Anticoagulant Drugs Gillian Tufts DNP CFNP Uses of




















- Slides: 54

Anticoagulant Drugs Gillian Tufts, DNP, C-FNP

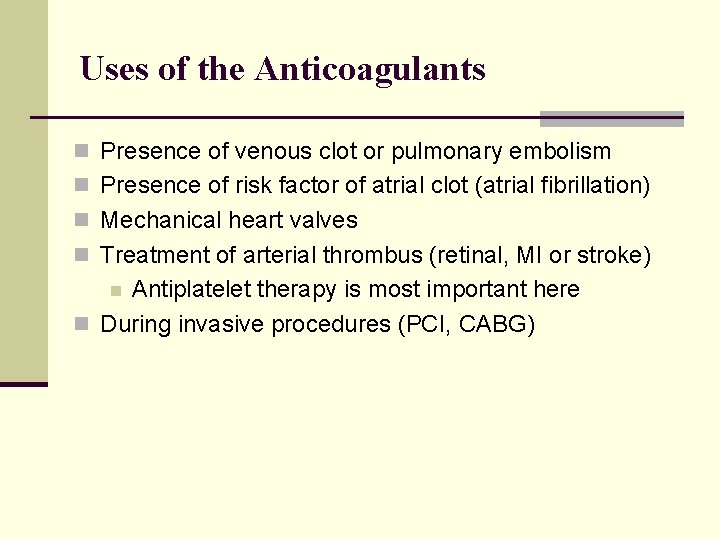
Uses of the Anticoagulants n Presence of venous clot or pulmonary embolism n Presence of risk factor of atrial clot (atrial fibrillation) n Mechanical heart valves n Treatment of arterial thrombus (retinal, MI or stroke) Antiplatelet therapy is most important here n During invasive procedures (PCI, CABG) n

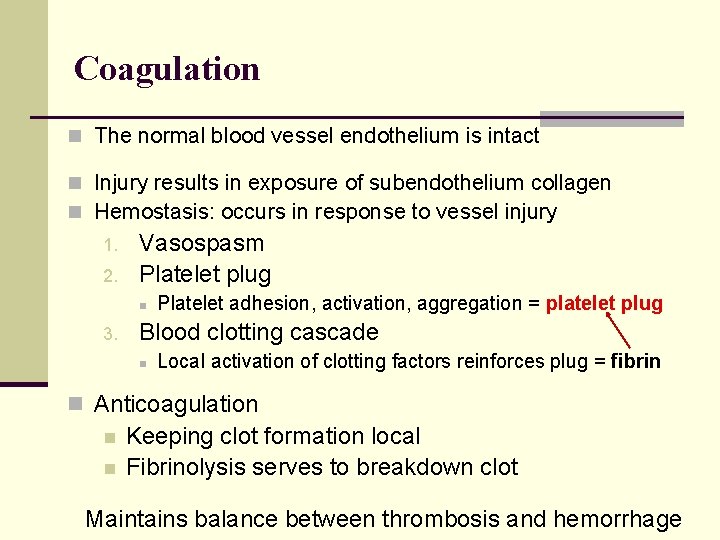
Coagulation n The normal blood vessel endothelium is intact n Injury results in exposure of subendothelium collagen n Hemostasis: occurs in response to vessel injury 1. 2. Vasospasm Platelet plug n 3. Platelet adhesion, activation, aggregation = platelet plug Blood clotting cascade n Local activation of clotting factors reinforces plug = fibrin n Anticoagulation n n Keeping clot formation local Fibrinolysis serves to breakdown clot Maintains balance between thrombosis and hemorrhage

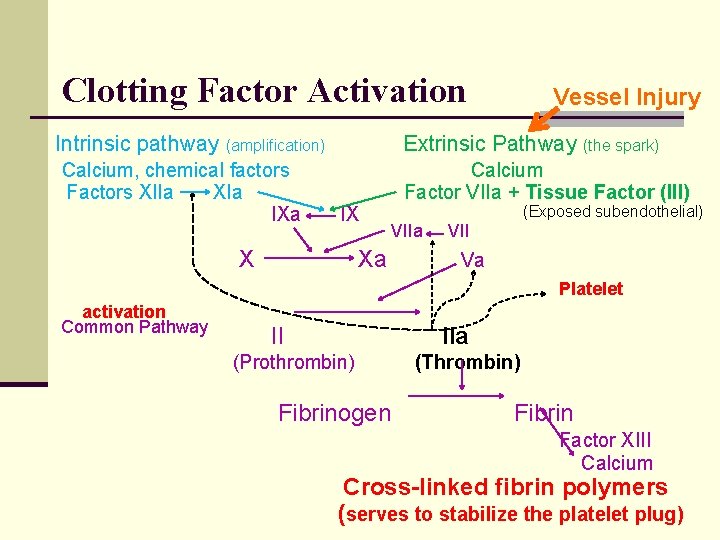
Coagulation Cascade n Local activation of clotting factors reinforces plug = fibrin n n Circulating inactive clotting factors With vessel injury, activation of the clotting cascade n Exposes tissue factor (clotting factor III) n One activated factor activates another n Function as catalysts n The pathways n n n Extrinsic: The spark Intrinsic: Amplification of numbers Common

Clotting Factor Activation Intrinsic pathway Extrinsic Pathway (the spark) (amplification) Calcium, chemical factors Factors XIIa XIa IXa Vessel Injury IX Calcium Factor VIIa + Tissue Factor (III) X (Exposed subendothelial) Xa VII Va Platelet activation Common Pathway II IIa (Prothrombin) Fibrinogen (Thrombin) Fibrin Factor XIII Calcium Cross-linked fibrin polymers (serves to stabilize the platelet plug)

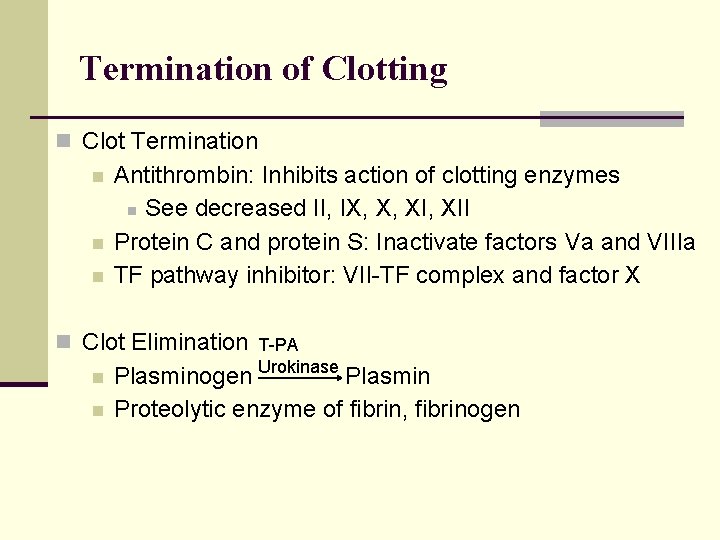
Termination of Clotting n Clot Termination n Antithrombin: Inhibits action of clotting enzymes n See decreased II, IX, X, XII Protein C and protein S: Inactivate factors Va and VIIIa TF pathway inhibitor: VII-TF complex and factor X n Clot Elimination T-PA n n Plasminogen Urokinase Plasmin Proteolytic enzyme of fibrin, fibrinogen

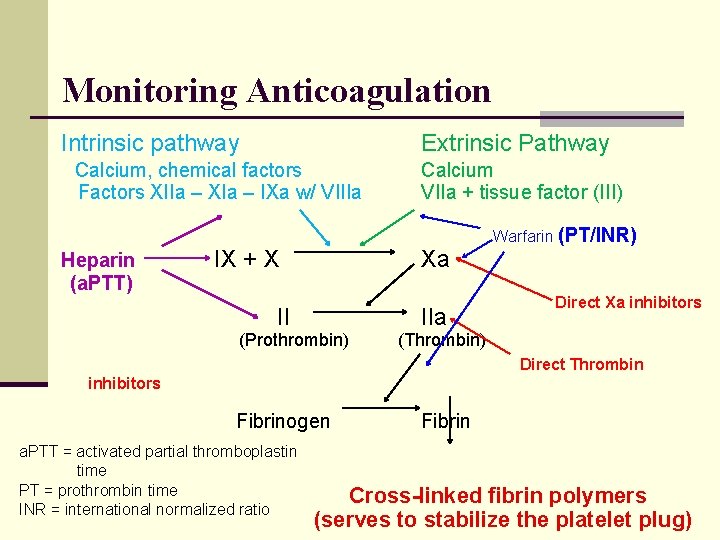
Monitoring Anticoagulation Intrinsic pathway Extrinsic Pathway Calcium, chemical factors Factors XIIa – XIa – IXa w/ VIIIa Heparin (a. PTT) IX + X Calcium VIIa + tissue factor (III) Xa II (Prothrombin) IIa Warfarin (PT/INR) Direct Xa inhibitors (Thrombin) Direct Thrombin inhibitors Fibrinogen a. PTT = activated partial thromboplastin time PT = prothrombin time INR = international normalized ratio Fibrin Cross-linked fibrin polymers (serves to stabilize the platelet plug)

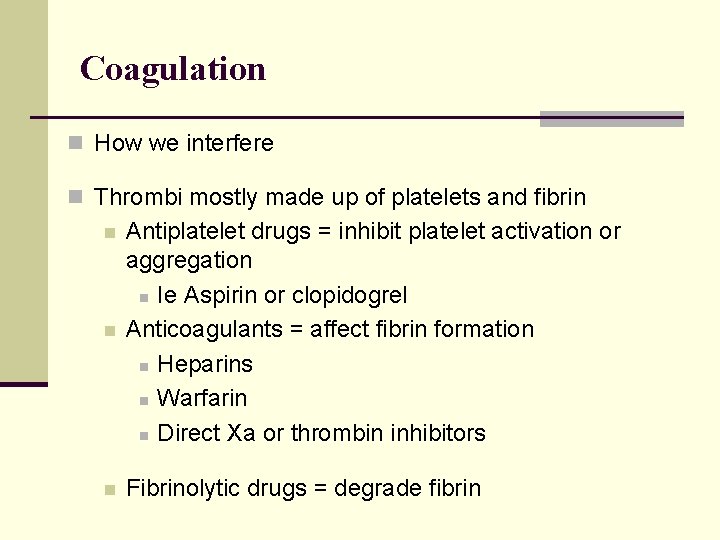
Coagulation n How we interfere n Thrombi mostly made up of platelets and fibrin n Antiplatelet drugs = inhibit platelet activation or aggregation n Ie Aspirin or clopidogrel Anticoagulants = affect fibrin formation n Heparins n Warfarin n Direct Xa or thrombin inhibitors n Fibrinolytic drugs = degrade fibrin n

Anticoagulant Drugs n Heparin n Low Molecular Weight Heparins n Coumadin (warfarin) n Direct Xa inhibitors n Direct Thrombin inhibitors n Antiplatelet agents

A. S is a 48 year old women with new onset (L) lower leg pain, swelling and redness. She is seen in the ED. Doppler study done = + (L) lower leg DVT PMHx: Rheumatoid Arthritis Surgeries: 1 month ago total hysterectomy What medication(s) do you think she was started on?

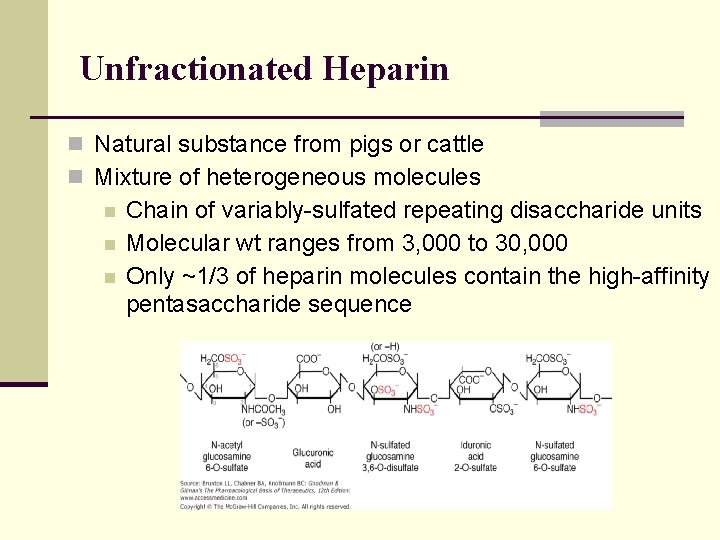
Unfractionated Heparin n Natural substance from pigs or cattle n Mixture of heterogeneous molecules n n n Chain of variably-sulfated repeating disaccharide units Molecular wt ranges from 3, 000 to 30, 000 Only ~1/3 of heparin molecules contain the high-affinity pentasaccharide sequence

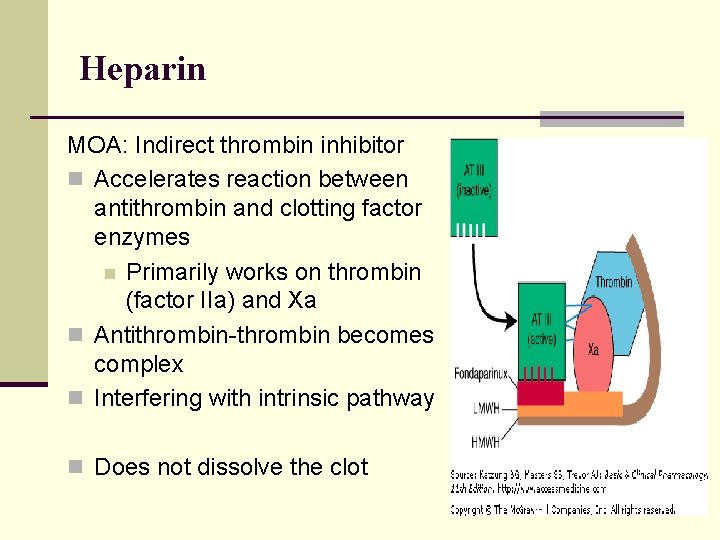
Heparin MOA: Indirect thrombin inhibitor n Accelerates reaction between antithrombin and clotting factor enzymes n Primarily works on thrombin (factor IIa) and Xa n Antithrombin-thrombin becomes complex n Interfering with intrinsic pathway n Does not dissolve the clot

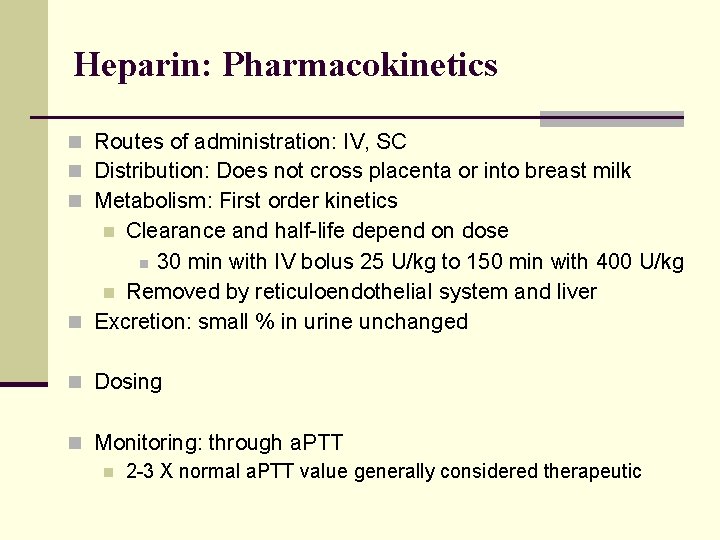
Heparin: Pharmacokinetics n Routes of administration: IV, SC n Distribution: Does not cross placenta or into breast milk n Metabolism: First order kinetics Clearance and half-life depend on dose n 30 min with IV bolus 25 U/kg to 150 min with 400 U/kg n Removed by reticuloendothelial system and liver n Excretion: small % in urine unchanged n n Dosing n Monitoring: through a. PTT n 2 -3 X normal a. PTT value generally considered therapeutic

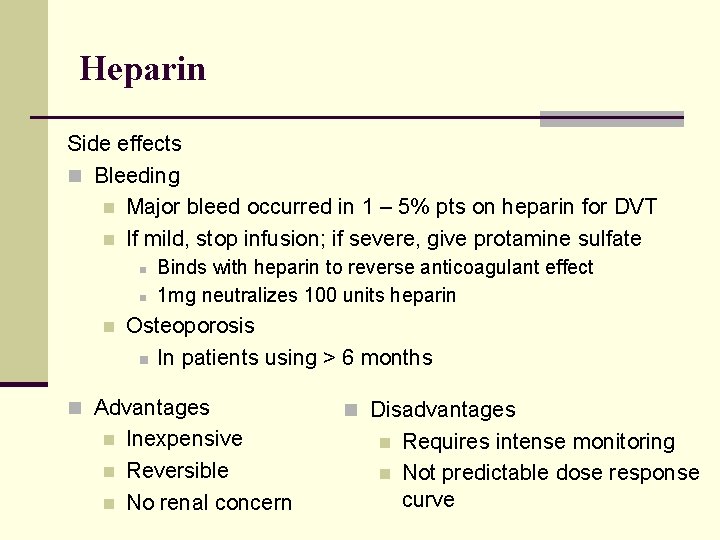
Heparin Side effects n Bleeding n Major bleed occurred in 1 – 5% pts on heparin for DVT n If mild, stop infusion; if severe, give protamine sulfate n n n Binds with heparin to reverse anticoagulant effect 1 mg neutralizes 100 units heparin Osteoporosis n In patients using > 6 months n Advantages n n n Inexpensive Reversible No renal concern n Disadvantages n n Requires intense monitoring Not predictable dose response curve

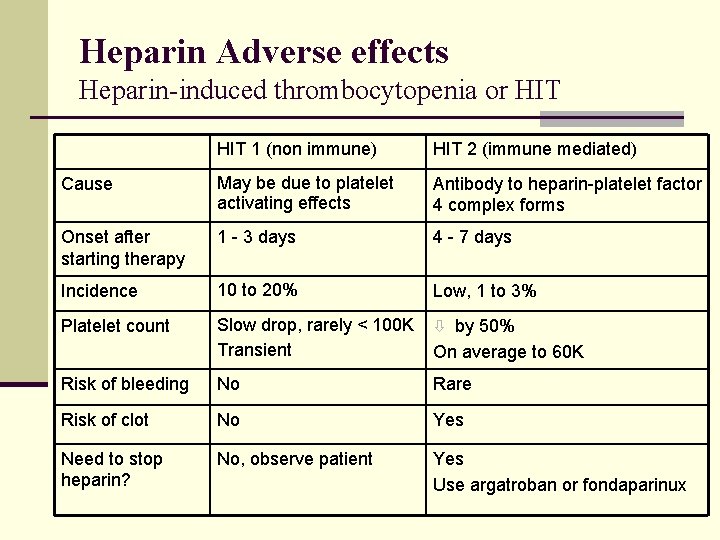
Heparin Adverse effects Heparin-induced thrombocytopenia or HIT 1 (non immune) HIT 2 (immune mediated) Cause May be due to platelet activating effects Antibody to heparin-platelet factor 4 complex forms Onset after starting therapy 1 - 3 days 4 - 7 days Incidence 10 to 20% Low, 1 to 3% Platelet count Slow drop, rarely < 100 K Transient ò by 50% Risk of bleeding No Rare Risk of clot No Yes Need to stop heparin? No, observe patient Yes Use argatroban or fondaparinux On average to 60 K

A. S. was sent home on enoxaparin SQ BID and warfarin. Her weight is 138 lbs. What enoxaparin dose would you start her on? Would you want to monitor her PT or PTT?

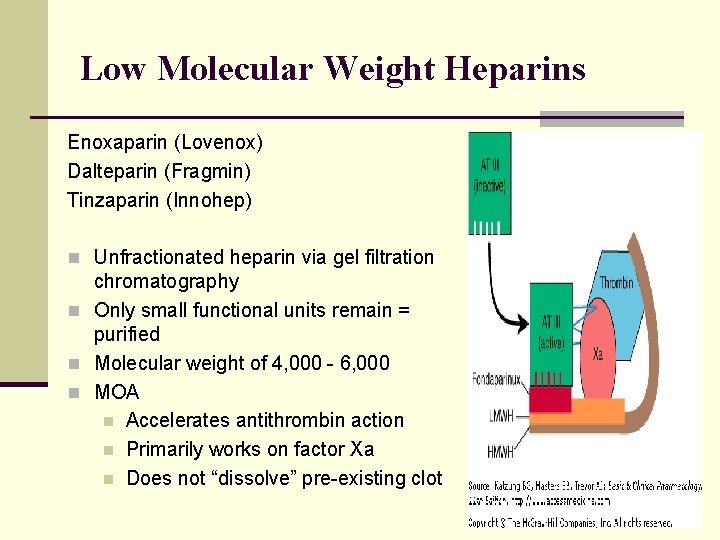
Low Molecular Weight Heparins Enoxaparin (Lovenox) Dalteparin (Fragmin) Tinzaparin (Innohep) n Unfractionated heparin via gel filtration chromatography n Only small functional units remain = purified n Molecular weight of 4, 000 - 6, 000 n MOA n Accelerates antithrombin action n Primarily works on factor Xa n Does not “dissolve” pre-existing clot

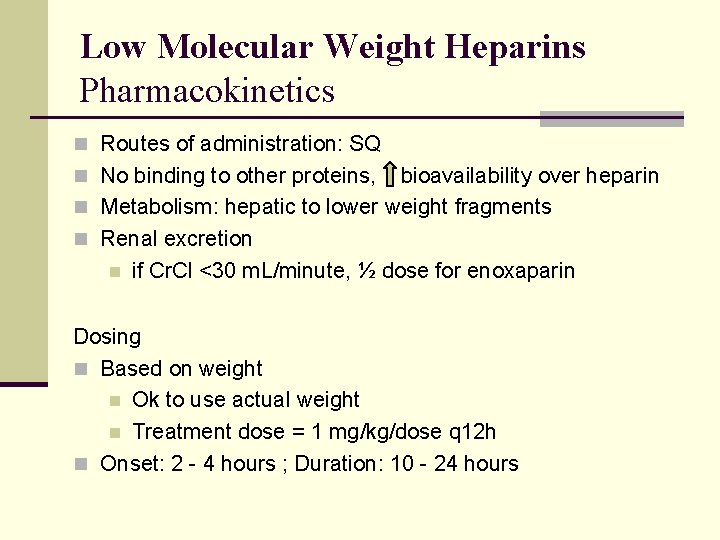
Low Molecular Weight Heparins Pharmacokinetics n Routes of administration: SQ n No binding to other proteins, bioavailability over heparin n Metabolism: hepatic to lower weight fragments n Renal excretion n if Cr. Cl <30 m. L/minute, ½ dose for enoxaparin Dosing n Based on weight n Ok to use actual weight n Treatment dose = 1 mg/kg/dose q 12 h n Onset: 2 - 4 hours ; Duration: 10 - 24 hours

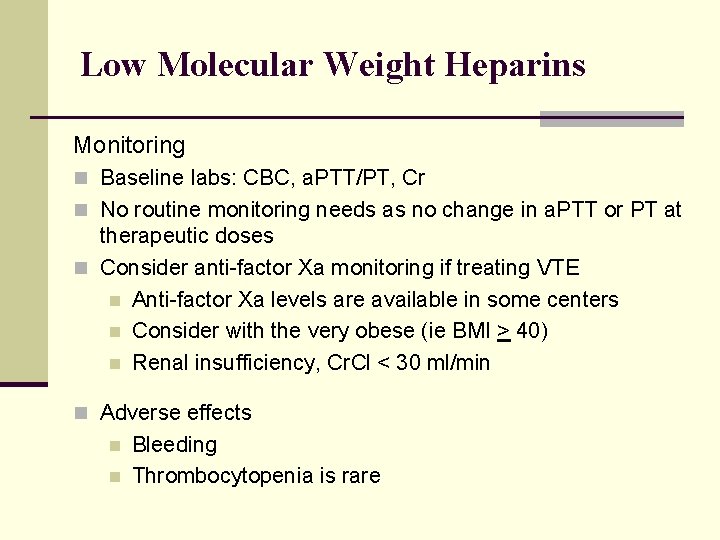
Low Molecular Weight Heparins Monitoring n Baseline labs: CBC, a. PTT/PT, Cr n No routine monitoring needs as no change in a. PTT or PT at therapeutic doses n Consider anti-factor Xa monitoring if treating VTE n Anti-factor Xa levels are available in some centers n Consider with the very obese (ie BMI > 40) n Renal insufficiency, Cr. Cl < 30 ml/min n Adverse effects n n Bleeding Thrombocytopenia is rare

Low Molecular Weight Heparins n Advantages!! n n n High SC bioavailability – close to 100% No routine monitoring, can be given at home Predictable dose response curve – correlated to patient’s weight Rare to no HIT Less effect on bones with long term use n Disadvantages n n Expensive Reversal: Protamine incompletely and unpredictably

Direct Xa and Thrombin Inhibitors Injectables

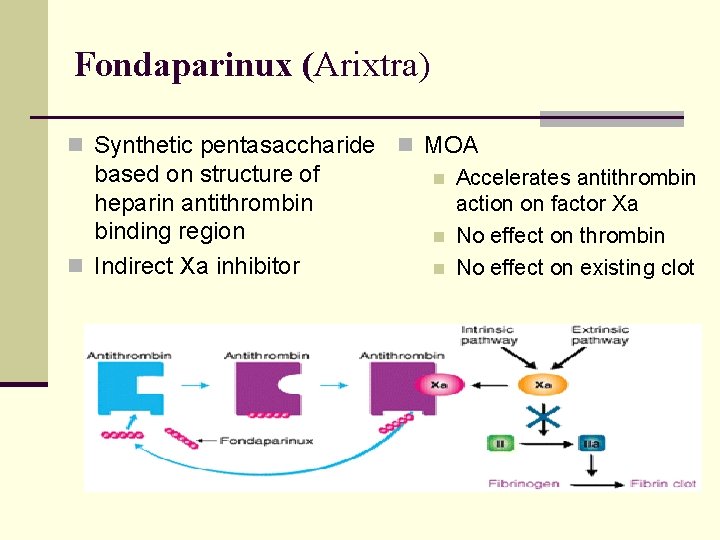
Fondaparinux (Arixtra) n Synthetic pentasaccharide n MOA based on structure of n Accelerates antithrombin heparin antithrombin action on factor Xa binding region n No effect on thrombin n Indirect Xa inhibitor n No effect on existing clot

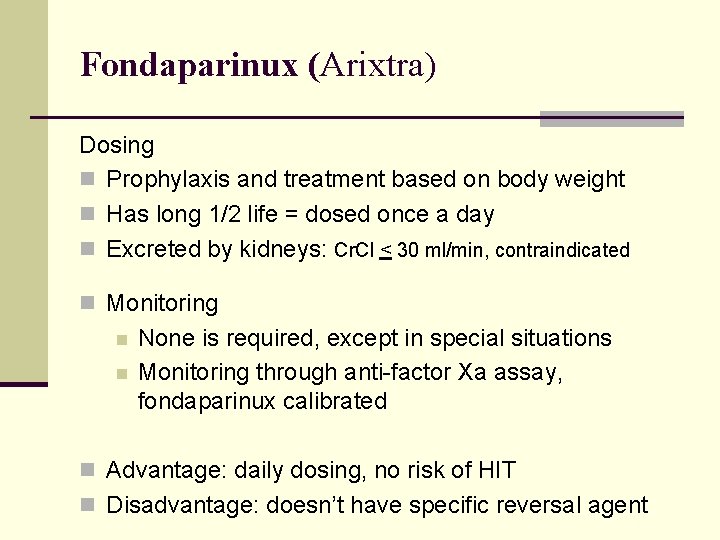
Fondaparinux (Arixtra) Dosing n Prophylaxis and treatment based on body weight n Has long 1/2 life = dosed once a day n Excreted by kidneys: Cr. Cl < 30 ml/min, contraindicated n Monitoring n n None is required, except in special situations Monitoring through anti-factor Xa assay, fondaparinux calibrated n Advantage: daily dosing, no risk of HIT n Disadvantage: doesn’t have specific reversal agent

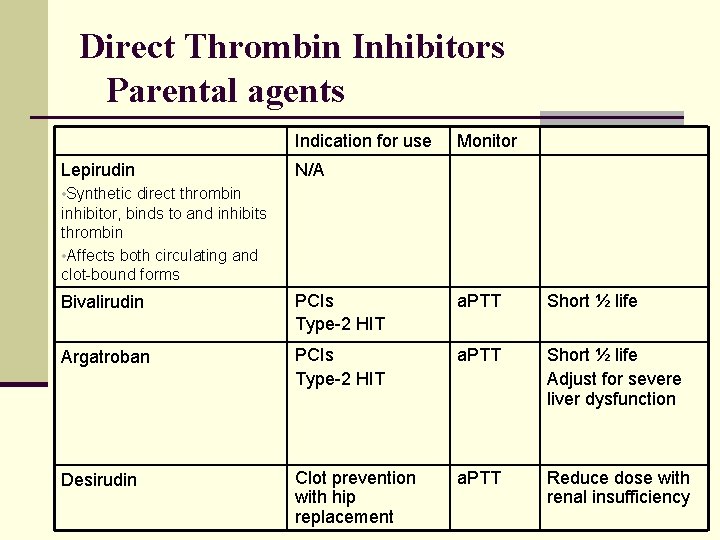
Direct Thrombin Inhibitors Parental agents Indication for use Lepirudin Monitor N/A • Synthetic direct thrombin inhibitor, binds to and inhibits thrombin • Affects both circulating and clot-bound forms Bivalirudin PCIs Type-2 HIT a. PTT Short ½ life Argatroban PCIs Type-2 HIT a. PTT Short ½ life Adjust for severe liver dysfunction Desirudin Clot prevention with hip replacement a. PTT Reduce dose with renal insufficiency

A. S. was sent home on enoxaparin SQ BID and warfarin. What warfarin dose would you start her on? Loading dose? When would you have her follow up?

Warfarin Vitamin K dependent clotting factors

Warfarin n Coumadin ® n Precursor dicoumarol n Introduced as rodenticide in 1948 n MOA n Interferes with n n hepatic synthesis of vitamin K-dependent coagulation factors (II, VII, IX, X) natural anticoagulant proteins C and S

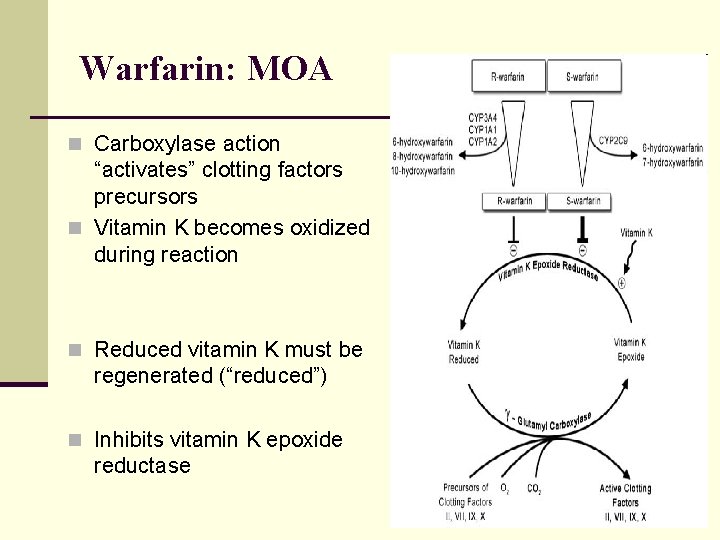
Warfarin: MOA n Carboxylase action “activates” clotting factors precursors n Vitamin K becomes oxidized during reaction n Reduced vitamin K must be regenerated (“reduced”) n Inhibits vitamin K epoxide reductase

Warfarin: OOA Protein VII IX X II Protein C Protein S ~ Half-life (hrs) 6 24 36 50 8 30 n Clotting factors are diminished n Does not “dissolve” pre-existing clot The onset of action of warfarin depends on the elimination half lives of the coagulation proteins

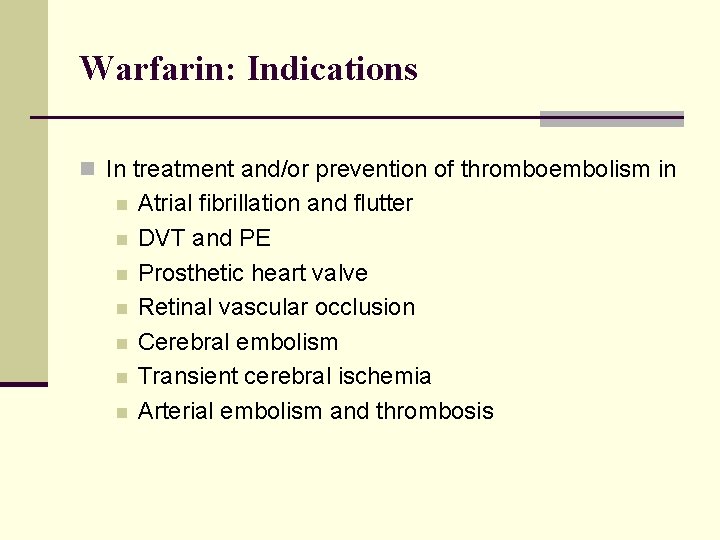
Warfarin: Indications n In treatment and/or prevention of thromboembolism in n n n Atrial fibrillation and flutter DVT and PE Prosthetic heart valve Retinal vascular occlusion Cerebral embolism Transient cerebral ischemia Arterial embolism and thrombosis

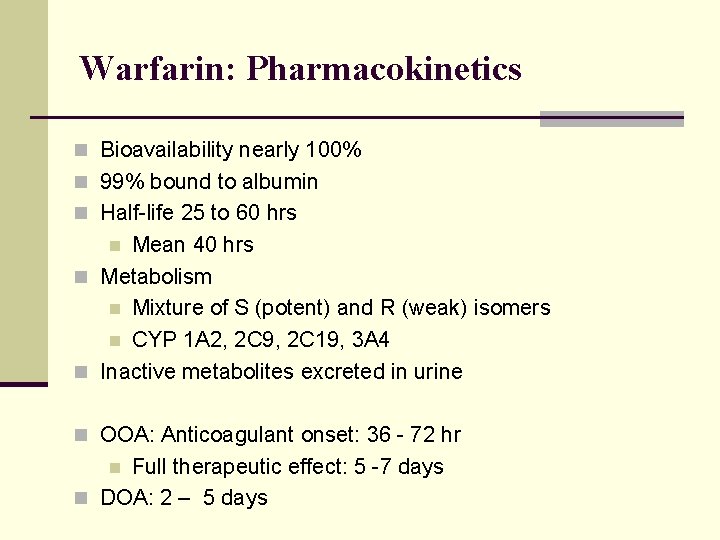
Warfarin: Pharmacokinetics n Bioavailability nearly 100% n 99% bound to albumin n Half-life 25 to 60 hrs Mean 40 hrs n Metabolism n Mixture of S (potent) and R (weak) isomers n CYP 1 A 2, 2 C 9, 2 C 19, 3 A 4 n Inactive metabolites excreted in urine n n OOA: Anticoagulant onset: 36 - 72 hr Full therapeutic effect: 5 -7 days n DOA: 2 – 5 days n

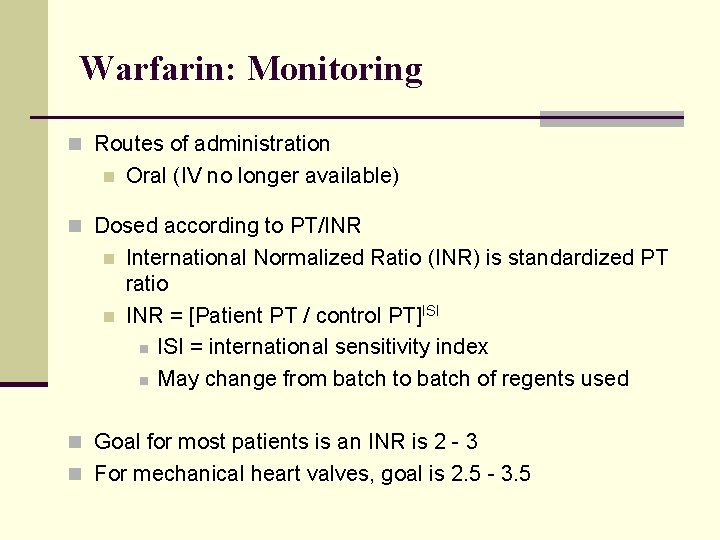
Warfarin: Monitoring n Routes of administration n Oral (IV no longer available) n Dosed according to PT/INR n n International Normalized Ratio (INR) is standardized PT ratio INR = [Patient PT / control PT]ISI n ISI = international sensitivity index n May change from batch to batch of regents used n Goal for most patients is an INR is 2 - 3 n For mechanical heart valves, goal is 2. 5 - 3. 5

Warfarin: Start dose n No loading dose given, most started at 5 mg po n Patients likely to require less dose – start at 2. 5 mg Advanced age, drug interactions, severe liver disease n If switching patients from heparin or LMWH n Start warfarin as soon as possible n Overlap therapeutic warfarin for 24 - 48 hrs n n Baseline PT/INR should be done n Then daily PT/INRs until they are stable n Keep in mind that level reflects dose given 2 -3 days ago

Warfarin: Dose adjustments n Once therapeutic, typically checked q 4 to 6 weeks n Making minor adjustments to the dose n n n Calculate weekly dose Changing the total weekly dose by 10 – 20% Use same strength pills n INR 1. 8/1. 9 or 3. 1/3. 2, consider repeating 1 week n INR 3. 3 - 4. 9, no bleeding: Lower warfarin dose (maybe hold a dose) n INR 5. 0 - 8. 9, no bleeding Hold a warfarin dose or two, restart at lower dose n Consider giving vitamin K, in patients at high risk of bleeding. n INR > 8. 9, no bleeding n Stop warfarin, give vitamin K n Restart at lower dose when INR < 3. 1 n

Warfarin: Dose adjustments n With impending surgery or procedure n Historically enoxaparin bridge was used n n Recent study showed more harm (more bleeds) None needed in low/moderate risk atrial fib patients Stop 5 days before procedure, restart later that day Needed in high risk patients n n Mechanical heart valves Venous thromboembolism within 3 months Atrial fib patient with stroke within 3 months A fib patient with CHA 2 DS 2 -VASc score > 6

Warfarin: Contraindications n Pregnancy - Fetal demise n Bleeding dyscrasias n Severe uncontrolled hypertension n History of warfarin hypersensitivity or skin necrosis n Alcoholism n Unreliable or noncompliant patient

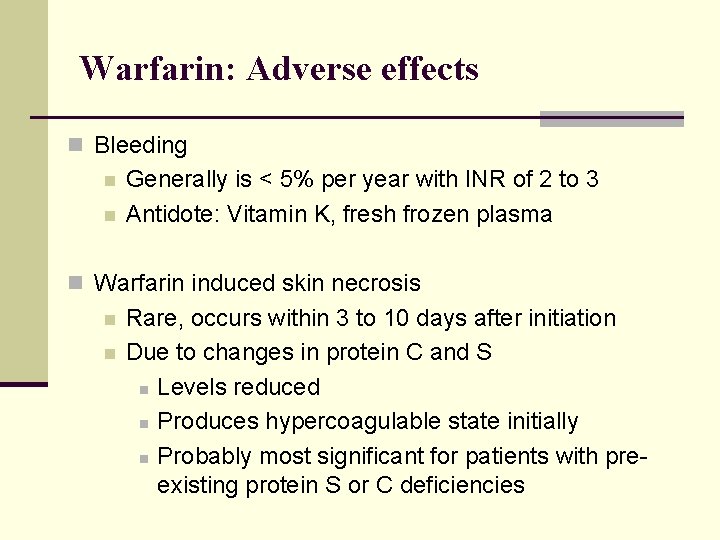
Warfarin: Adverse effects n Bleeding n n Generally is < 5% per year with INR of 2 to 3 Antidote: Vitamin K, fresh frozen plasma n Warfarin induced skin necrosis n n Rare, occurs within 3 to 10 days after initiation Due to changes in protein C and S n Levels reduced n Produces hypercoagulable state initially n Probably most significant for patients with preexisting protein S or C deficiencies

Warfarin: Drug interactions n MANY, MANY n Always check for drug interactions BEFORE starting any drug in patients on warfarin therapy n Pharmacokinetic: Other highly protein bound drugs n n Fluoxetine Statins (not pravastatin) Sulfonylureas Phenytoin

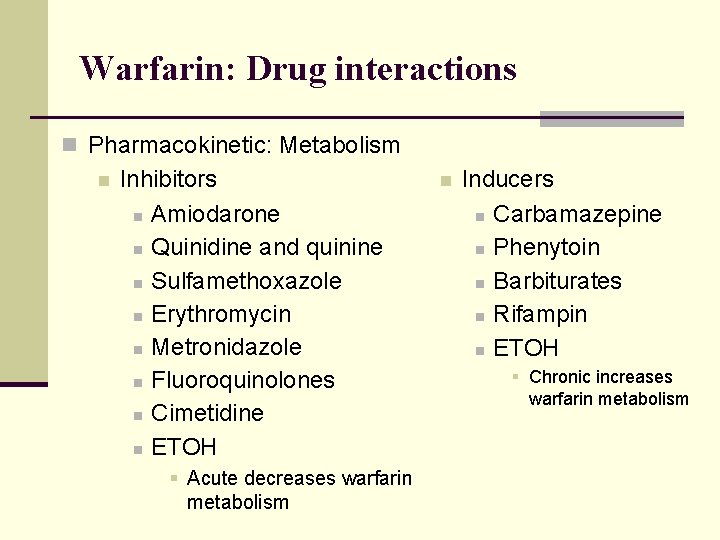
Warfarin: Drug interactions n Pharmacokinetic: Metabolism n Inhibitors n Amiodarone n Quinidine and quinine n Sulfamethoxazole n Erythromycin n Metronidazole n Fluoroquinolones n Cimetidine n ETOH § Acute decreases warfarin metabolism n Inducers n Carbamazepine n Phenytoin n Barbiturates n Rifampin n ETOH § Chronic increases warfarin metabolism

Warfarin: Interactions n Pharmacodynamic interactions n Drugs effecting vitamin K metabolism n n n Ie antibiotics Any drug that inhibits platelets or anticoagulants Drugs that might cause bleeding n NSAIDS or aspirin, GI irritation

Warfarin: Dietary Interactions n Pharmacokinetic Grapefruit juice n Pharmacodynamic n Foods affecting vitamin K levels n OK to eat, but consistently n Lettuce n Asparagus n Cauliflower n Turnip greens n Brussels sprouts n Spinach n Cabbage n Broccoli n Watercress n Green tea n

Warfarin n Advantages n n n Inexpensive oral agent Effective anticoagulant oral agent Ambulatory population n Disadvantages n n Unpredictable dose response curve Frequent monitoring Many drug interactions Many dietary interactions

Warfarin: Dose adjustments n Make minor adjustments to the dose n n Calculate weekly dose Changing the total weekly dose by 10 – 20% 2 months later A. S. ’s INR = 3. 7 She is taking 5 mg tablets: ½ tab daily except 1 tab on Tuesdays and Thursdays What is her weekly dose? How much would you change her dose by?

Direct Xa and Thrombin Inhibitors Oral Agents

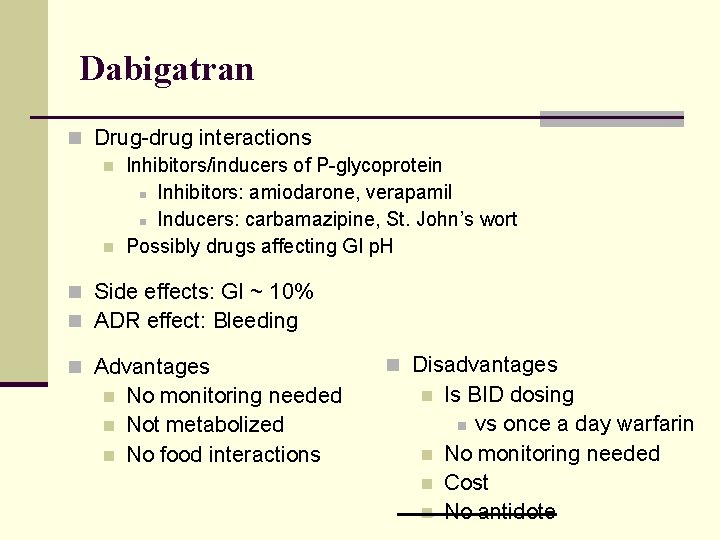
Oral Direct Thrombin Inhibitor n Dabigatran (Pradaxa) n Direct competitive thrombin inhibitor Decrease conversion of fibrinogen n Decrease self-amplification n Decrease in platelet activation n Can bind to both free and bound thrombin n n Indications n Thromboembolism prevention n Stroke prevention in non-valvular atrial fibrillation DVT/PE prevention and treatment Not indicated in patients with artificial valves

Dabigatran n Pharmacokinetics n Bioavailabilty: 6% (needs acidic environment) n n n Protein binding: 34 -35% Metabolism: no CYP 450! n n n Absorption dependent on P-glycoproteins Is a prodrug, activated by esterases in liver Excretion: 85% unchanged in urine ½ life 14 -17 hrs n Dosing (but depends on Cr. Cl) n n Treatment dosing, BID Prevention dosing (post ortho surgery), daily

Dabigatran n Drug-drug interactions n Inhibitors/inducers of P-glycoprotein n Inhibitors: amiodarone, verapamil n Inducers: carbamazipine, St. John’s wort n Possibly drugs affecting GI p. H n Side effects: GI ~ 10% n ADR effect: Bleeding n Advantages n n n No monitoring needed Not metabolized No food interactions n Disadvantages n n Is BID dosing n vs once a day warfarin No monitoring needed Cost No antidote

Praxbind (Idarucizumab) n Monoclonal antibody to dabigatran only, not Xa inhibitors n IV formulation only n Approval for reversal in Emergent or urgent surgery or procedure n Life-threatening or uncontrolled bleeding n Reversal takes ~ 4 hrs; bleeding stops ~ 11 hrs n Not needed if last dose was > 48 hours n n Issues n n n Now leaves patient unprotected from clot risk Not clear when to restart anticoagulant Doesn’t reverse any damage from bleed

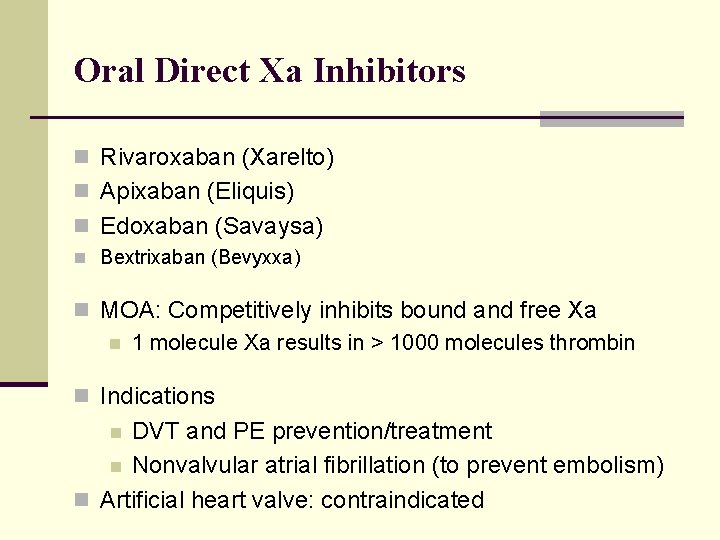
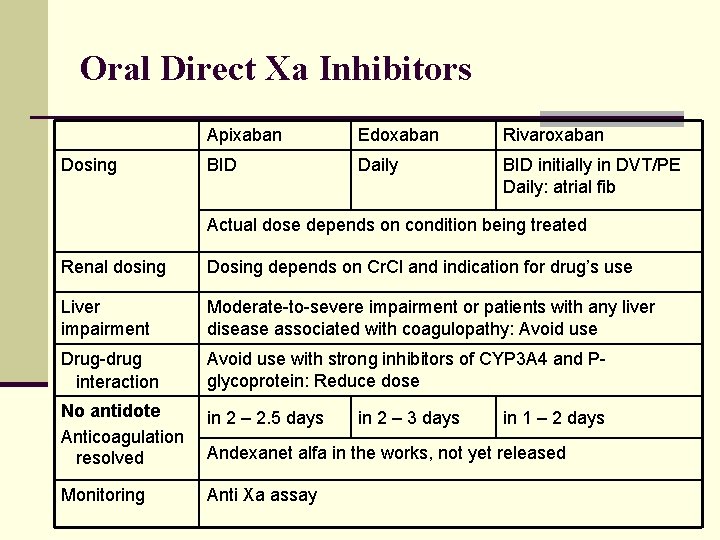
Oral Direct Xa Inhibitors n Rivaroxaban (Xarelto) n Apixaban (Eliquis) n Edoxaban (Savaysa) n Bextrixaban (Bevyxxa) n MOA: Competitively inhibits bound and free Xa n 1 molecule Xa results in > 1000 molecules thrombin n Indications DVT and PE prevention/treatment n Nonvalvular atrial fibrillation (to prevent embolism) n Artificial heart valve: contraindicated n

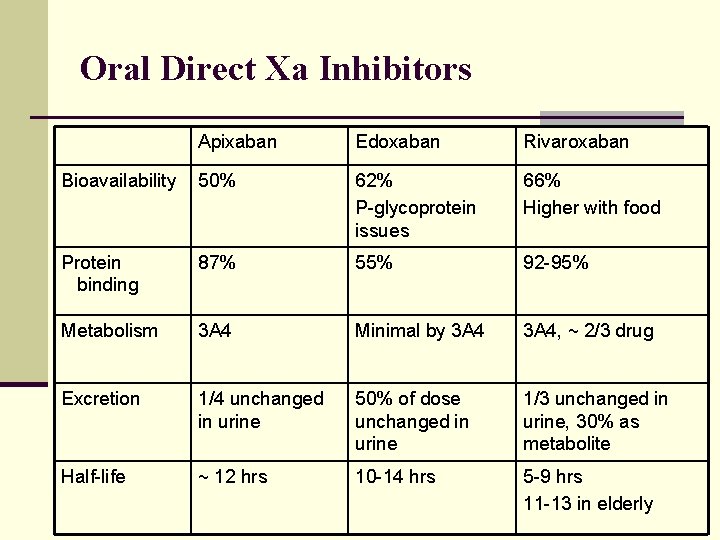
Oral Direct Xa Inhibitors Apixaban Edoxaban Rivaroxaban Bioavailability 50% 62% P-glycoprotein issues 66% Higher with food Protein binding 87% 55% 92 -95% Metabolism 3 A 4 Minimal by 3 A 4, ~ 2/3 drug Excretion 1/4 unchanged in urine 50% of dose unchanged in urine 1/3 unchanged in urine, 30% as metabolite Half-life ~ 12 hrs 10 -14 hrs 5 -9 hrs 11 -13 in elderly

Oral Direct Xa Inhibitors Dosing Apixaban Edoxaban Rivaroxaban BID Daily BID initially in DVT/PE Daily: atrial fib Actual dose depends on condition being treated Renal dosing Dosing depends on Cr. Cl and indication for drug’s use Liver impairment Moderate-to-severe impairment or patients with any liver disease associated with coagulopathy: Avoid use Drug-drug interaction Avoid use with strong inhibitors of CYP 3 A 4 and Pglycoprotein: Reduce dose No antidote Anticoagulation resolved in 2 – 2. 5 days Monitoring Anti Xa assay in 2 – 3 days in 1 – 2 days Andexanet alfa in the works, not yet released

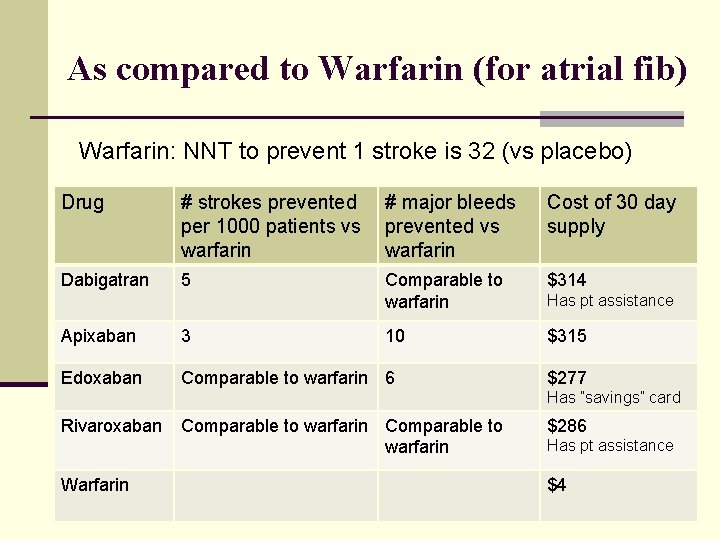
As compared to Warfarin (for atrial fib) Warfarin: NNT to prevent 1 stroke is 32 (vs placebo) Drug # strokes prevented per 1000 patients vs warfarin # major bleeds prevented vs warfarin Cost of 30 day supply Dabigatran 5 Comparable to warfarin $314 10 $315 Apixaban 3 Edoxaban Comparable to warfarin 6 Has pt assistance $277 Has ”savings” card Rivaroxaban Warfarin Comparable to warfarin $286 Has pt assistance $4

Black Box Warning With all anticoagulants: there is an increased risk of spinal / epidural hematoma in patients undergoing epidural or spinal anesthesia or spinal puncture

Questions?
 Anticoagulant drugs
Anticoagulant drugs Warfarin strengths and colors
Warfarin strengths and colors Anticoagulant examples
Anticoagulant examples Anticoagulant
Anticoagulant Heparin anticoagulant
Heparin anticoagulant Anticoagulant
Anticoagulant Types of anticoagulants
Types of anticoagulants An anticoagulant is a substance that prevents
An anticoagulant is a substance that prevents Thrombolytic vs anticoagulant
Thrombolytic vs anticoagulant Chamberlain practicum coordinator
Chamberlain practicum coordinator Uca dnp program
Uca dnp program Bpun unal
Bpun unal Sicodis sgp 2022
Sicodis sgp 2022 Bronchoconstrictor drugs uses
Bronchoconstrictor drugs uses Gillian jessurun overleden
Gillian jessurun overleden Dr gillian jessurun
Dr gillian jessurun Andrew unsworth
Andrew unsworth Gillian ryan model
Gillian ryan model Gillian dell
Gillian dell Gillian roehrig
Gillian roehrig Gillian hazell
Gillian hazell Gillian lord uf
Gillian lord uf Gillian swan
Gillian swan Nnn captions
Nnn captions The egan report
The egan report Esli osmanlliu
Esli osmanlliu Gillian unsworth
Gillian unsworth Gillian reed
Gillian reed Gillian leng
Gillian leng Gillian gunn
Gillian gunn Miracle on st david's day
Miracle on st david's day Dr. gillian kernaghan
Dr. gillian kernaghan Gillian hamilton
Gillian hamilton Gillian plant
Gillian plant Csos recovery dell
Csos recovery dell Gillian anderson manager
Gillian anderson manager Gillian dennehy
Gillian dennehy Gillian foulger
Gillian foulger Carla brodley
Carla brodley Mem8000
Mem8000 Tufts navigator gym reimbursement
Tufts navigator gym reimbursement Lionel zupan
Lionel zupan Tufts anesthesia residency
Tufts anesthesia residency Norepinephrine
Norepinephrine Enamel tufts
Enamel tufts Ilvs tufts
Ilvs tufts Enamel lamellae types
Enamel lamellae types Tufts blast
Tufts blast Tufts tusk
Tufts tusk Borders
Borders Parasympathomimetic drugs
Parasympathomimetic drugs And the drink and the drugs won't flush him out analysis
And the drink and the drugs won't flush him out analysis Drugs that affect basic driving skills are
Drugs that affect basic driving skills are Anti diarrheal drugs list
Anti diarrheal drugs list Themes about drugs
Themes about drugs