Enzymes Coenzymes And Energy Nutrients Nutrients are molecules

























- Slides: 30

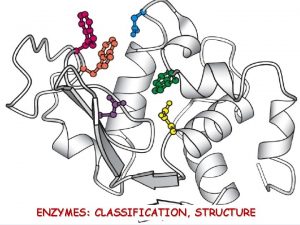
Enzymes, Coenzymes, And Energy

Nutrients • Nutrients are molecules required by organisms for growth, reproduction, or repair. • Nutrients are a source of energy. • Nutrients are a source of the building blocks for new molecules in the body.

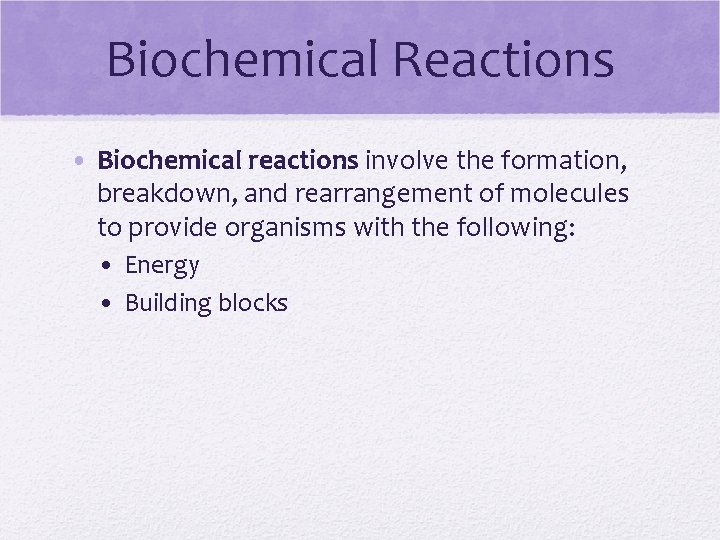
Biochemical Reactions • Biochemical reactions involve the formation, breakdown, and rearrangement of molecules to provide organisms with the following: • Energy • Building blocks

Activation Energy • Activation Energy is the amount of energy input required to get the reaction started. • Random chemical processes would take millions of years to break down a candy bar and release its energy. • We can increase the rate of a chemical reaction by increasing its temperature. • Cellular proteins become denatured at high temperatures.


Catalyst • A catalyst is a chemical that speeds up the rate of the chemical reaction. • Catalysts lower the amount of activation energy required to start a reaction. • The catalyst is not used up in the chemical reaction. • The catalyst is unchanged when the chemical reaction is complete. • The cell manufactures specific proteins to act as catalysts. • An Enzyme is a protein molecule that acts as a catalyst to speed the rate of a chemical reaction. • The DNA regulates which enzymes are produced by the cell.

Formation Of Enzymes • The DNA guides the production of the enzyme. • A specific sequence of amino acids is linked together at the ribosomes. • The chain of amino acids folds and twists to form a particular three-dimensional shape.

Enzyme-Substrate Complex Formation • The specific three-dimensional shape, size, and charge of the enzyme allows it to combine with a specific reactant. • This combination lowers the activation energy required for chemical reaction. • The enzyme physically fits with the reactant.

Enzyme-Substrate Complex Formation • The substrate is the molecule (reactant) to which the enzyme attaches itself. • A new, temporary molecule called the enzymesubstrate complex is formed. • Enzymes are specific to certain substrate molecules because their shape can only combine with specific parts of certain molecules.


Binding Site • The binding site or attachment site is the specific location on the enzyme where the substrate attaches.

Enzymes • Enzymes can be used again and again. • Eventually they break down and new ones need to be synthesized. • Enzymes have a particular surface geometry that matches the geometry of their respective substrates.

Induced Fit Hypothesis • The induced fit hypothesis states that enzymes can fold to fit the substrate. • The enzyme molds or adjusts itself to fit the substrate when the two come into contact with each other.

Active Site • The active site on the enzyme is the place that causes a specific part of a substrate to change. • Chemical bonds are either formed or broken here. • Activation energy is lowered at this site. • Electrons are shifted to change the bonds.


Naming Enzymes • The 1 st part of an enzymes name is usually the name of the molecule to which it can become attached. • The 2 nd part of an enzymes name tells us the type of reaction it facilitates. • The 3 rd part of an enzymes name is “-ase”. This indicates that the molecule is an enzyme.

Naming Enzymes • Examples: • Glycogen synthetase. • DNA polymerase. • Amylose hydrolase (amylase)

Cofactors • Some enzymes need an additional molecule to help them function. • Cofactors are either inorganic or organic molecules that help enzymes. • Ions such as zinc, iron, and magnesium assist enzymes. • A Coenzyme is an organic molecule that acts as a cofactor.

Vitamins • Vitamins are organic molecules that are used to make certain coenzymes. • Vitamins help to regulate gene action. • Vitamins are either water soluble or fat soluble (A, D, E, K) • We are not able to manufacture vitamins; therefore, it is necessary to obtain them from our diet.

Turnover Number • The turnover number is the number of molecules of substrate with which a single enzyme molecule can react in a given time.

Turnover Number • Some enzymes can perform incredible numbers of reactions in a single minute. • Enzymes can facilitate chemical reactions anywhere from one thousand (103) to ten thousand trillion (1016) times faster than uncatalyzed reactions)

Factors That Affect Enzyme Function • Temperature • Increased temperature increases molecular motion and therefore the number of reactions. • Temperature that is too high will denature the protein structure of the enzyme and slow or even stop the chemical reaction. • p. H determines how many hydrogen ions are present and will attach the enzyme side chains.

Factors That Affect Enzyme Function • The positively charged hydrogen ions will affect the reactivity of the enzyme. • Many enzymes function best at a neutral p. H of around 7; however, some work better in more acidic or more alkaline environments. • Pepsin – works well in stomach acid p. H 1. 5 to 2. 2

Factors That Affect Enzyme Function • Arginase – works well in basic p. H of 9. 5 – 9. 9 • Enzyme-Substrate concentration • If all of the enzymes are occupied, the reaction time will be limited.

Enzymatic Competition • Enzymatic competition occurs when there are several kinds of enzymes available to combine with the same kind of substrate molecule • Different enzymes have different effects upon the same substrate.

Gene Regulation • Gene regulator proteins are chemical messengers that inform the genes of the cell’s need for enzymes. • Gene-repressor proteins decrease protein production. • Gene-activator proteins increase protein production.

Inhibition • An inhibitor is a molecule that attaches itself to an enzyme and interferes with that enzyme’s ability to form an enzyme-substrate complex.

Competitive Inhibition • Some inhibitors have a shape that closely resembles that of the substrate. • They compete with the substrate for binding sites.

Negative Feedback Inhibition • Certain end product molecules with stop the enzyme from functioning. • As the concentration of end product increases, it slows the rate of reaction of the enzyme, which ultimately decreases the rate of the reaction.

 Mikael ferm
Mikael ferm Organic molecules vs inorganic molecules
Organic molecules vs inorganic molecules An example of prosthetic group
An example of prosthetic group Ariboflavinosis adalah
Ariboflavinosis adalah Thiamin
Thiamin Preparatory reaction inputs outputs
Preparatory reaction inputs outputs Coenzymes
Coenzymes Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Radiation examples
Radiation examples Kinetic energy of gas molecules
Kinetic energy of gas molecules Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes Galactosemia
Galactosemia Non functional plasma enzyme example
Non functional plasma enzyme example Enzymes in plasma
Enzymes in plasma Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes What is the enzyme of small intestine
What is the enzyme of small intestine Enzymes and lactose intolerance lab answers
Enzymes and lactose intolerance lab answers List of enzymes and their functions
List of enzymes and their functions What are enxymes
What are enxymes Enzyme characteristics
Enzyme characteristics Buprenorphine and elevated liver enzymes
Buprenorphine and elevated liver enzymes Unit 2 physiology of fitness
Unit 2 physiology of fitness Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes Relationship between atoms and molecules
Relationship between atoms and molecules Cecil writes the equation for the reaction of hydrogen
Cecil writes the equation for the reaction of hydrogen Solid
Solid Substance
Substance Chiral achiral
Chiral achiral How can you count atoms and molecules
How can you count atoms and molecules Similarities between polar and nonpolar molecules
Similarities between polar and nonpolar molecules