FLUID AND ELECTROLYTE MANAGEMENT in paediatrics Dr Audu




















![Severe hyponatraemia • Serum [Na+] <121 m. Eq/L • Lethargy and seizures • Correct Severe hyponatraemia • Serum [Na+] <121 m. Eq/L • Lethargy and seizures • Correct](https://slidetodoc.com/presentation_image_h/0aab78e8aaecde3bebf5a4fa6803e913/image-30.jpg)



- Slides: 35

FLUID AND ELECTROLYTE MANAGEMENT in paediatrics Dr Audu Lamidi Isah Chief Consultant Peadiatrician © NITMED TUTORIALS

Objectives • Understand Normal Fluid and electrolyte distribution and homeostasis • Derangement in Fluid and electrolyte balance • Severity and types of dehydration • Fluid and electrolyte therapy in children

Introduction • Total body water (TBW) changes from birth up to the age of 1 year. • At birth TBW constitute 80% of total body weight and this slowly decreases to 60% at one year. • TBW in adult is about 60% of body weight • Baby loses weight (5 -10%) in the first week of life, mainly from body fluid.

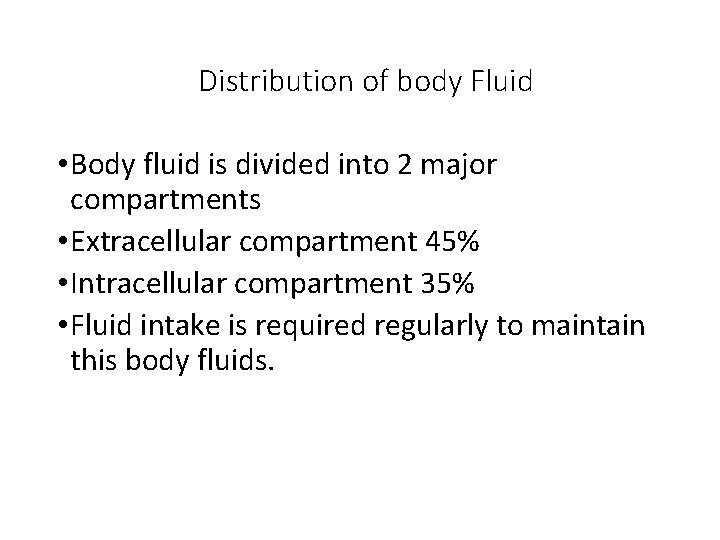
Distribution of body Fluid • Body fluid is divided into 2 major compartments • Extracellular compartment 45% • Intracellular compartment 35% • Fluid intake is required regularly to maintain this body fluids.

Body fluid and electrolyte distribution • ECF (intravascular and interstitial) • Na+ is Principal ECF cation while Cl - and HCO 3 - are principal anions • Regulation of ECF is primarily related to Na balance; total body Na affects ECF vol • ICF: water within the cells. • Principal cation is K. and anion = HPO 4 -2 • ICF and ECF separated by cell membrane permeable to H 2 O but not to solutes

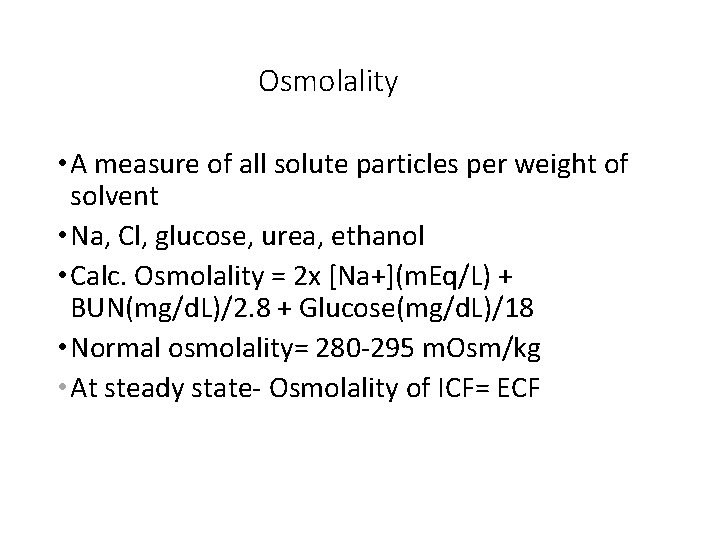
Osmolality • A measure of all solute particles per weight of solvent • Na, Cl, glucose, urea, ethanol • Calc. Osmolality = 2 x [Na+](m. Eq/L) + BUN(mg/d. L)/2. 8 + Glucose(mg/d. L)/18 • Normal osmolality= 280 -295 m. Osm/kg • At steady state- Osmolality of ICF= ECF

The need for fluids and electrolytes §Routinely: Fluids and electrolytes are required on daily basis to maintain optimal body functions §Therapeutically administered fluids become necessary in the following conditions: 1. Absence or diminished oral fluid intake (surgical or critically ill patients or patients with diminished drive for food) 2. Increased fluid losses- e. g. Patients with vomiting, diarrhoea, polyuria as in diabetic ketoacidosis, diabetes insipidus, etc

Goals of Body Fluid and Electrolyte Maintenance • Prevent dehydration • Prevent electrolyte disorders • Prevent ketoacidosis • Prevent protein degradation

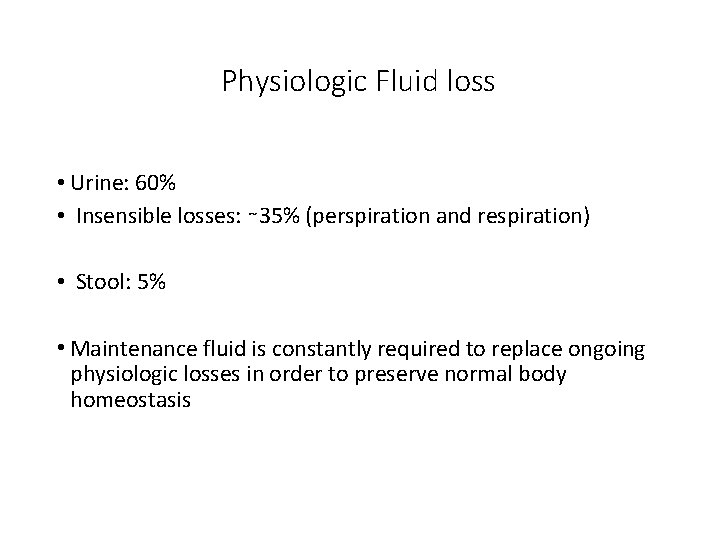
Physiologic Fluid loss • Urine: 60% • Insensible losses: ∼ 35% (perspiration and respiration) • Stool: 5% • Maintenance fluid is constantly required to replace ongoing physiologic losses in order to preserve normal body homeostasis

Pathologic loss of fluid • Significant amount of fluid is lost (at least 5%) • May be mild moderate or severe • When severe, it may compromise tissue perfusion • It is important to be able to identify signs and define the level of dehydration in patients that have lost significant amount of fluid

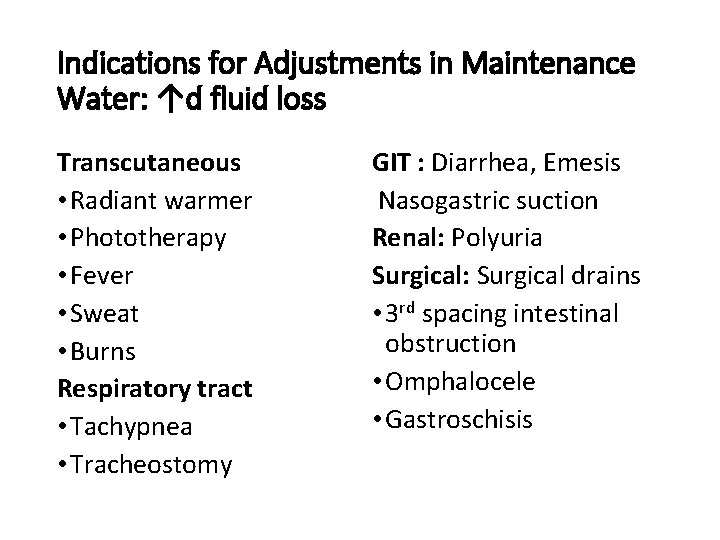
Indications for Adjustments in Maintenance Water: ↑d fluid loss Transcutaneous • Radiant warmer • Phototherapy • Fever • Sweat • Burns Respiratory tract • Tachypnea • Tracheostomy GIT : Diarrhea, Emesis Nasogastric suction Renal: Polyuria Surgical: Surgical drains • 3 rd spacing intestinal obstruction • Omphalocele • Gastroschisis

Fluid therapy • Goal: To achieve Normal volume and electrolyte composition of body fluids • Consists of : Maintenance, Deficit and Ongoing losses. • Note that the body compensates for excess water intake by DIURESIS and that this mechanism may be inhibited by inappropriate ADH secretion in certain situations: critical illnesses such as Perinatal Asphyxia, peri-operatively. Cautious fluid therapy is required to prevent cerebral oedema.

Maintenance • Replacement of physiologic losses(sweat, urine, respiration, stools) to preserve homeostasis. • The volume may vary when there are changes in the clinical status of the patient. • Children need higher maintenance fluids than adults because of higher metabolic rate, higher body surface area: weight ratio and higher respiratory rate.

Maintenance fluid therapy contd • Holliday-Segar formula for calculating maintenance fluid: ØFirst 10 kg: 100 mls/kg/day (4 ml/kg/hr) ØSecond 10 kg: 50 mls/kg/day (2 ml/kg/hr) ØEvery kg thereafter: 20 mls/kg/day (1 ml/kg/hr)

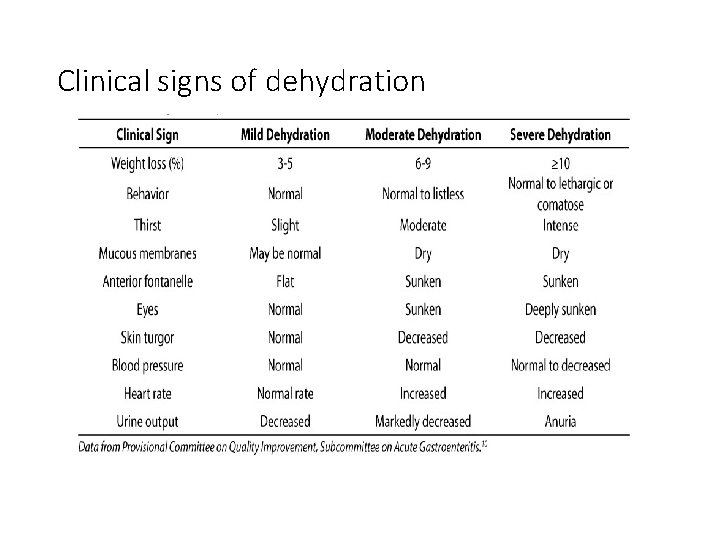
Repletion (deficit) therapy • This refers to the correction of established losses to return the body to normal fluid and electrolyte status. • Usually due to diarrhea/vomiting, traumatic haemorrhage, or inadequate intake. • Requires an initial assessment: ØClinical signs correlate with degree of dehydration: mild, moderate, severe ØBiochemical tests type of dehydration

Clinical signs of dehydration

Types of Dehydration • Sodium is the most important electrolyte in defining type of dehydration • Isotonic (Na level 135 -145 mmol/L): This is the most commonly encountered type • Hypotonic/hyponatraemic <135 mmol/L • Hypernatraemic (Na >145 mmol/l): least commonly encountered but most difficult to identify because of the peculiar clinical signs.

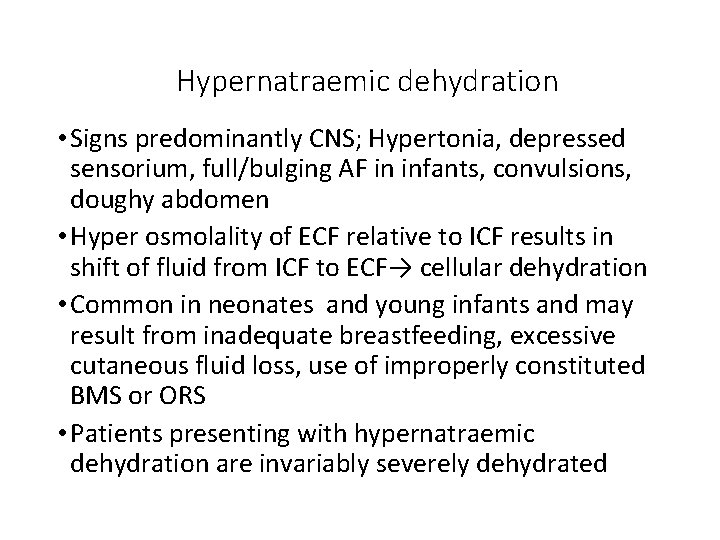
Hypernatraemic dehydration • Signs predominantly CNS; Hypertonia, depressed sensorium, full/bulging AF in infants, convulsions, doughy abdomen • Hyper osmolality of ECF relative to ICF results in shift of fluid from ICF to ECF→ cellular dehydration • Common in neonates and young infants and may result from inadequate breastfeeding, excessive cutaneous fluid loss, use of improperly constituted BMS or ORS • Patients presenting with hypernatraemic dehydration are invariably severely dehydrated

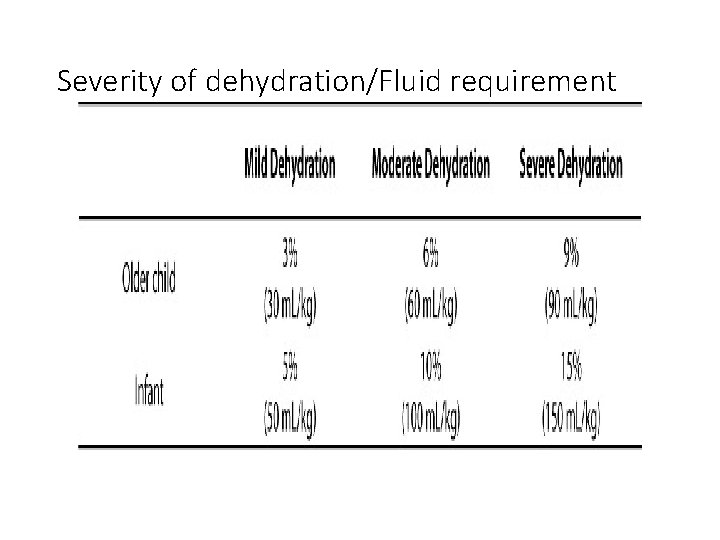
Severity of dehydration/Fluid requirement

Mild to moderate dehydration • Oral rehydration is usually adequate • Available fluids contain calories (carbohydrate 140 mml/l), Sodium (45 mmol/l), Potassium (20 mmol/L) • Requires 50 -75 mls/Kg and this can be given over 4 -8 hours • Supervise administration and monitor closely

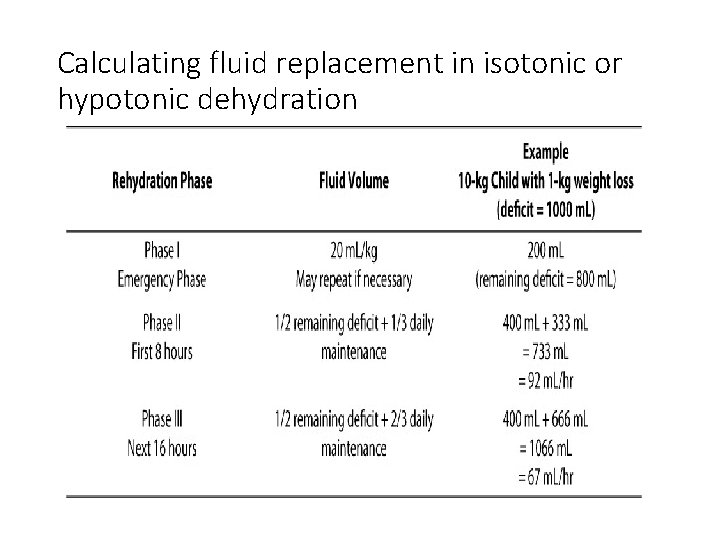
Severe dehydration • Fluid correction in severe dehydration is effected in phases: total deficit 100 mls/kg • Phase 1: Øto expand extravascular volume for adequate tissue perfusion. ØIsotonic solution is used (Normal saline or Ringers lactate) ØGive as 20 mls/kg over 1 hour. This may need to be repeated.

Severe dehydration contd • Phase 2: 1. Subtract phase 1 fluid from total deficit 2. Give ½ of what remains over 8 hours 3. This is added to 1/3 of the daily maintenance 4. Fluid type for phases 2 : 5% Dextrose in ½ N/Saline (. 45 Saline)

• Phase 3: 1. give 2 nd ½ over 16 hours 2. This is added to 2/3 of daily maintenance 3. Fluid type 4. 3%D in. 18 Saline 4. Add 20 -30 m. Eq/L of potassium (ensure patient has made urine before adding K+1 ) beginning from phase 2

Calculating fluid replacement in isotonic or hypotonic dehydration

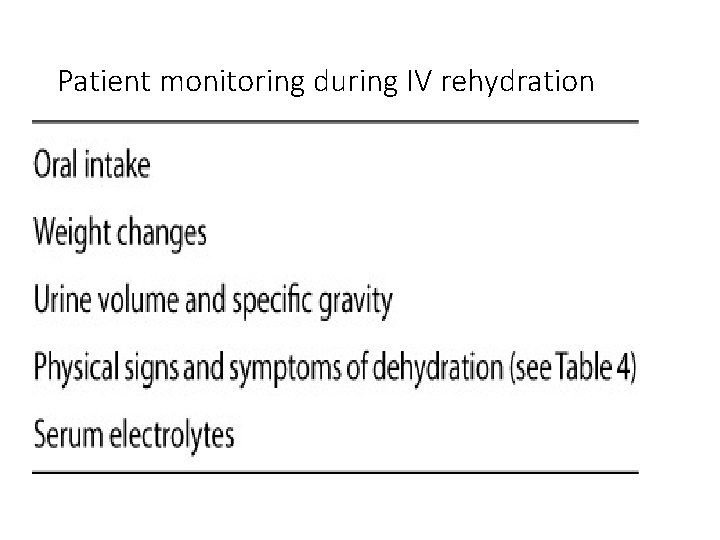
Patient monitoring during IV rehydration

Hypernatraemic dehydration • Initial step as for severe dehydration for ECF expansion: 20 mls/kg over 1 hour • The remaining deficit fluid volume is added to the maintenance fluid volume for 48 hours, • Total should be administered over 48 hours;

hypernatraemia • administering the deficit fluid too rapidly causes osmotic fluid shifts which can result in 1. cerebral edema, 2. haemorrhge and 3. convulsions. • Serum sodium should be corrected by no more than 10 m. Eq/L/day. • Use hypotonic fluid such as 4. 5% dextrose with 0. 18% saline for rehydration

Medically significant ongoing fluid loss • Examples of clinical situations where replacement fluids are needed include 1. patients with chest tubes in place, 2. uncontrolled vomiting, 3. continuing diarrhea, or 4. externalized cerebrospinal fluid shunts • These losses can not be met by the correction of established deficit at admission • Correction is based on estimated fluid loss

Electrolyte considerations • Electrolyte replacement in intravenous fluids generally includes: • sodium: 3 m. Eq/kg/ day • Potassium: 2 m. Eq/kg/day • Chloride: 5 m. Eq/kg/day usually met by administering sodium and potassium as sodium chloride and potassium chloride salts. • Kidney status should always be considered before administering fluid containing potassium.
![Severe hyponatraemia Serum Na 121 m EqL Lethargy and seizures Correct Severe hyponatraemia • Serum [Na+] <121 m. Eq/L • Lethargy and seizures • Correct](https://slidetodoc.com/presentation_image_h/0aab78e8aaecde3bebf5a4fa6803e913/image-30.jpg)
Severe hyponatraemia • Serum [Na+] <121 m. Eq/L • Lethargy and seizures • Correct with hypertonic saline 3% saline +] – current [Na+] X [ • Sodium deficit (SD) =desired [Na 0. 6 X Wt (kg). • Volume of 3%Nacl required = SD X 2 • Give over a few hours and monitor serum [Na+]; avoid rapid increase beyond 12 m. Eq/day

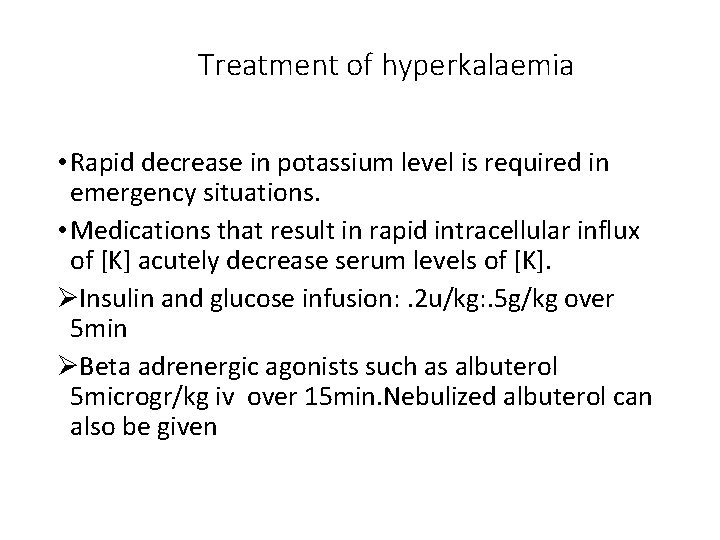
Hyperkalaemia • Serum Potassium level > 6 m. Eq/L • Common in severe dehydration with reduced renal perfusion • Always rule out falsely elevated serum K before commencement of therapy • There are multiple mechanisms for decreasing serum potassium in hyperkaelaemia, • Medications are chosen based upon their mechanism and the level of urgency of the clinical situation

Treatment of hyperkalaemia • Rapid decrease in potassium level is required in emergency situations. • Medications that result in rapid intracellular influx of [K] acutely decrease serum levels of [K]. ØInsulin and glucose infusion: . 2 u/kg: . 5 g/kg over 5 min ØBeta adrenergic agonists such as albuterol 5 microgr/kg iv over 15 min. Nebulized albuterol can also be given

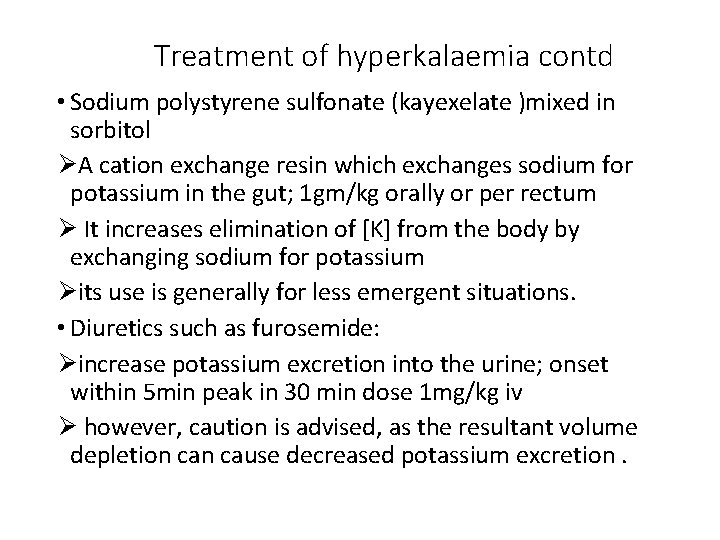
Treatment of hyperkalaemia contd • Sodium polystyrene sulfonate (kayexelate )mixed in sorbitol ØA cation exchange resin which exchanges sodium for potassium in the gut; 1 gm/kg orally or per rectum Ø It increases elimination of [K] from the body by exchanging sodium for potassium Øits use is generally for less emergent situations. • Diuretics such as furosemide: Øincrease potassium excretion into the urine; onset within 5 min peak in 30 min dose 1 mg/kg iv Ø however, caution is advised, as the resultant volume depletion cause decreased potassium excretion.

Treatment of hyperkalaemia contnd • Calcium gluconate: Ø is used in symptomatic patients for cardio-protective effects. Ø as it antagonizes the membrane effects of potassium. For life threatening hyperkalaemia • Renal replacement therapy; haemodyalysis or peritoneal dialysis. • Sodium bicarbonate: this is useful in patients with metabolic acidosis. It causes intracellular transfer of potassium as well as increased tubular excretion of potassium. Dose 1 -2 meq/kg iv over 5 -15 min

END • THANK YOU