ENTEROPATHOGENIC ESCHERICHIA COLI I Hinchung Wong Department of
































- Slides: 55

ENTEROPATHOGENIC ESCHERICHIA COLI I Hin-chung Wong Department of Microbiology Soochow University

Content Ø INTRODUCTION Ø HEAT-LABILE ENTEROTOXINS p p p General Characteristics Gene and Regulation Mode of Action Ø HEAT-STABLE ENTEROTOXINS p p General Characteristics Mode of Action and Regulation Ø ENTEROTOXIN PLASMIDS p p p SHIGA-LIKE TOXINS Purification and Structure Mode of Action Production and Regulation Genetics Role in Disease Ø HEMOLYSINS Ø Production and Purification p p p Characteristics Genetics Role in Virulence and Mode of Action

Content Ø ADHERENCE p p p In Enterotoxigenic E. coli In Enteropathogenic E. coli In Enterohemorrhagic E. coli Ø INVASIVENESS Ø DETECTION p p p p Using glucuronidase assay Animal Tissue Culture Animal Assays Immunological Methods Enzymatic bio-nanotransduction Nucleic Acid Probes Using polymerase chain reaction

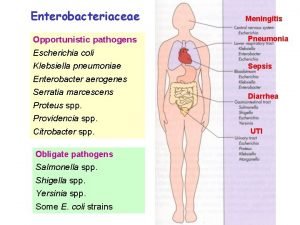
INTRODUCTION Ø E. coli is usually considered to be an opportunistic pathogen which constitutes a large portion of the normal intestinal flora of humans Ø This organism can, however, contaminate, colonize, and subsequently cause infection of extraintestinal sites and is a major cause of septicemia, peritonitis, abscesses, meningitis, and urinary tract infections in humans

INTRODUCTION Ø E. coli was first incriminated as an enteropathogen in 1945, responsible for an outbreak of infantile diarrhea Ø Enteropathogenic E. coli (EEC) has been associated with diarrhea in developing countries and localities having poor sanitation Ø In the developed countries, EEC has been historically associated primarily with infantile diarrhea, but it was later recognized that adults also may suffer from the illness Ø E. coli are also enteropathogenic in animals

INTRODUCTION Ø E. coli O 157: H 7 causes severe illneses (hemorrhagic) and it does possess distinguishing characteristics, e. g. does not ferment sorbitol within 24 h, does not possess α-glucuronidase activity, and does not grow well at all at 44 -45. 5 C

INTRODUCTION Ø There are several subgroups of EEC p p (A) enterotoxigenic (ETEC) (B) enteroinvasive (EIEC) (C) hemorrhagic (EHEC) (D) enteropathogenic (EPEC) strains Ø Some authors classify them into six different pathotypes: ETEC, EIEC, EPEC, enteroaggregative E. coli, diffusely adherent E. coli, and Shiga toxin-producing E. coli (STEC)

INTRODUCTION Ø In 1995, the enteropathogenic E. coli (EPEC) pathotype is divided into two groups, typical EPEC (t. EPEC) and atypical EPEC (a. EPEC) Ø The property that distinguishes these two groups is the presence of the EPEC adherence factor plasmid (p. EAF), which is only found in t. EPEC Ø a. EPEC strains are emerging enteropathogens that have been detected worldwide Ø The large variety of serotypes and genetic virulence properties of a. EPEC strains from nonclassical EPEC serogroups makes it difficult to determine which strains are truly pathogenic

INTRODUCTION Ø Humans are thought to be the principal if not the only reservoir of toxigenic and invasive strains of E. coli, contaminating foods via contact with food or via contact of processing equipment with water contaminated by human feces Ø In contrast, animals are reservoirs of the hemorrhagic strain (O 157: H 7); hence, foods of animal origin may become contaminated via slaughter procedures or postprocessing recontamination Ø However, when E. coli is isolated from foods, pathogenic serotypes are usually absent or represent a very low percentage of the total isolates

HEAT-LABILE ENTEROTOXINS Ø Probably the most common type of EEC strains is the enterotoxigenic type. Heat-labile (LT) and heat -stable (ST) enterotoxins are produced. Ø Two partially cross-reacting antigenic variants of plasmid-coded LT, designated LTh and LTp, have been described in E. coli Ø LTh is associated with E. coli isolates from humans, and LTp is associated with E. coli isolates from pigs Ø The LT family from restricted geographical region exhibited a segregated pattern of dissemination that was revealed by a restriction enzyme site polymorphism

HEAT-LABILE ENTEROTOXINS Ø Another heat-labile enterotoxin was discovered in extracts of E. coli SA 53, a strain isolated from water buffalo Ø It activated adenylate cyclase. Ø Hyperimmune antisera prepared against LTh and LTp or CT do not neutralize the crude LT-like toxin in Y 1 adrenal cell assays

HEAT-LABILE ENTEROTOXINS Ø Subcloning and minicell experiments indicated that the toxin is composed of two polypeptide subunits that are encoded by two genes Ø The two toxin subunits exhibited mobilities on PAGE gels that are similar to those of CT and LT

HEAT-LABILE ENTEROTOXINS

HEAT-LABILE ENTEROTOXINS Ø The LT gene was cloned into E. coli and two proteins of molecular weights 11, 500 (B subunit) and 25, 500 (A subunits) were produced Ø The LT A subunit structureal gene (elt. A) was sequenced and the amino acid sequence deduced

HEAT-LABILE ENTEROTOXINS Ø It is proposed by Pickett et al. that the LTp and LTh (antigenic variants of LT will both be included in serogroup I and should be designated LTp-I and LTh-I and the LT-like toxin will be the prototype for serogroup II enterotoxins and should be renamed LT-II. Ø Two distinct members of the LT-II family, LT -IIa and LT-IIb, are now known, and both have A and B subunits which are similar in size to those of CT and LT-I

HEAT-LABILE ENTEROTOXINS Ø It starts with methionine, ends with leucine, and comprises 254 amino acids Ø The computed molecular weight of LT A is 29, 673. Ø The A subunit genes of CT and LT (LT-I) are 78. 6% homologous, and the B subunit genes are 78% homologous Ø The NH 2 -terminal regions exhibit the highest degree of homology (91%) as compared with CT subunit A, while the COOH-terminal region, containing the sole cystine residue in each toxin is less conserved (52%) Ø Alignment of homologous residues in the COOH-terminal regions of LT A and CT A indicates that a likely site for proteolytic cleavage of LT A is after Arg residue 188

HEAT-LABILE ENTEROTOXINS Ø The gene of LT-IIa was studied. It is organized in a transcriptional unit similar to those of CT and LT -I Ø The A subunit gene of LT-IIa was found to be 57% homologous with the A subunit gene of LTh-I and 55% homologous with the A gene of CT Ø Most of the homology derived from the region of the A gene which encodes the A 1 fragment Ø The B gene of LT-IIa was not homologous with the B gene of LTh-I or CT

HEAT-LABILE ENTEROTOXINS Ø The LT-IIb gene was also cloned analysed. Ø The A genes of LT-IIa and LT-IIb exhibited 71% DNA sequence homology with each other and 55 to 57% homology with the A genes of CT and LT-I. Ø The B subunits of LT-IIa and LT-IIb differ from the LT-I in their carbohydrate-binding specificities. Ø The B genes of LT-IIa and LT-IIb were 66% homologous, but neither had significant homology with the B genes of CT and LT-Is. Ø The A subunits of the heat-labile enterotoxins also have limited homology with other ADP-ribosylating toxins, including pertussis toxin, diphtheria toxin, and Pseudomonas aeruginosa exotoxin A ADP-ribosylation is the addition of one or more ADP-ribose moieties to a protein

HEAT-LABILE ENTEROTOXINS Ø The carboxy-terminal domain of Etx. B (encodes B subunit) mediates A subunit-B subunit interaction Ø The gene encoding the B subunit of LT was mutated at its 3' end by targeted addition of random nucleotide sequences Ø The functional and structural properties of the gene products were analysed, that these mutants were defective in their ability to associate stably with A subunits and form holotoxin

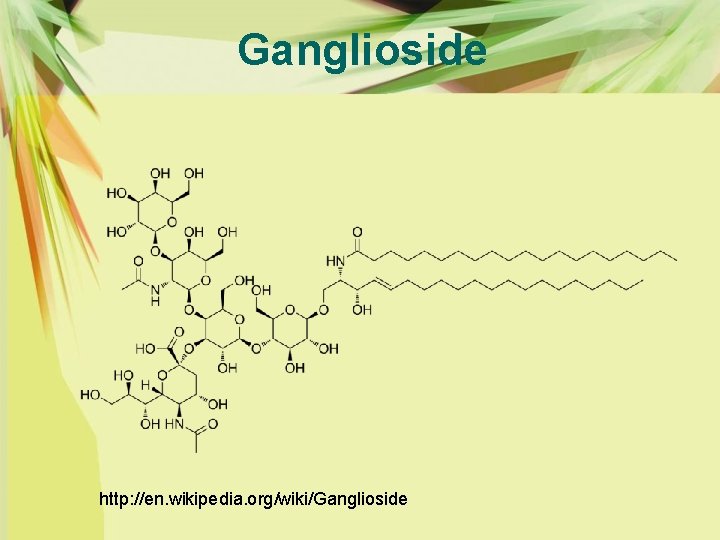
HEAT-LABILE ENTEROTOXINS Ø Mode of Action Ø LT from E. coli is a protein of approximately 86, 000 daltons that consists of one A polypeptide and five B polypeptides held together by noncovalent bonds Ø LT is closely related to cholera enterotoxin (CT) in structure, antigenicity, and mode of action. Both LT and CT bind to ganglioside GM 1 receptors on eukaryotic target cells via their B subunits

Ganglioside http: //en. wikipedia. org/wiki/Ganglioside

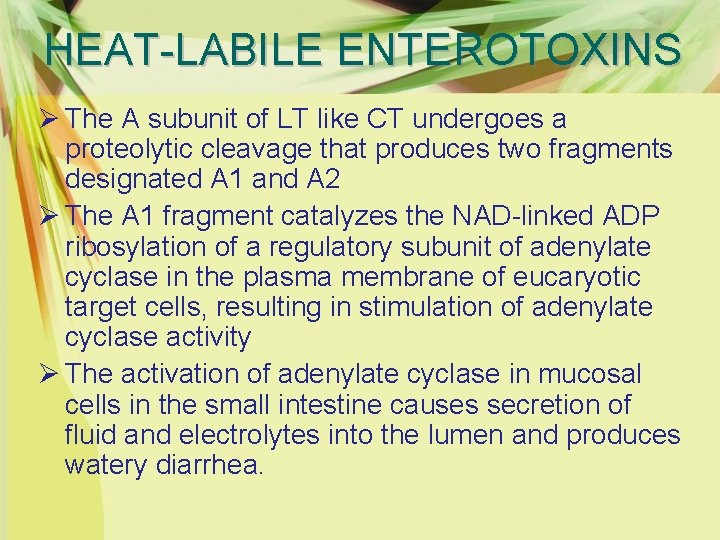
HEAT-LABILE ENTEROTOXINS Ø The A subunit of LT like CT undergoes a proteolytic cleavage that produces two fragments designated A 1 and A 2 Ø The A 1 fragment catalyzes the NAD-linked ADP ribosylation of a regulatory subunit of adenylate cyclase in the plasma membrane of eucaryotic target cells, resulting in stimulation of adenylate cyclase activity Ø The activation of adenylate cyclase in mucosal cells in the small intestine causes secretion of fluid and electrolytes into the lumen and produces watery diarrhea.

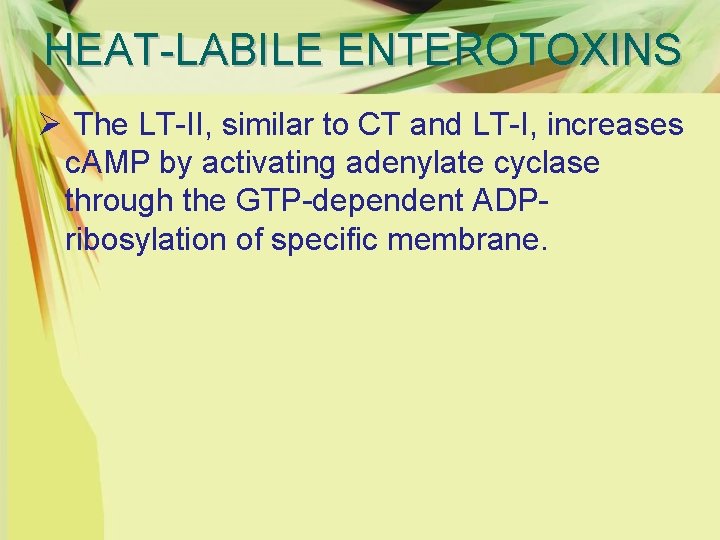
HEAT-LABILE ENTEROTOXINS Ø The LT-II, similar to CT and LT-I, increases c. AMP by activating adenylate cyclase through the GTP-dependent ADPribosylation of specific membrane.

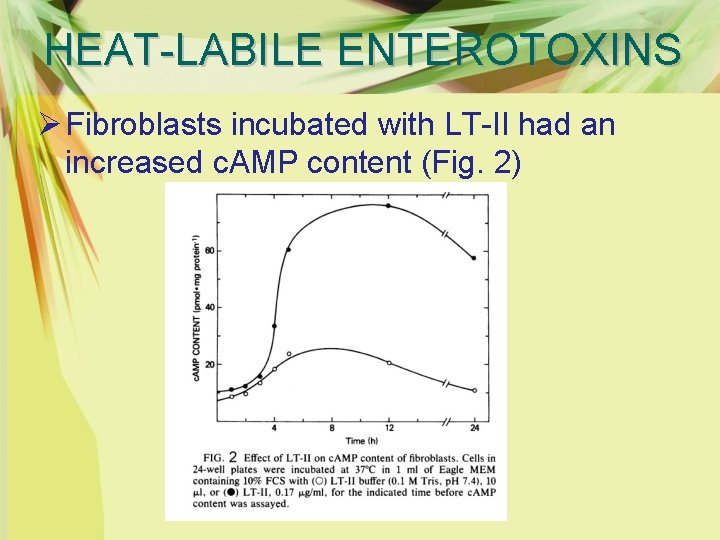
HEAT-LABILE ENTEROTOXINS Ø Fibroblasts incubated with LT-II had an increased c. AMP content (Fig. 2)

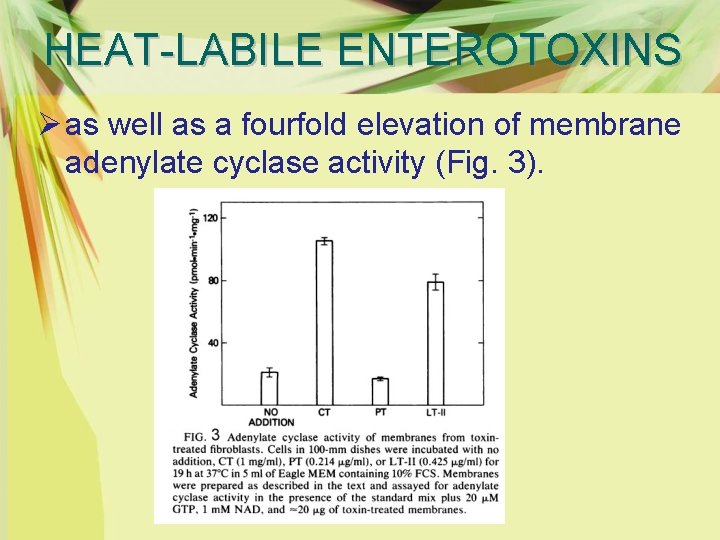
HEAT-LABILE ENTEROTOXINS Ø as well as a fourfold elevation of membrane adenylate cyclase activity (Fig. 3).

HEAT-LABILE ENTEROTOXINS Ø The B subunit of LTh (Human) is also hemagglutinating Ø Very strong hemagglutination of both neuraminidase- and pronase-treated human erythrocytes was induced by the B subunit of LTh. Ø Different blood groups reacted differently to such enhancement Ø Combining site of the B subunit may gain access to the receptor exposed on erythrocytes more easily by enzyme treatment. Ø Neuraminidase and pronase are suppose to convert major gangliosides to GM 1 and/or expose masked receptors for the B subunit. Both CT and LT strongly react with ganglioside GM 1.

HEAT-LABILE ENTEROTOXINS Ø Separation of ETEC bacteria from target intestinal epithelial monolayers by semipermeable filters prevented activation of adenylate cyclase suggesting that pathogen-host cell contact is required for efficient toxin delivery Ø Likewise, a non-motile strain bearing a mutation in the flagellar fli. D gene was deficient in delivery of LT relative to the ETEC prototype Ø Although LT secretion via the type II secretion system (T 2 SS) was responsive to a variety of environmental factors, neither toxin release nor delivery depended on transcriptional activation of genes encoding LT or the T 2 SS

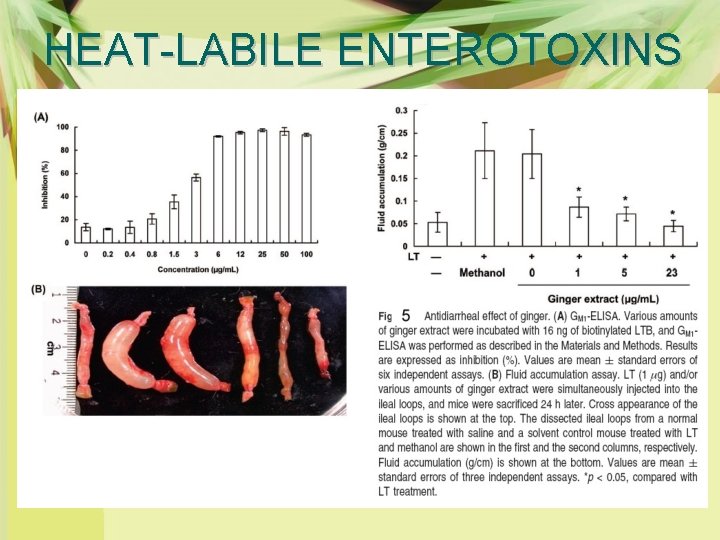
HEAT-LABILE ENTEROTOXINS Ø Enterotoxigenic E. coli LT-induced diarrhea is the leading cause of infant death in developing countries. Ø Ginger significantly blocked the binding of LT to cell-surface receptor G M 1 (Fig. 4), resulting in the inhibition of fluid accumulation in the closed ileal loops of mice (Fig. 5).

HEAT-LABILE ENTEROTOXINS

HEAT-LABILE ENTEROTOXINS

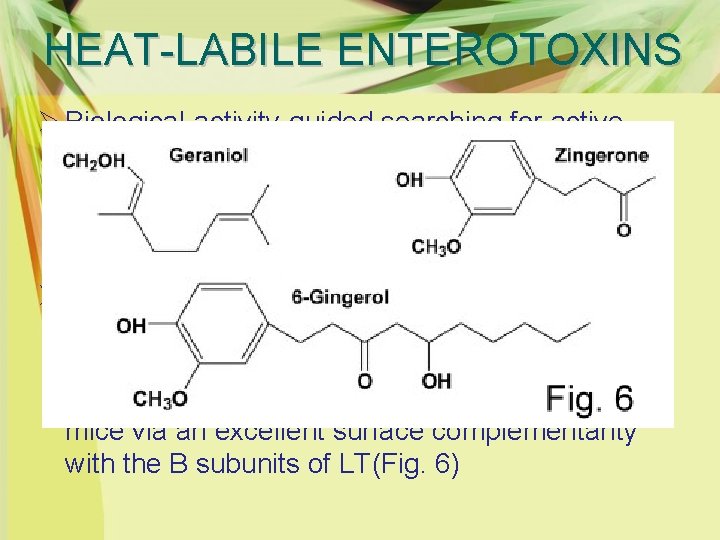
HEAT-LABILE ENTEROTOXINS Ø Biological-activity-guided searching for active components showed that zingerone (vanillylacetone) was the likely active constituent responsible for the antidiarrheal efficacy of ginger. Ø Further analysis of chemically synthesized zingerone derivatives revealed that compound 31 (2 -[(4 -methoxybenzyl)oxy] benzoic acid) significantly suppressed LT-induced diarrhea in mice via an excellent surface complementarity with the B subunits of LT(Fig. 6)

HEAT-STABLE ENTEROTOXINS Ø The heat-stable enterotoxins are low-molecularweight, heat-stable, nonantigenic proteins which do not cause intestinal secretion by activation of adenylate cyclase Ø At least two types have been described, one with biological activity in suckling mice and piglets (STa, or named as STh, or ST-I) due to stimulation of particulate intestinal guanylate cyclase and a second which induces secretion by an unknown mechanism only in piglets (STb, or known as STp, or ST-II).

HEAT-STABLE ENTEROTOXINS Ø Generally STs are known to be small peptide toxins consisting of 18 (STb) or 19 (STa) amino acids. Ø The different STa (M. W. about 2, 000) from different animal origins are remarkably homogeneous. Ø The amino acid composition of STa of porcine, bovine, and human origins were identical and consisted of 10 of the 18 amino acids usually present in proteins. Ø Six of the 18 amino acids were half-cystines which appear to be present as three disulfide bonds in the native form of the toxin. These disulfide bonds are important for toxic activity.

HEAT-STABLE ENTEROTOXINS Ø STb is a heat-stable enterotoxin which does not cause intestinal fluid secretion in the suckling mouse as STa does, but does cause intestinal fluid secretion in pig intestinal loop assays. Ø It is insoluble in methanol, while STa is methanol-soluble

HEAT-STABLE ENTEROTOXINS Ø Since STs are small peptides and are nonantigenic, fusion proteins (e. g. STa with outer membrane protein C, etc. ) (Saarilahti et al. , 1989) or synthetic ST peptide conjugated with ovalbumin could be use as the immunoprophylactic agents against diarrhea caused by STs.

HEAT-STABLE ENTEROTOXINS Ø In a study, these two toxins were examined in terms of importance for piglets >1 week old with the construction of isogenic single- and double-deletion mutants and inoculation of 9 -day-old F 4 ac receptor-positive gnotobiotic piglets. Ø Based on the postinoculation percent weight change per h and serum bicarbonate concentrations, the virulence of the STb- mutant (Delta est. B) did not significantly differ from that of the parent. Ø However, deletion of the LT genes (Delta elt. AB) in the STb(-) mutant resulted in a complete abrogation of weight loss, dehydration, and metabolic acidosis in inoculated pigs, and LT complementation restored the virulence of this strain.

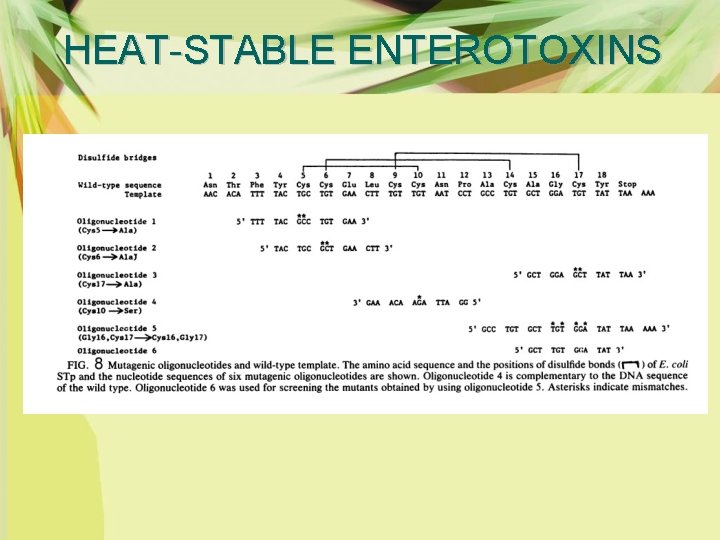
HEAT-STABLE ENTEROTOXINS Ø The cysteine residues were substituted in vivo by oligonucleotide-directed site-specific mutangenesis to dissociate each disulfide bond and examined the biological activitites of the resulting mutants (Fig. 7, 8). Ø All three disulfide bonds formed at fixed positions are required for full expression of the biological activity of STb. Ø It has some fexibilities in its conformation to exert toxic activity and that the role of each disulfide bond in exerting toxic activity is not quite the same

HEAT-STABLE ENTEROTOXINS

HEAT-STABLE ENTEROTOXINS

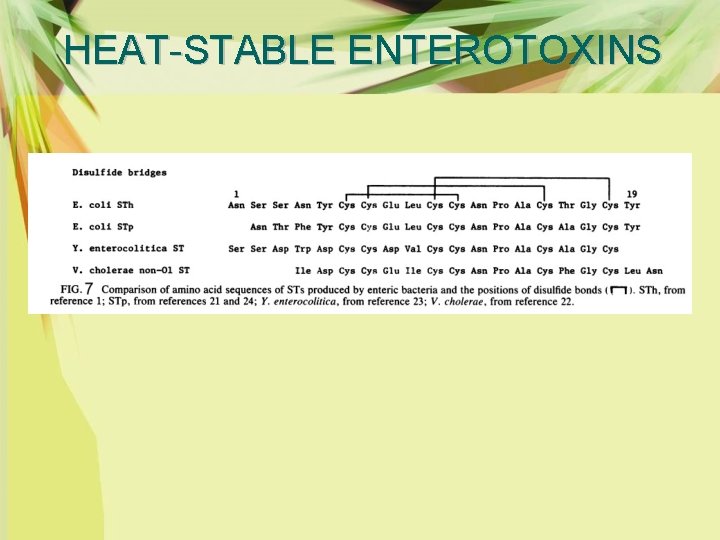
HEAT-STABLE ENTEROTOXINS Ø The STs share biologically active sequences which reside in the C-terminal 13 amino acid residues. Substitution of the asparagines residue at position 11 of STb by other amino acids resulted in significant decrease in enterotoxic activities, although the conformation was not changed (Okamoto et al. , 1988). Ø The amino acid sequences and disulfide bonds of the heat-stable enterotoxins of E. coli, Yersinia enterocolitica, and Vibrio cholerae non-O 1 are shown in Fig. 9

HEAT-STABLE ENTEROTOXINS

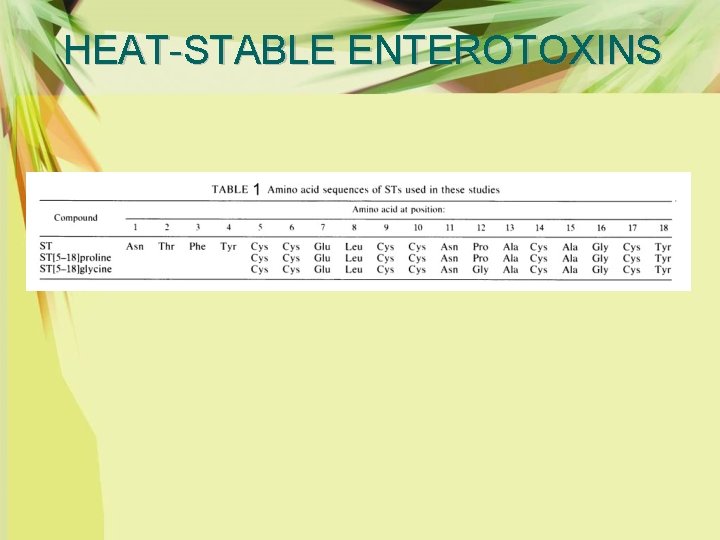
HEAT-STABLE ENTEROTOXINS Ø Analogs of ST were made, including the native 18 -amino-acid ST, the 14 -amino-acid carboxy terminus of this native peptide with a proline at position 12, and the 14 -aminoacid carboxy terminus of in which the proline at position 12 was substituted with glycine (Table 1).

HEAT-STABLE ENTEROTOXINS

HEAT-STABLE ENTEROTOXINS Ø Each analog bound to the receptor in a dose-dependent fashion, native ST with the highest adherence (Fig. 10).

HEAT-STABLE ENTEROTOXINS

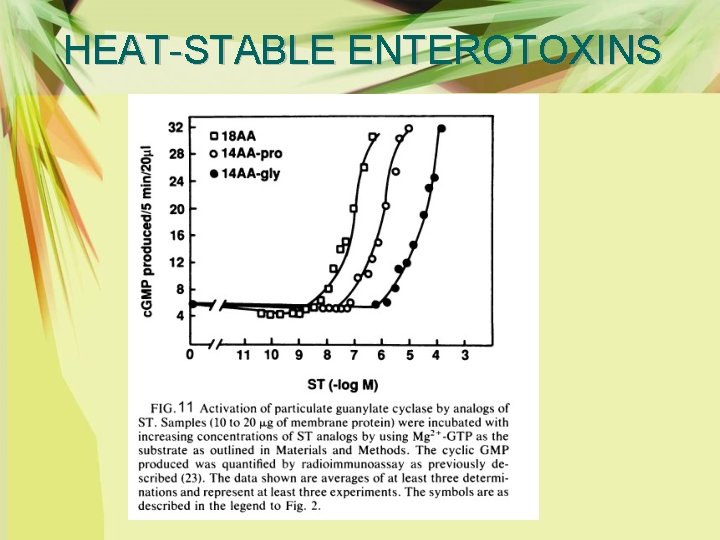
HEAT-STABLE ENTEROTOXINS Ø Similarly, these peptides maximally activated particulate guanylate cyclase and stimulate intestinal secretion in suckling mice, and native ST with the highest potency (Fig. 11, Table 2).

HEAT-STABLE ENTEROTOXINS

HEAT-STABLE ENTEROTOXINS

HEAT-STABLE ENTEROTOXINS Ø It demonstrats that the four amino-terminal residues contribute significantly to the potency of these peptides. Ø In addition, the turn imposed by the proline residue at position 12 is not absolutely required for receptor occupancy or activation of the biochemical cascade that results in intestinal secretion. Ø However, it significantly increases the potency of the toxin

HEAT-STABLE ENTEROTOXINS Ø The STb structural gene (est. A) was cloned into high-expression vector p. KC 30 downstream from the strong PL promoter and the expression was studied Ø 10 -20 -fold increase in m. RNA was produced by the recombinant strain.

HEAT-STABLE ENTEROTOXINS Ø Both STs are synthesized as precursor proteins and are then converted to the active forms with intramolecular disulfide bonds after being released into the periplasm. Ø The active STs are finally translocated across the outer membrane through a tunnel made by Tol. C.

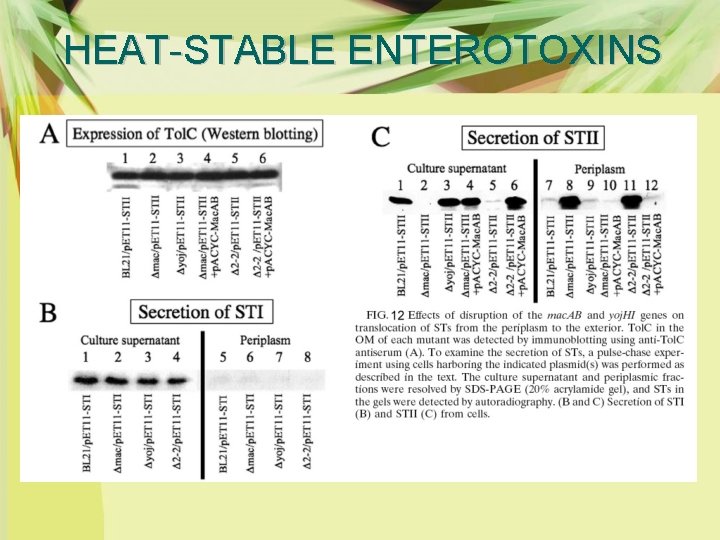
HEAT-STABLE ENTEROTOXINS Ø Several transporters in the inner membrane and their periplasmic accessory proteins are known to combine with Tol. C and form a tripartite transport system. Ø Pulse-chase experiments using E. coli BL 21(DE 3) mutants in which various transporter genes (acr. AB, acr. EF, emr. AB, emr. KY, mdt. EF, mac. AB, and yoj. HI) had been knocked out and analyzed the secretion of STs in those strains. Ø The results revealed that the extracellular secretion of STII was largely decreased in the mac. AB mutant and the toxin molecules were accumulated in the periplasm, although the secretion of STI was not affected in any mutant

HEAT-STABLE ENTEROTOXINS Ø The periplasmic stagnation of STII in the mac. AB mutant was restored by the introduction of p. ACYC 184, containing the mac. AB gene, into the cell (Fig. 12). Ø These results indicate that Mac. AB, an ATPbinding cassette transporter of Mac. B and its accessory protein, Mac. A, participates in the translocation of STII from the periplasm to the exterior.

HEAT-STABLE ENTEROTOXINS

ENTEROTOXIN PLASMIDS Ø Enterotoxin plasmids from classical strains (frequently associated with diarrhea, e. g. O 6, O 25, O 27, O 128, and O 159) did not transfer by conjugation from clinical isolates, whereas those from rare strains (rarely associated with diarrhea, e. g. O 7, O 17, O 80, O 98, O 139, and O 153) transferred almost always from the clinical isolates by conjugation. Ø Analyses of enterotoxin plasmids by restriction endonucleases and hybridization with the enterotoxin probes revealed that the strains with the same O serotype and toxigenicity carry closely related enterotoxin plasmids
 Tsi agar
Tsi agar Flagella
Flagella Medio butzler
Medio butzler Virus monera
Virus monera Escherichia coli unicellular or multicellular
Escherichia coli unicellular or multicellular Escherichia coli mobile ou immobile
Escherichia coli mobile ou immobile Construction of a genetic toggle switch in escherichia coli
Construction of a genetic toggle switch in escherichia coli Materi tembang gambuh kelas 8
Materi tembang gambuh kelas 8 Genul escherichia
Genul escherichia Escherichia spp
Escherichia spp Temperate phage
Temperate phage Trajcov ligament
Trajcov ligament Amuboit
Amuboit Flexura coli dextra
Flexura coli dextra Balantidium trophozoite
Balantidium trophozoite Is e coli unicellular or multicellular
Is e coli unicellular or multicellular Mitochondria double membrane function
Mitochondria double membrane function Boda coli
Boda coli Enteric definition biology
Enteric definition biology Phage typing
Phage typing Coli
Coli Prequiste de entamoeba coli
Prequiste de entamoeba coli E. coli organelles
E. coli organelles Arteria interlobularis
Arteria interlobularis Api 20e test results e coli
Api 20e test results e coli E coli
E coli Bordel coli
Bordel coli Huevo de entamoeba histolytica
Huevo de entamoeba histolytica Blantidium coli
Blantidium coli E coli tca cycle
E coli tca cycle Nama ff coli
Nama ff coli Anal canal tissue
Anal canal tissue Subkingdom protozoa
Subkingdom protozoa Plica longitudinalis
Plica longitudinalis Cestodes
Cestodes E coli treatment
E coli treatment Entamoeba coli hospedero
Entamoeba coli hospedero E coli starch hydrolysis test
E coli starch hydrolysis test E coli form elevation margin
E coli form elevation margin Enterotoxin e coli
Enterotoxin e coli Giardia lamblia ciclo biologico
Giardia lamblia ciclo biologico Mastigophora
Mastigophora Taenia coli
Taenia coli Caecum
Caecum E coli in sarcina
E coli in sarcina Torace in platosa
Torace in platosa Pertama kali coli
Pertama kali coli Entamoeba histolytica eucariota o procariota
Entamoeba histolytica eucariota o procariota Caruncula sublingualis
Caruncula sublingualis Teniae coli
Teniae coli E coli treatment
E coli treatment Pars superior duodeni
Pars superior duodeni Lobozea
Lobozea Lake of the ozarks e coli
Lake of the ozarks e coli Balantidium coli genus
Balantidium coli genus Teniae coli
Teniae coli