contraceptives Combination products Progestin alone Control menstrual cycle: amenorrhea, dysmenorrhea, endometriosis Hyperandrogenism: PCOS Menopausal hormone replacement Antagonist Breast cancer Endometriosis

Estrogen and Progesterone Preparations Estrogens Natural: estradiol, estrone, estriol, equilin Synthetic: ethinyl estradiol, mestranol, estradiol valerate Progesterone and Progestins Natural: progesterone Synthetic progestins 1 st generation: norethindrone, medroxyprogesterone 2 nd generation: levonorgestrel, norgestrel 3 rd generation: norgestimate, desogestrel, etonogestrel 4 th generation: drospirenone, dienogest Alone or in combination

Estrogen and Progestin Use: Contraception Oral contraceptives: Most widely used contraceptive agents Convenient, affordable, reliable First pills approved by FDA 1959: Envoid – norethynodrel/mestranol 1962: Orthonovum – norethindrone/mestranol 1970 s adverse effects recognized 1980 s (second generation pills) Low-dose pills introduced Phasic pills Benefits recognized 1990 s (third generation pills) Lower androgenic pills introduced Other form of contraceptives discovered

Estrogen and Progestin Use: Contraception Mechanism of action of combination pills Reduction of Gn. RH pulses Decrease anterior pituitary response to Gn. RH Reduced levels of LH and FSH Decreased FSH, prevent follicle maturation Prevention of ovulation: Inhibits mid-cycle surge of LH Organ effect Impairment of tubal motility Viscous cervical mucous Endometrium less receptive to implantation

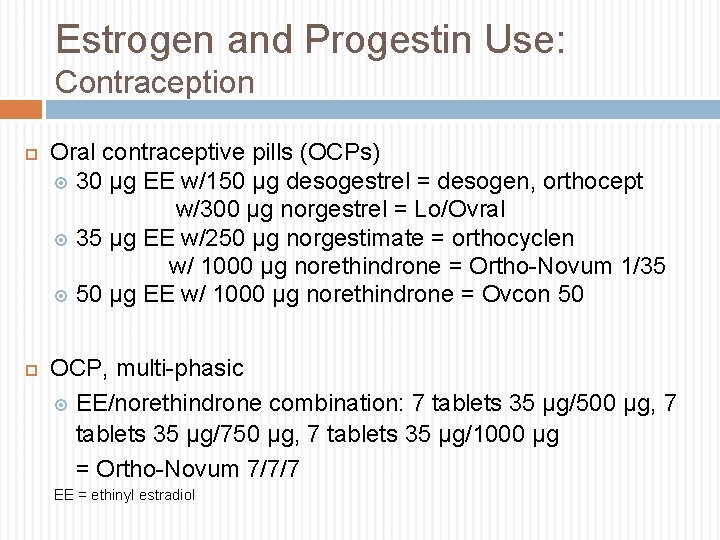
Estrogen and Progestin Use: Contraception Oral contraceptive pills (OCPs) 30 µg EE w/150 µg desogestrel = desogen, orthocept w/300 µg norgestrel = Lo/Ovral 35 µg EE w/250 µg norgestimate = orthocyclen w/ 1000 µg norethindrone = Ortho-Novum 1/35 50 µg EE w/ 1000 µg norethindrone = Ovcon 50 OCP, multi-phasic EE/norethindrone combination: 7 tablets 35 µg/500 µg, 7 tablets 35 µg/750 µg, 7 tablets 35 µg/1000 µg = Ortho-Novum 7/7/7 EE = ethinyl estradiol

Estrogen and Progestin Use: Contraception Patch: Othro Evra® Norelgestromin/ethinyl estradiol transdermal System Intravaginal device: Nuva ring Etonogestrel/ethinyl estradiol vaginal ring delivers 0. 120 mg/15 mcg per day Continuous cycle Seasonale with 1 week of placebo every 3 months Lybrel with no placebo Indications: endometriosis, premenstrual dysphoric disorder

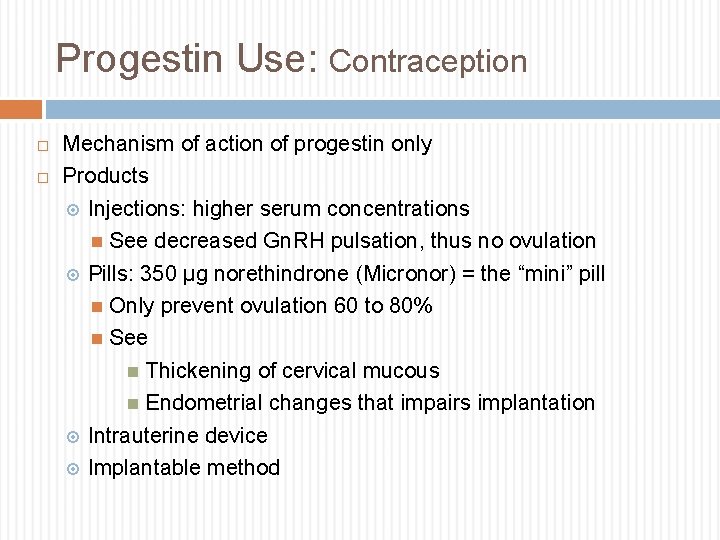
Progestin Use: Contraception Mechanism of action of progestin only Products Injections: higher serum concentrations See decreased Gn. RH pulsation, thus no ovulation Pills: 350 µg norethindrone (Micronor) = the “mini” pill Only prevent ovulation 60 to 80% See Thickening of cervical mucous Endometrial changes that impairs implantation Intrauterine device Implantable method

Progestins Only Products Implantable: Etonogestrel 68 mg Nexplanon: Lasts for 3 years, provides ~65 25 mcg daily Injectable: Depot medroxyprogesterone acetate (depo-provera) 150 mg injected every 12 weeks Intrauterine device: Levonorgestrel Mirena: last 5 years, provides 20 10 mcg daily Skyla: lasts 3 years, provides 14 5 mcg daily Emergency contraception, prevention after unprotected intercourse Plan B, levonorgestrel (OTC): One pill within 72 hours Ella, Ulipristal (progesterone receptor modulator) (by Rx): 1 tablet within 5 days

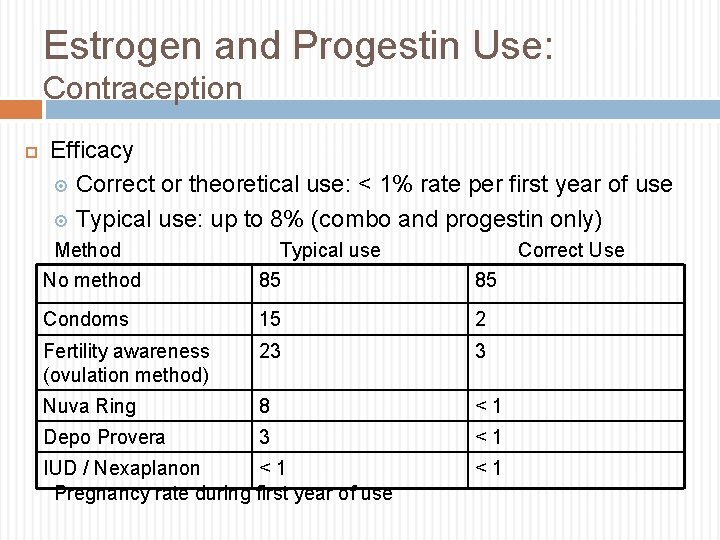
Estrogen and Progestin Use: Contraception Efficacy Correct or theoretical use: < 1% rate per first year of use Typical use: up to 8% (combo and progestin only) Method Typical use Correct Use No method 85 85 Condoms 15 2 Fertility awareness (ovulation method) 23 3 Nuva Ring 8 <1 Depo Provera 3 <1 IUD / Nexaplanon <1 Pregnancy rate during first year of use <1

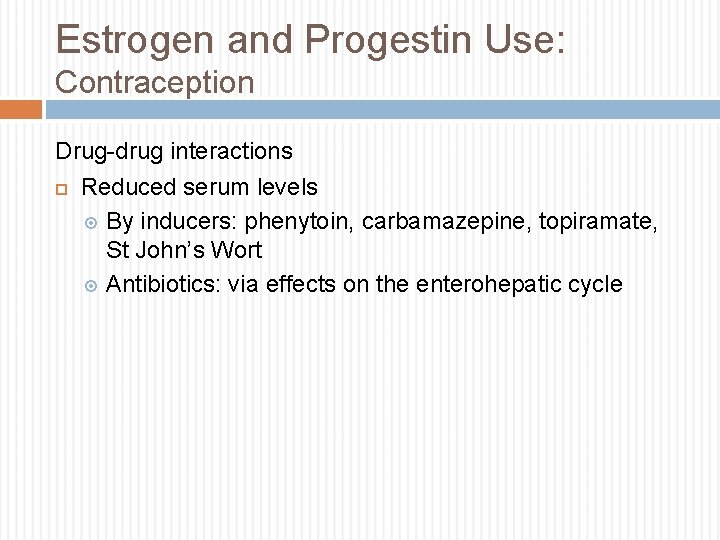
Estrogen and Progestin Use: Contraception Drug-drug interactions Reduced serum levels By inducers: phenytoin, carbamazepine, topiramate, St John’s Wort Antibiotics: via effects on the enterohepatic cycle

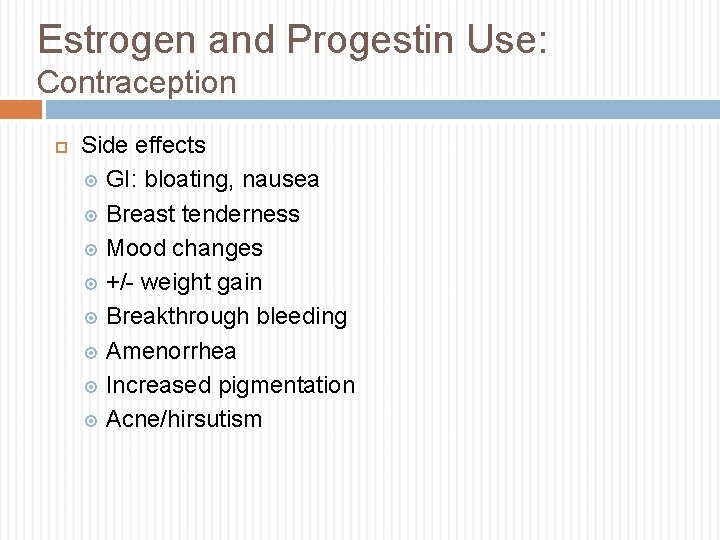
Estrogen and Progestin Use: Contraception Side effects GI: bloating, nausea Breast tenderness Mood changes +/- weight gain Breakthrough bleeding Amenorrhea Increased pigmentation Acne/hirsutism

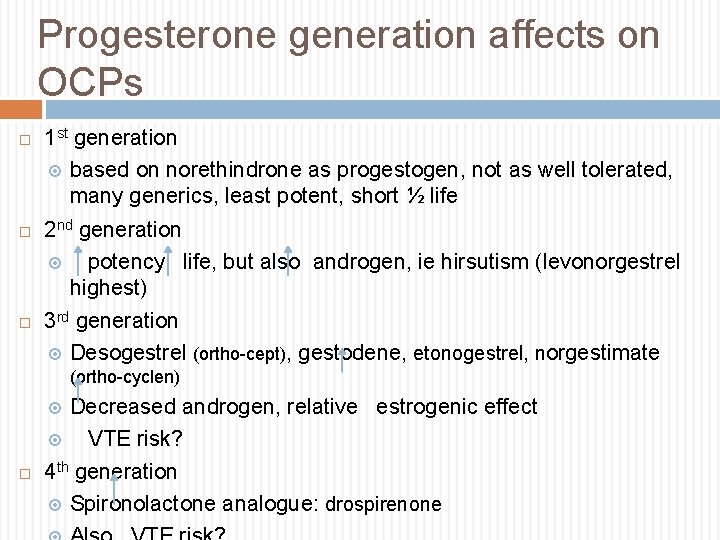
Progesterone generation affects on OCPs 1 st generation based on norethindrone as progestogen, not as well tolerated, many generics, least potent, short ½ life 2 nd generation potency, life, but also androgen, ie hirsutism (levonorgestrel highest) 3 rd generation Desogestrel (ortho-cept), gestodene, etonogestrel, norgestimate (ortho-cyclen) Decreased androgen, relative estrogenic effect VTE risk? 4 th generation Spironolactone analogue: drospirenone

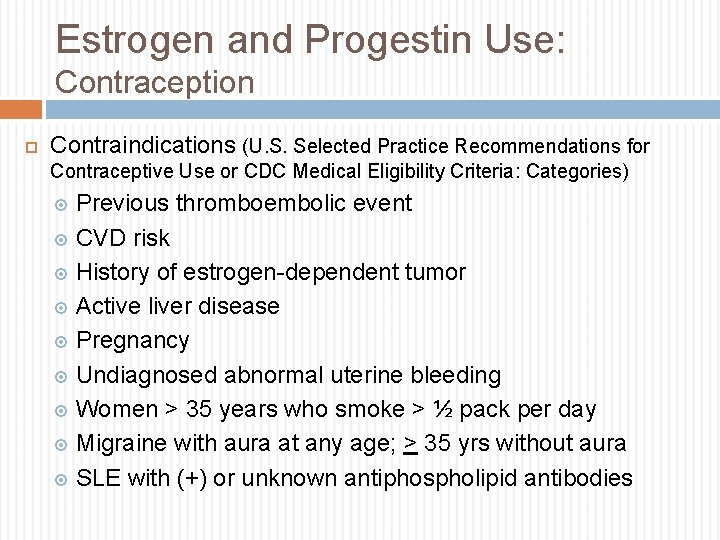
Estrogen and Progestin Use: Contraception Contraindications (U. S. Selected Practice Recommendations for Contraceptive Use or CDC Medical Eligibility Criteria: Categories) Previous thromboembolic event CVD risk History of estrogen-dependent tumor Active liver disease Pregnancy Undiagnosed abnormal uterine bleeding Women > 35 years who smoke > ½ pack per day Migraine with aura at any age; > 35 yrs without aura SLE with (+) or unknown antiphospholipid antibodies

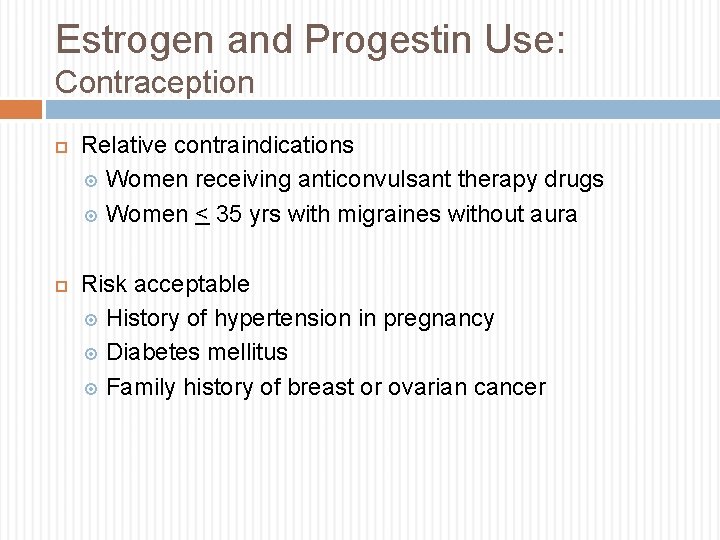
Estrogen and Progestin Use: Contraception Relative contraindications Women receiving anticonvulsant therapy drugs Women < 35 yrs with migraines without aura Risk acceptable History of hypertension in pregnancy Diabetes mellitus Family history of breast or ovarian cancer

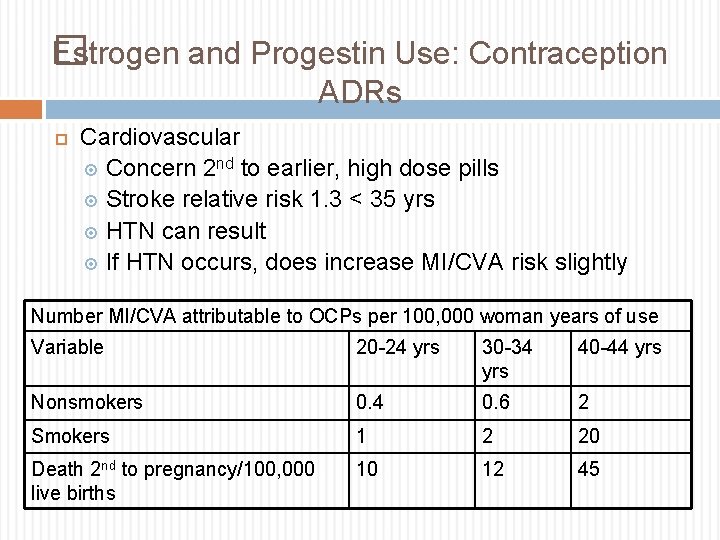
Estrogen and Progestin Use: Contraception � ADRs Cardiovascular Concern 2 nd to earlier, high dose pills Stroke relative risk 1. 3 < 35 yrs HTN can result If HTN occurs, does increase MI/CVA risk slightly Number MI/CVA attributable to OCPs per 100, 000 woman years of use Variable 20 -24 yrs 30 -34 yrs 40 -44 yrs Nonsmokers 0. 4 0. 6 2 Smokers 1 2 20 Death 2 nd to pregnancy/100, 000 live births 10 12 45

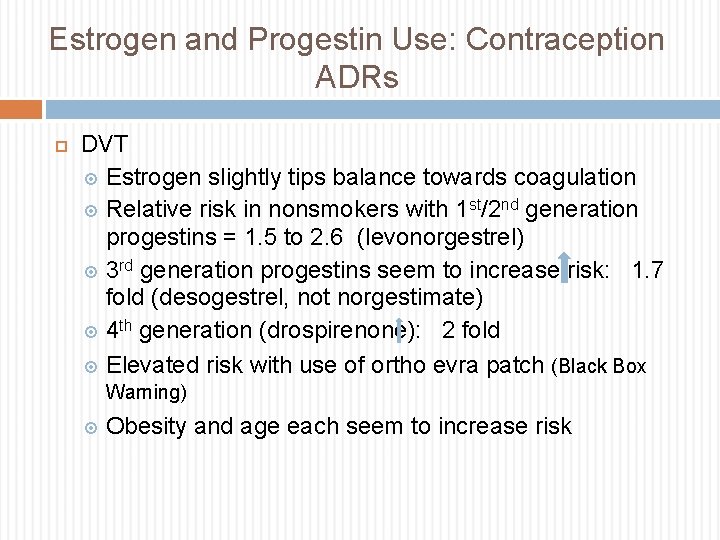
Estrogen and Progestin Use: Contraception ADRs DVT Estrogen slightly tips balance towards coagulation Relative risk in nonsmokers with 1 st/2 nd generation progestins = 1. 5 to 2. 6 (levonorgestrel) 3 rd generation progestins seem to increase risk: 1. 7 fold (desogestrel, not norgestimate) 4 th generation (drospirenone): 2 fold Elevated risk with use of ortho evra patch (Black Box Warning) Obesity and age each seem to increase risk

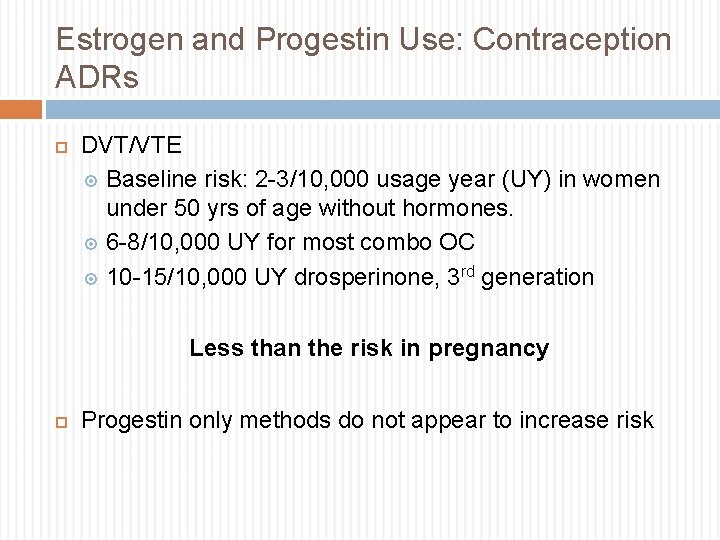
Estrogen and Progestin Use: Contraception ADRs DVT/VTE Baseline risk: 2 -3/10, 000 usage year (UY) in women under 50 yrs of age without hormones. 6 -8/10, 000 UY for most combo OC 10 -15/10, 000 UY drosperinone, 3 rd generation Less than the risk in pregnancy Progestin only methods do not appear to increase risk

Estrogen and Progestin Use: Contraception ADRs Cancer risk Overall reduced cancer risk Reduced risk for colorectal, uterine, ovarian cancers No effect on breast cancer Increases in cervical cancer seen, but ? ? related to HPV Liver/gallbladder Increase risk for gallstones

Hormone Replacement Products Hormone replacement Estradiol preparations Oral: Estrace, Gynodiol Transdermal preparation: Alora, Vivelle, Menostar Gel: Estrogel Vaginal ointment: Estrace Vaginal Cream Vaginal ring: Estring (estradiol acetate), Femring Conjugated equine estrogen preparations: premarin oral, vaginal Primarily estrone Estropipate: Ogen tablets, vaginal cream

Estrogen Replacement Products Hormone replacement Estrogen/progestin combinations Oral: prempro (medroxyprogesterone), activella (norethinidrone) Patch: combi-patch (norethindrone), climara pro (levnonrgestrel) Progesterone replacement Oral micronized progesterone: Prometrium, generic Medroxyprogesterone acetate: Provera, generic Estrogen/testosterone combos Estratest, syntest (esterified estrogen/methyltestosterone)

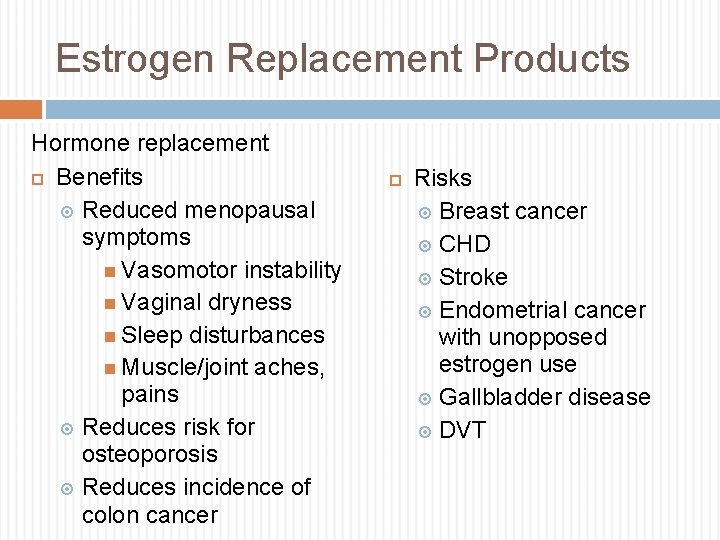
Estrogen Replacement Products Hormone replacement Benefits Reduced menopausal symptoms Vasomotor instability Vaginal dryness Sleep disturbances Muscle/joint aches, pains Reduces risk for osteoporosis Reduces incidence of colon cancer Risks Breast cancer CHD Stroke Endometrial cancer with unopposed estrogen use Gallbladder disease DVT

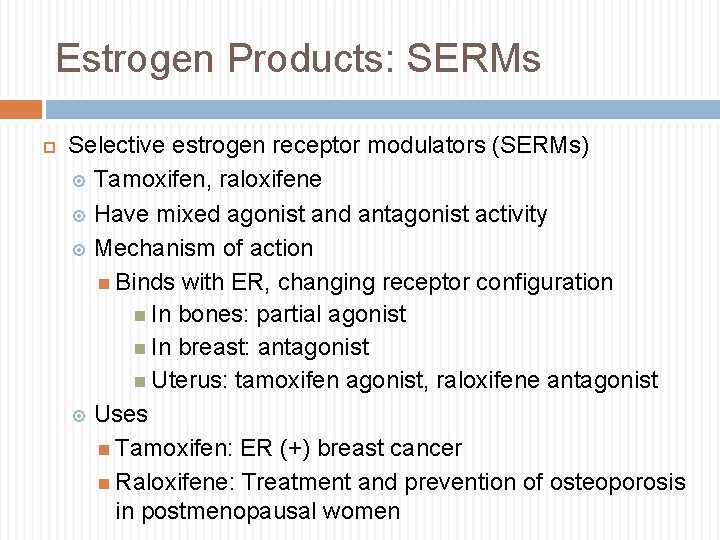
Estrogen Products: SERMs Selective estrogen receptor modulators (SERMs) Tamoxifen, raloxifene Have mixed agonist and antagonist activity Mechanism of action Binds with ER, changing receptor configuration In bones: partial agonist In breast: antagonist Uterus: tamoxifen agonist, raloxifene antagonist Uses Tamoxifen: ER (+) breast cancer Raloxifene: Treatment and prevention of osteoporosis in postmenopausal women

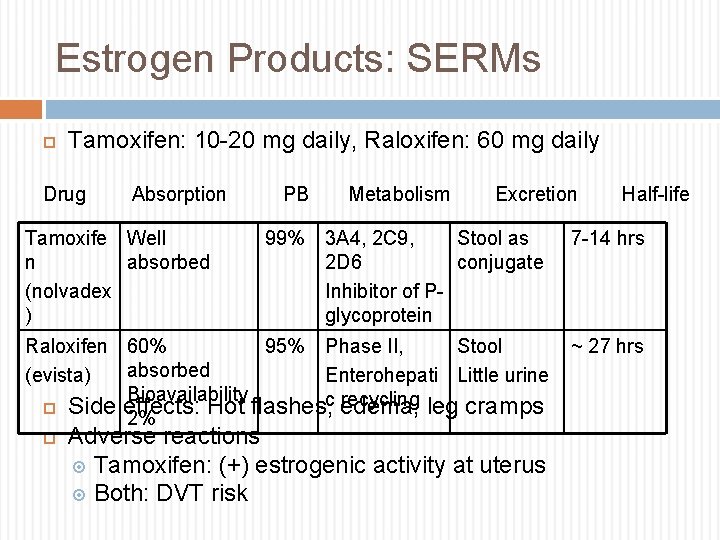
Estrogen Products: SERMs Tamoxifen: 10 -20 mg daily, Raloxifen: 60 mg daily Drug Absorption Tamoxife Well n absorbed (nolvadex ) PB Metabolism Excretion Half-life 99% 3 A 4, 2 C 9, Stool as 2 D 6 conjugate Inhibitor of Pglycoprotein 7 -14 hrs Raloxifen 60% 95% Phase II, Stool absorbed (evista) Enterohepati Little urine Bioavailability c recycling Side effects: Hot flashes, edema, leg cramps 2% ~ 27 hrs Adverse reactions Tamoxifen: (+) estrogenic activity at uterus Both: DVT risk

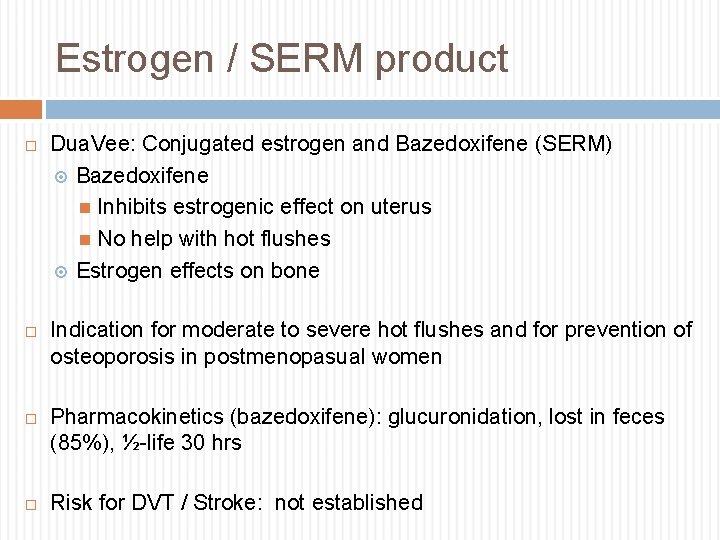
Estrogen / SERM product Dua. Vee: Conjugated estrogen and Bazedoxifene (SERM) Bazedoxifene Inhibits estrogenic effect on uterus No help with hot flushes Estrogen effects on bone Indication for moderate to severe hot flushes and for prevention of osteoporosis in postmenopasual women Pharmacokinetics (bazedoxifene): glucuronidation, lost in feces (85%), ½-life 30 hrs Risk for DVT / Stroke: not established

Progestin Products: PRMs Progesterone receptor modulators or SPRMs Mifepristone or RU 486: inhibits receptors, antagonizes endometrial and myometrial effects Ella: Ulipristal (emergency contraception)

In-Class Presentations Over semester: asthma, lipid lowering agents, antiarrhythmic agents, antipsychotic agents, GI drugs Schedule posted in Modules, under course information Next Tuesday, some of you will do an in-class presentation around the various delivery methods in asthma and COPD Under In-class Asthma Presentations are general and some specific articles to use in developing the short presentation Please make note and prepare some as you do your readings over the next week
 Gonadal hormones
Gonadal hormones Hyperandrogenism
Hyperandrogenism Androgens meaning
Androgens meaning Glucocorticoids mineralocorticoids and androgens
Glucocorticoids mineralocorticoids and androgens Estrogen and gallstones
Estrogen and gallstones Estrogen effect
Estrogen effect Difference between estrogen and progesterone
Difference between estrogen and progesterone Estrogen
Estrogen Estrogen and progesterone
Estrogen and progesterone Corpus luteum
Corpus luteum Function of testes
Function of testes Testosterone endocrine gland
Testosterone endocrine gland Estrogen positive and negative feedback
Estrogen positive and negative feedback Kryptorchidism
Kryptorchidism Hipotalamo hipofizer gonadal aks
Hipotalamo hipofizer gonadal aks Varicocele
Varicocele Sex chromosome aneuploidy
Sex chromosome aneuploidy Hermaphroditism
Hermaphroditism Left gonadal vein
Left gonadal vein Adrenarş pubarş
Adrenarş pubarş Uca dnp program
Uca dnp program Spi dnp
Spi dnp Sicodis sgp 2022
Sicodis sgp 2022 Chamberlain msn fnp
Chamberlain msn fnp Gillian ryan model
Gillian ryan model Language focus
Language focus Gillian dell
Gillian dell Gillian petrie
Gillian petrie Gillian lord uf
Gillian lord uf Gillian swan
Gillian swan Gillian unsworth
Gillian unsworth Gill leng nice
Gill leng nice Esli osmanlliu
Esli osmanlliu Gillian kernaghan
Gillian kernaghan Gillian reed
Gillian reed Gillian gunn
Gillian gunn Gillian plant
Gillian plant St davids day poem
St davids day poem Dell download csos for recovery
Dell download csos for recovery Gillian hamilton education scotland
Gillian hamilton education scotland Gillian hazell
Gillian hazell Gillian anderson manager
Gillian anderson manager Gillian jesserun
Gillian jesserun Gillian dennehy
Gillian dennehy Gillian unsworth
Gillian unsworth Gillian foulger
Gillian foulger Gillian jessurun overleden
Gillian jessurun overleden Gillian roehrig
Gillian roehrig Mem8000
Mem8000 Lionel zupan
Lionel zupan Enamel tufts
Enamel tufts Tufts anesthesia residency
Tufts anesthesia residency