Electrons The Octet Rule Periodic Trends Electrons The








- Slides: 20

Electrons, The Octet Rule & Periodic Trends

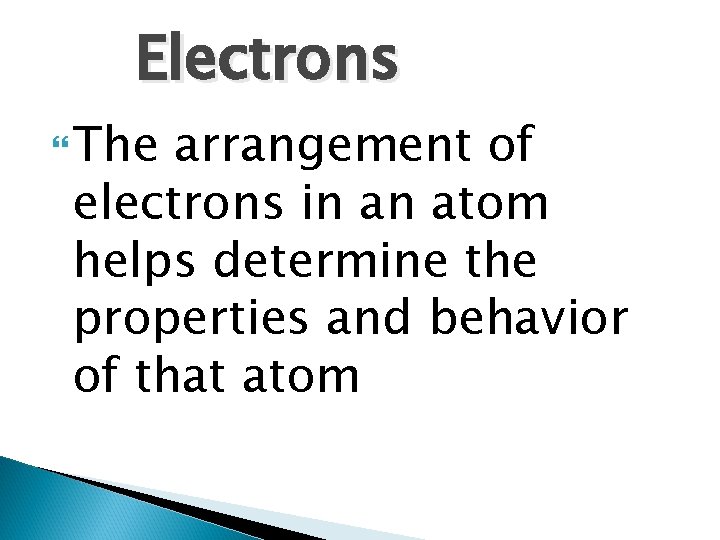
Electrons The arrangement of electrons in an atom helps determine the properties and behavior of that atom

Electrons have special rules…. Electrons live in something called energy levels. Only so many electrons can be in any energy level. The electrons in the outer most energy level of any element are called valence electrons.

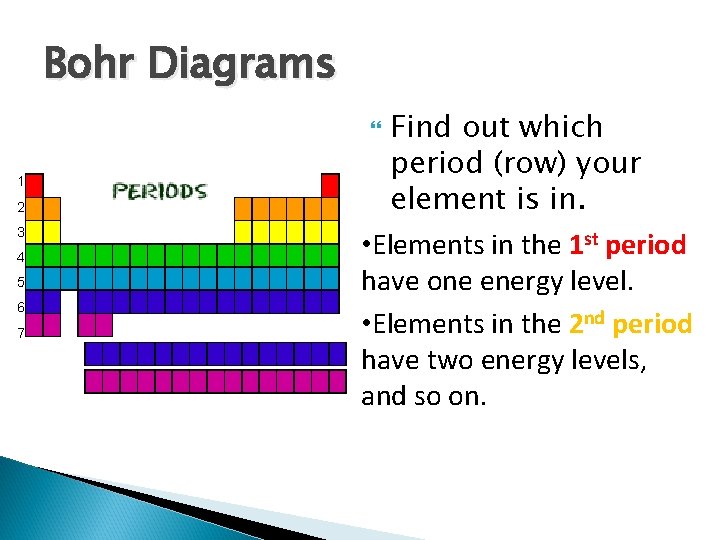
Bohr Diagrams Find out which period (row) your element is in. • Elements in the 1 st period have one energy level. • Elements in the 2 nd period have two energy levels, and so on. 1 2 3 4 5 6 7

The electron shells surrounding the nucleus each hold a particular number of electrons. Shells are named with letters: 1 = K shell 2 = L shell 3 = M shell 4 = N shell 5 -7 = O, P, Q = 2 electrons = 8 electrons = 18 electrons = up to 32 electrons Remember: The outer shell of an atom (no matter what letter) can only hold 8 electrons!

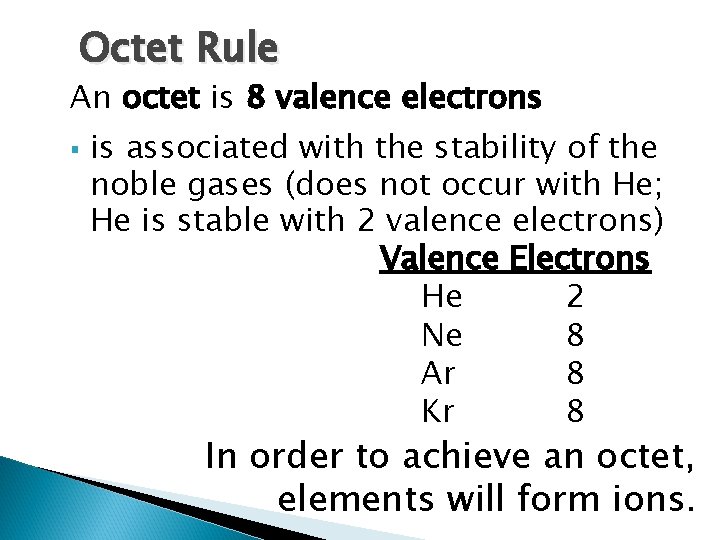
The Octet Rule

Octet Rule An octet is 8 valence electrons § is associated with the stability of the noble gases (does not occur with He; He is stable with 2 valence electrons) Valence Electrons He 2 Ne 8 Ar 8 Kr 8 In order to achieve an octet, elements will form ions.

Metals want to be happy. Metals form cations § § § by losing their valence electrons resemble the nearest noble gas have fewer electrons than protons Group 1 metals Group 2 metals Group 3 metals ion ion 1+ 2+ 3+

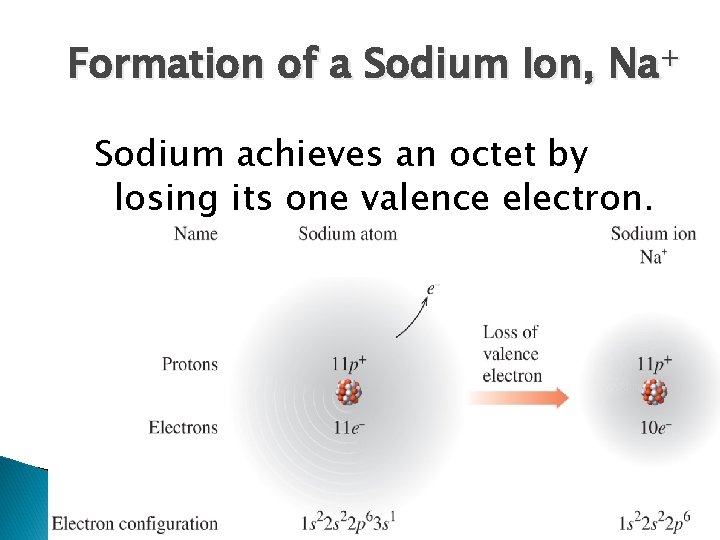
Formation of a Sodium Ion, Na+ Sodium achieves an octet by losing its one valence electron.

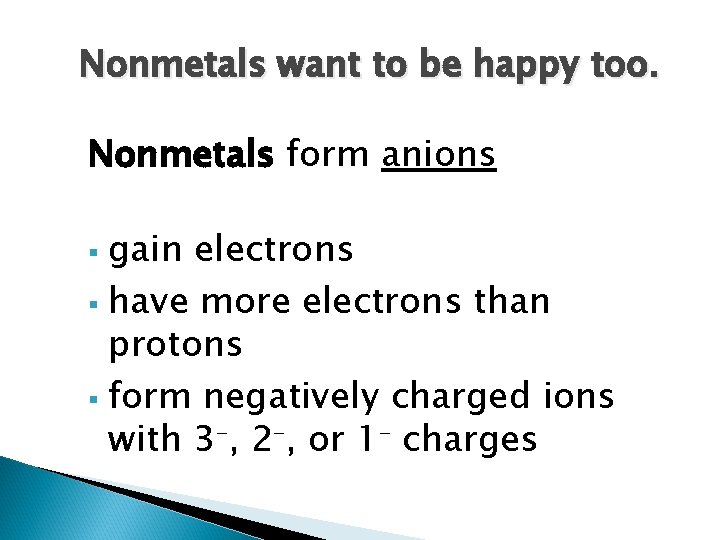
Nonmetals want to be happy too. Nonmetals form anions gain electrons § have more electrons than protons § form negatively charged ions with 3–, 2–, or 1– charges §

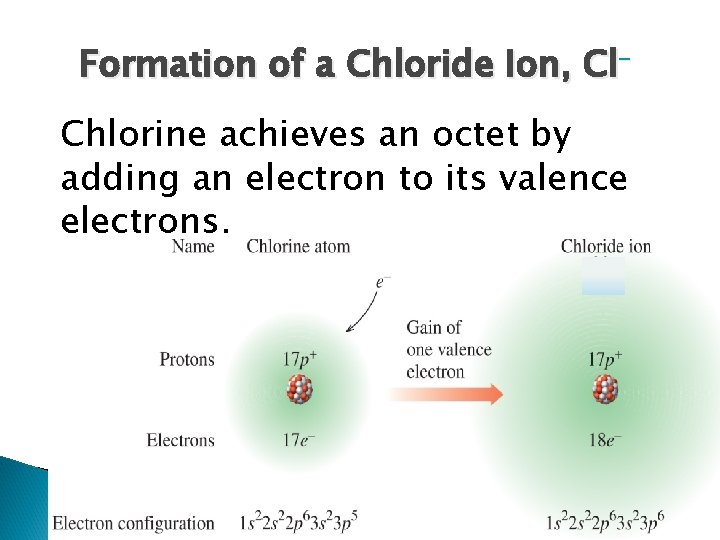
Formation of a Chloride Ion, Cl– Chlorine achieves an octet by adding an electron to its valence electrons.

Periodic trends

Ionization Energy needed to remove one of atom’s electrons from its outermost shell Atoms with high ionization energies hold onto their electrons very tightly. Atoms with low ionization energies are more likely to lose one or more of their outermost electron.

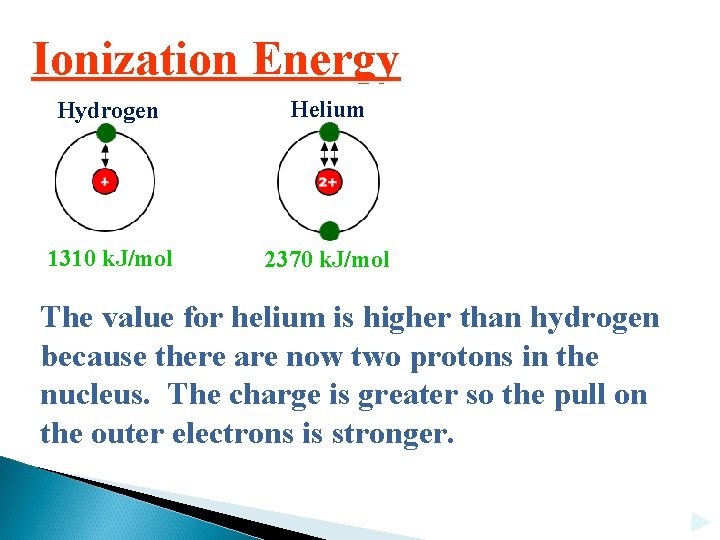
Ionization Energy Hydrogen Helium 1310 k. J/mol 2370 k. J/mol The value for helium is higher than hydrogen because there are now two protons in the nucleus. The charge is greater so the pull on the outer electrons is stronger.

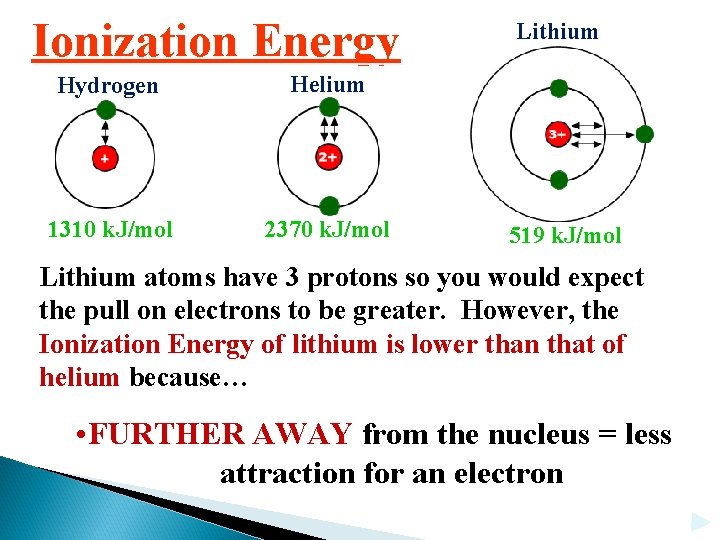
Ionization Energy Hydrogen Helium 1310 k. J/mol 2370 k. J/mol Lithium 519 k. J/mol Lithium atoms have 3 protons so you would expect the pull on electrons to be greater. However, the Ionization Energy of lithium is lower than that of helium because… • FURTHER AWAY from the nucleus = less attraction for an electron

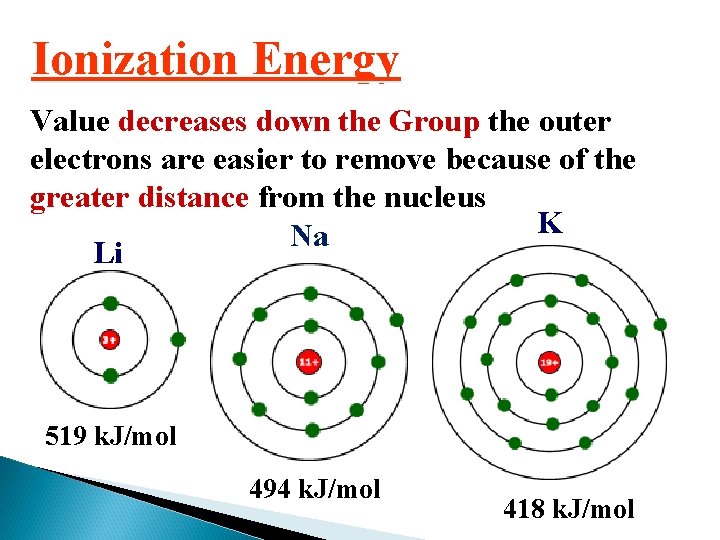
Ionization Energy Value decreases down the Group the outer electrons are easier to remove because of the greater distance from the nucleus K Na Li 519 k. J/mol 494 k. J/mol 418 k. J/mol

Electronegativity is a chemical property that describes the tendency of an atom to attract electrons towards itself.

ELECTRONEGATIVITY An atom's electronegativity is affected by its atomic number. Higher atomic number elements tend to be less electronegative because of the distance from their nucleus to their valence electrons.

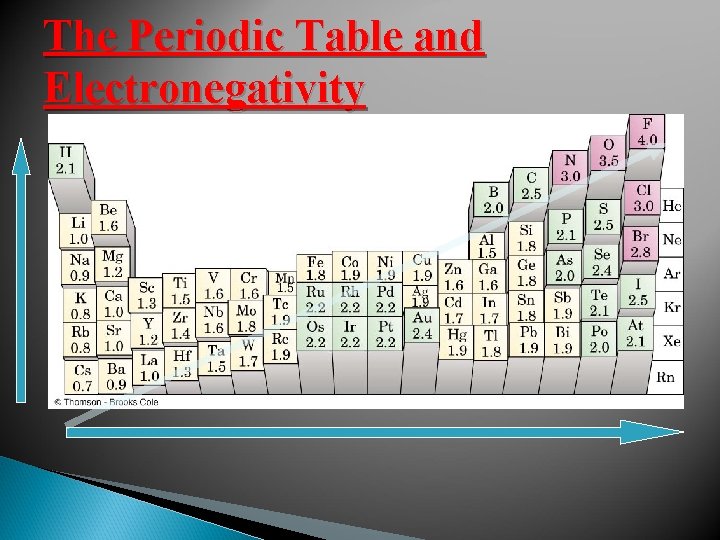
ELECTRONEGATIVITY Pauling’s electronegativity scale Fluorine is the most electronegative element It has an electronegativity value of 4. 0

The Periodic Table and Electronegativity
 Periodic table with electronegativity and ionization energy
Periodic table with electronegativity and ionization energy Elements in period 2
Elements in period 2 The octet rule states that
The octet rule states that Octet rule
Octet rule Metoda vsepr
Metoda vsepr Abe notation
Abe notation Whats an ionic bond
Whats an ionic bond Exceptions to the octet rule
Exceptions to the octet rule Bent molecular shape
Bent molecular shape The octet rule states that
The octet rule states that Sodium octet rule
Sodium octet rule Atoms tend to gain lose or share electrons
Atoms tend to gain lose or share electrons Honc rules
Honc rules Octet rule exceptions
Octet rule exceptions Hnc lewis structure
Hnc lewis structure Ionic bond
Ionic bond Mayan periodic table
Mayan periodic table Atomic trends
Atomic trends Mendeleev
Mendeleev Valence electron cheat sheet
Valence electron cheat sheet Oxygen periodic trends
Oxygen periodic trends