Electrons and Quantum Mechanics Unit 5 Electrons Rutherford








- Slides: 35

Electrons and Quantum Mechanics Unit 5

Electrons • Rutherford described the dense center of the atom called the nucleus. • But the Electrons spin around the outside of that nucleus. – Provide the chemical properties of the atoms. – Responsible for color and reactivity.

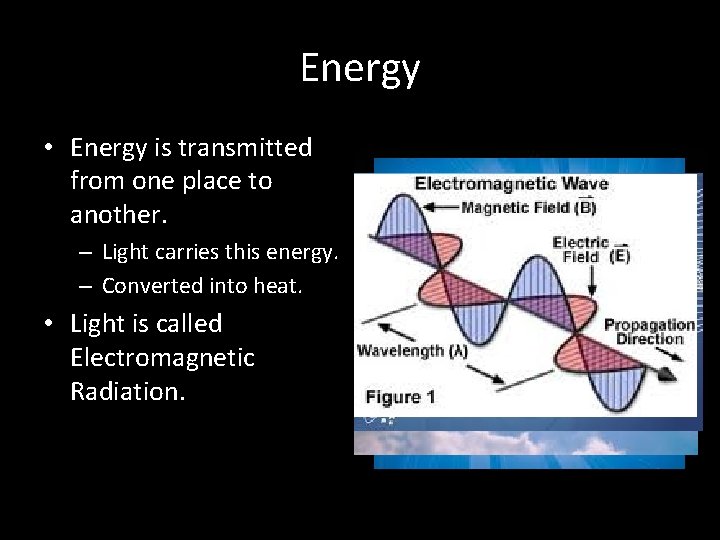
Energy • Energy is transmitted from one place to another. – Light carries this energy. – Converted into heat. • Light is called Electromagnetic Radiation.

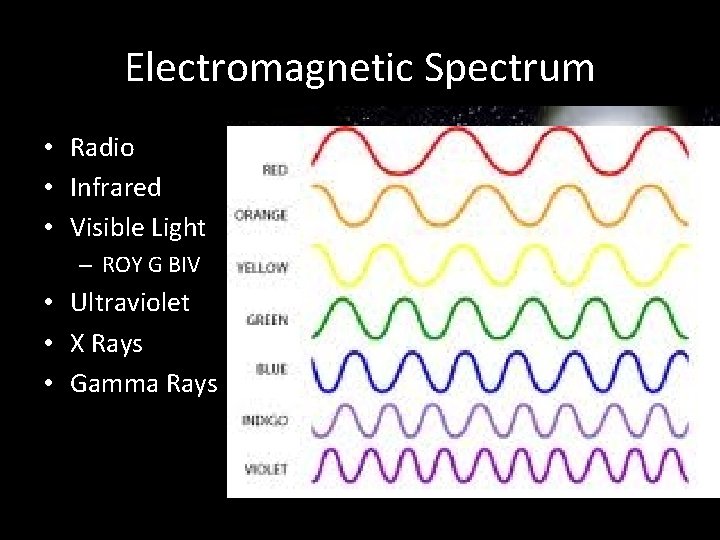
Electromagnetic Spectrum • Radio • Infrared • Visible Light – ROY G BIV • Ultraviolet • X Rays • Gamma Rays

Light • Light travels as a wave. • Wave Properties – Wavelength (λ) = distance between two waves (m) – Frequency (f) = number of peaks per second (Hz) – Speed of Light (c) = how fast light moves.

Light • Light Equation c= ƒλ • Speed of light is a constant = 3 x 108 m/s • Nothing travel faster than the speed of light! – Maybe? !? !?

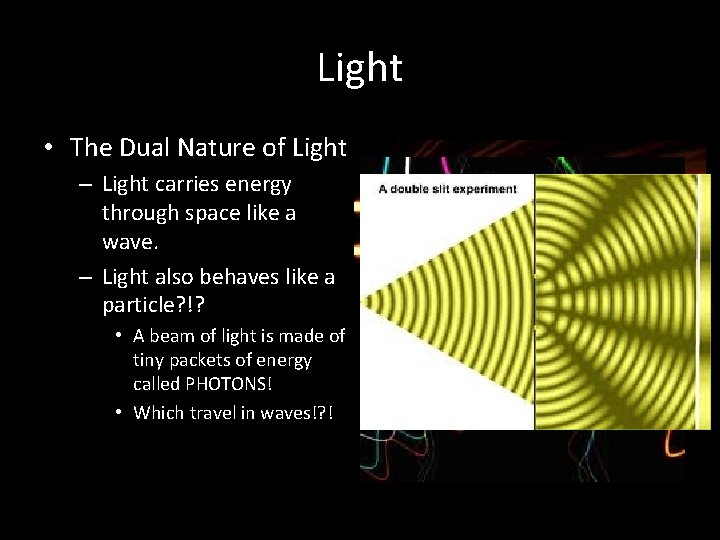
Light • The Dual Nature of Light – Light carries energy through space like a wave. – Light also behaves like a particle? !? • A beam of light is made of tiny packets of energy called PHOTONS! • Which travel in waves!? !

Light • The Energy of a photon depends on its frequency. – So is the color of light!!! E = hƒ • ELECTRONS are like photons! – Act as waves and particles. – Orbit the nucleus in a wave -like motion.

Blackbody Radiation • Rutherford could never explain why objects change colors when they are heated. • As the object heats, it must give off electrons of certain frequencies and energies.

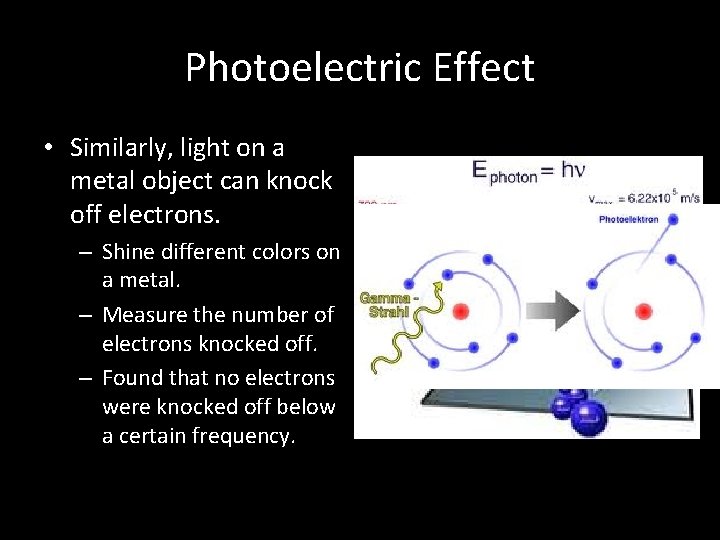
Photoelectric Effect • Similarly, light on a metal object can knock off electrons. – Shine different colors on a metal. – Measure the number of electrons knocked off. – Found that no electrons were knocked off below a certain frequency.

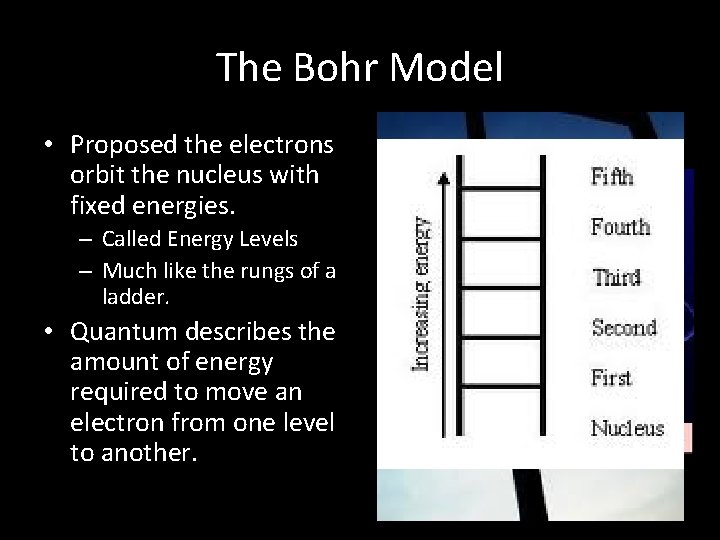
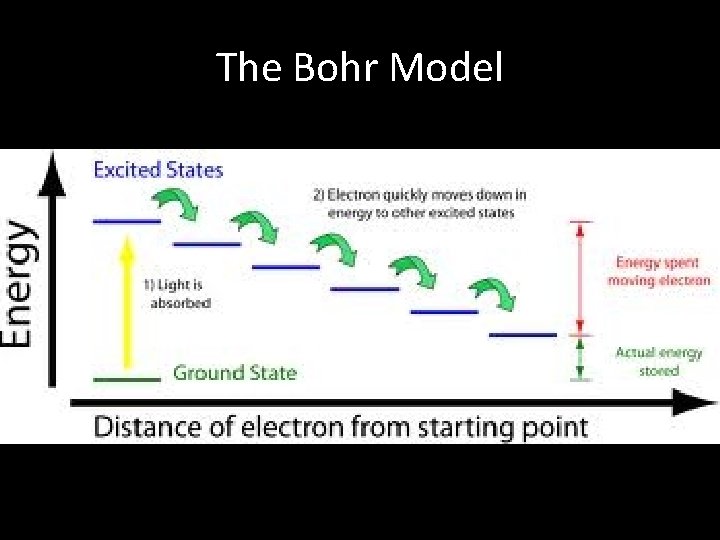
The Bohr Model • Proposed the electrons orbit the nucleus with fixed energies. – Called Energy Levels – Much like the rungs of a ladder. • Quantum describes the amount of energy required to move an electron from one level to another.

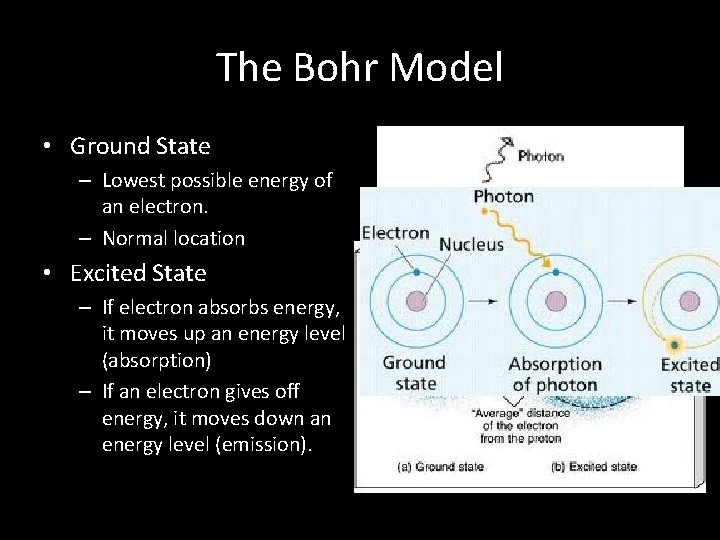
The Bohr Model • Ground State – Lowest possible energy of an electron. – Normal location • Excited State – If electron absorbs energy, it moves up an energy level (absorption) – If an electron gives off energy, it moves down an energy level (emission).

The Bohr Model

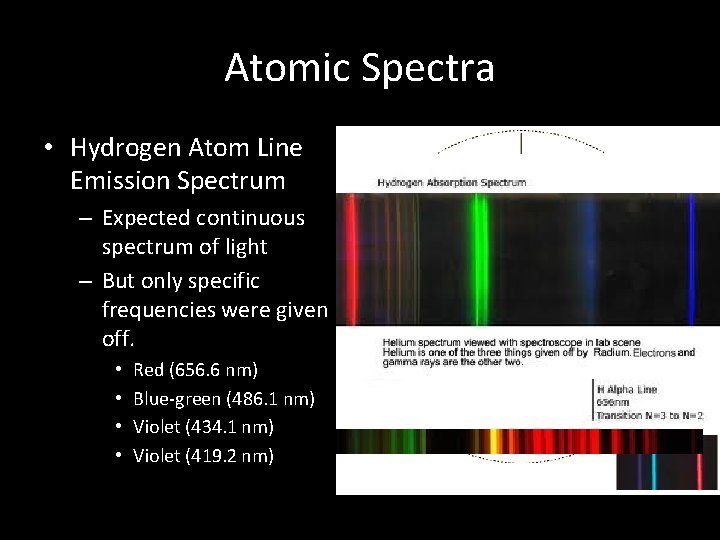
Atomic Spectra • Hydrogen Atom Line Emission Spectrum – Expected continuous spectrum of light – But only specific frequencies were given off. • • Red (656. 6 nm) Blue-green (486. 1 nm) Violet (434. 1 nm) Violet (419. 2 nm)

Atomic Spectra • Shine a light on an Atom – When atoms absorb energy, electrons move to higher energy levels. – When atoms release the energy, electrons return to the lower energy level. • Atomic Spectra – Frequencies of light emitted by a certain element. – No two elements have the same spectrum. http: //student. fizika. org/~nnctc/spectra. htm

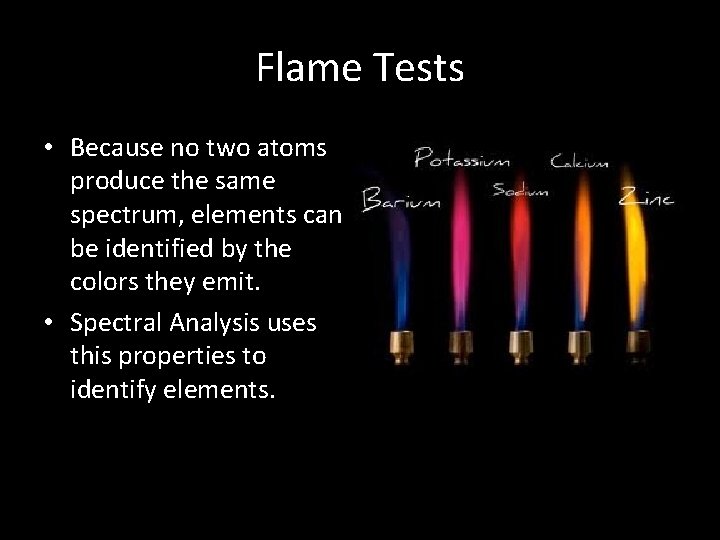
Flame Tests • Because no two atoms produce the same spectrum, elements can be identified by the colors they emit. • Spectral Analysis uses this properties to identify elements.

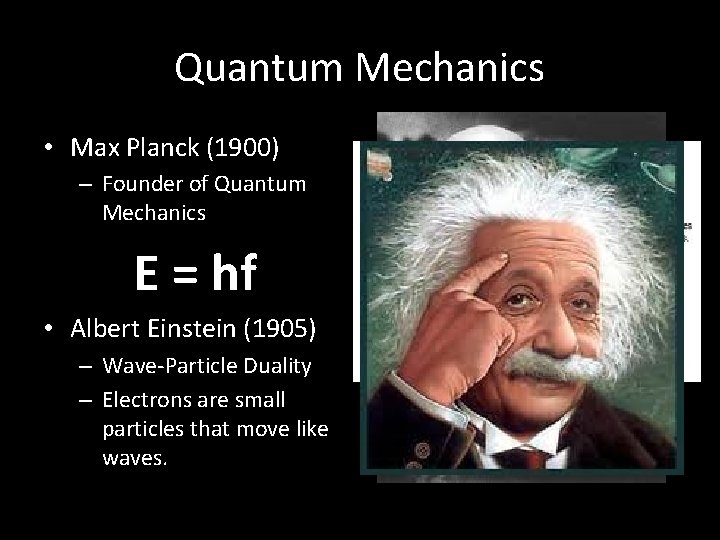
Quantum Mechanics • Max Planck (1900) – Founder of Quantum Mechanics E = hf • Albert Einstein (1905) – Wave-Particle Duality – Electrons are small particles that move like waves.

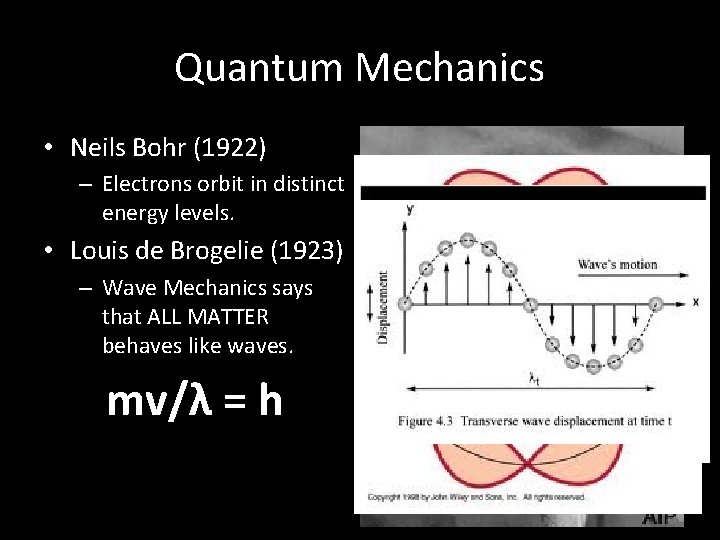
Quantum Mechanics • Neils Bohr (1922) – Electrons orbit in distinct energy levels. • Louis de Brogelie (1923) – Wave Mechanics says that ALL MATTER behaves like waves. mv/λ = h

Quantum Mechanics • Werner Heisenberg (1927) – Principle of Indeterminacy – You can’t know both the position and the velocity of an electron. • Erwin Schrödinger (1930) – Used wave mechanics to show the PROBABLE location of an electron. – Electrons exist in 3 D clouds of probability!!!


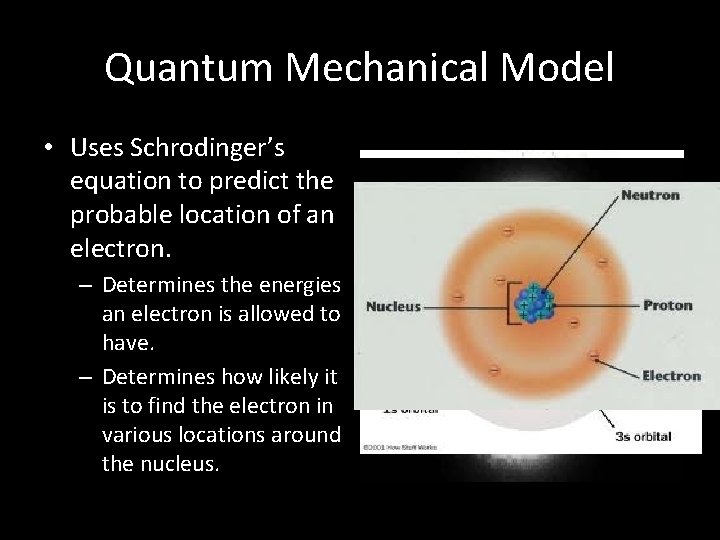
Quantum Mechanical Model • Uses Schrodinger’s equation to predict the probable location of an electron. – Determines the energies an electron is allowed to have. – Determines how likely it is to find the electron in various locations around the nucleus.

Quantum Numbers • Describes the location and behavior of an electron – Like an electron’s address – No two electrons can have the same quantum numbers. • Four Numbers

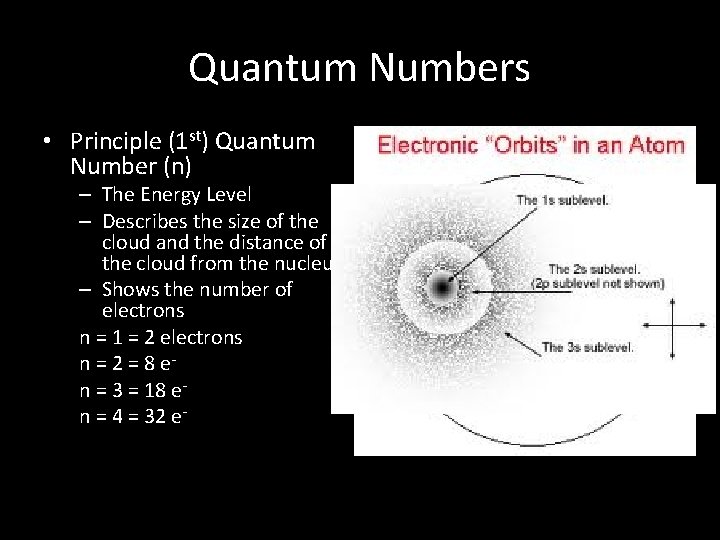
Quantum Numbers • Principle (1 st) Quantum Number (n) – The Energy Level – Describes the size of the cloud and the distance of the cloud from the nucleus. – Shows the number of electrons n = 1 = 2 electrons n = 2 = 8 en = 3 = 18 en = 4 = 32 e-


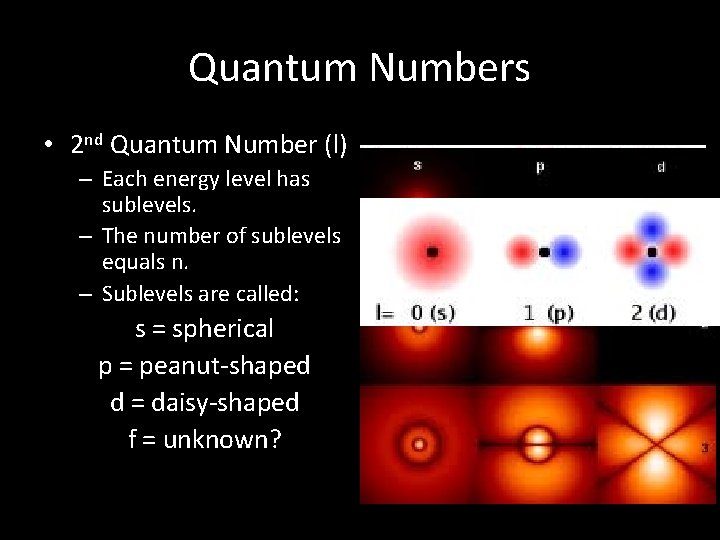
Quantum Numbers • 2 nd Quantum Number (l) – Each energy level has sublevels. – The number of sublevels equals n. – Sublevels are called: s = spherical p = peanut-shaped d = daisy-shaped f = unknown?


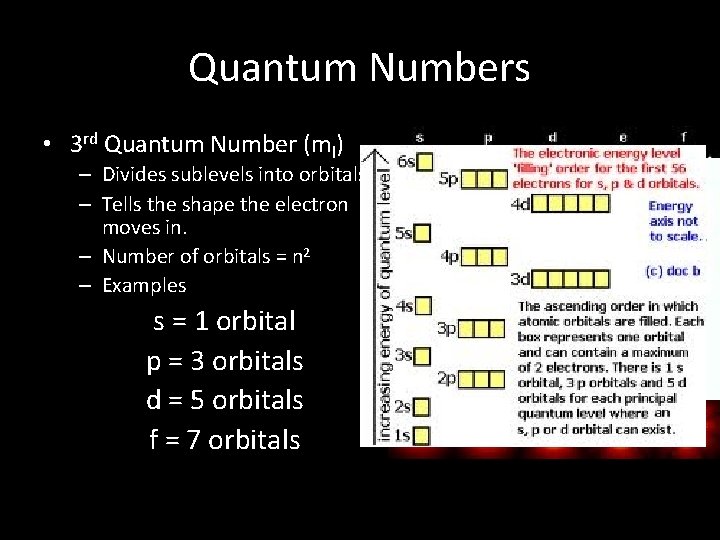
Quantum Numbers • 3 rd Quantum Number (ml) – Divides sublevels into orbitals. – Tells the shape the electron moves in. – Number of orbitals = n 2 – Examples s = 1 orbital p = 3 orbitals d = 5 orbitals f = 7 orbitals

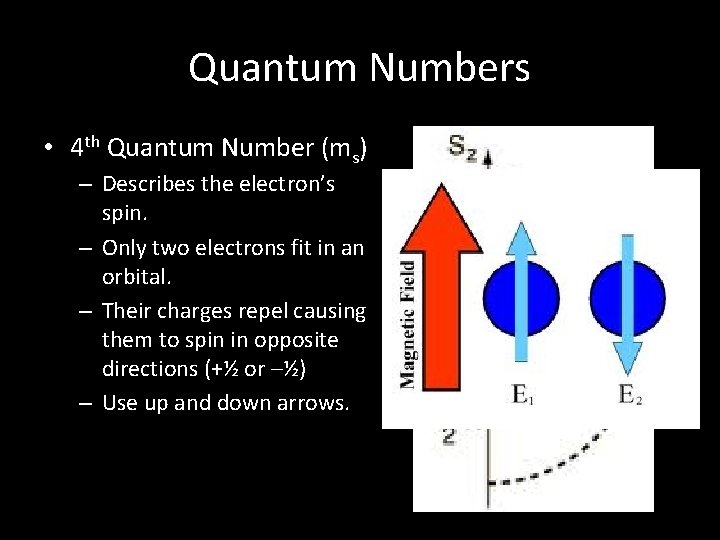
Quantum Numbers • 4 th Quantum Number (ms) – Describes the electron’s spin. – Only two electrons fit in an orbital. – Their charges repel causing them to spin in opposite directions (+½ or –½) – Use up and down arrows.


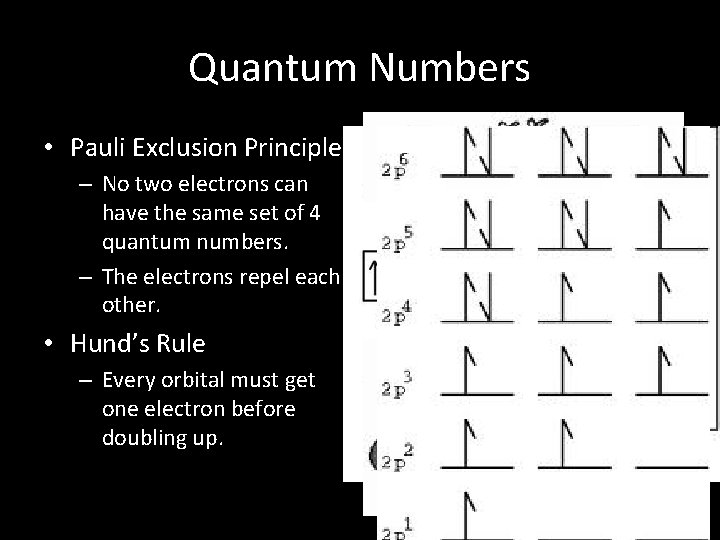
Quantum Numbers • Pauli Exclusion Principle – No two electrons can have the same set of 4 quantum numbers. – The electrons repel each other. • Hund’s Rule – Every orbital must get one electron before doubling up.

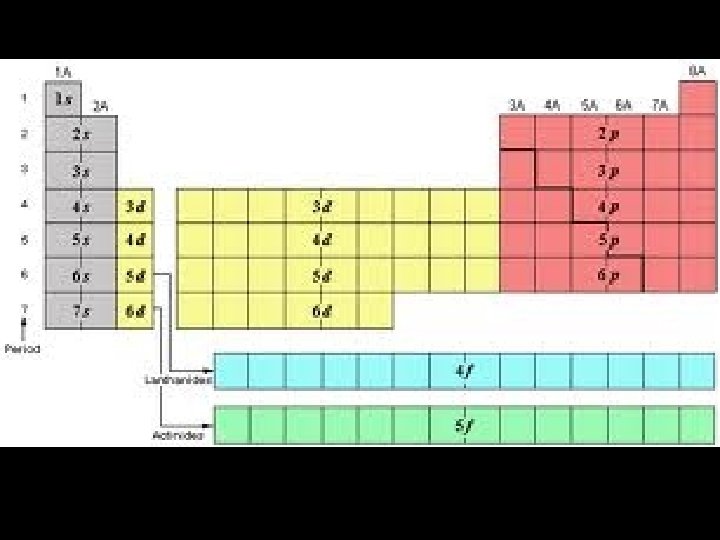
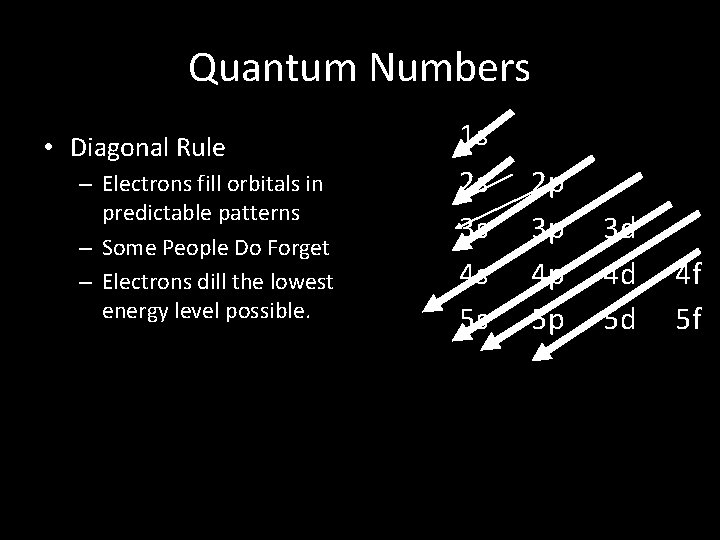
Quantum Numbers • Diagonal Rule – Electrons fill orbitals in predictable patterns – Some People Do Forget – Electrons dill the lowest energy level possible. 1 s 2 s 3 s 4 s 5 s 2 p 3 p 4 p 5 p 3 d 4 d 5 d 4 f 5 f

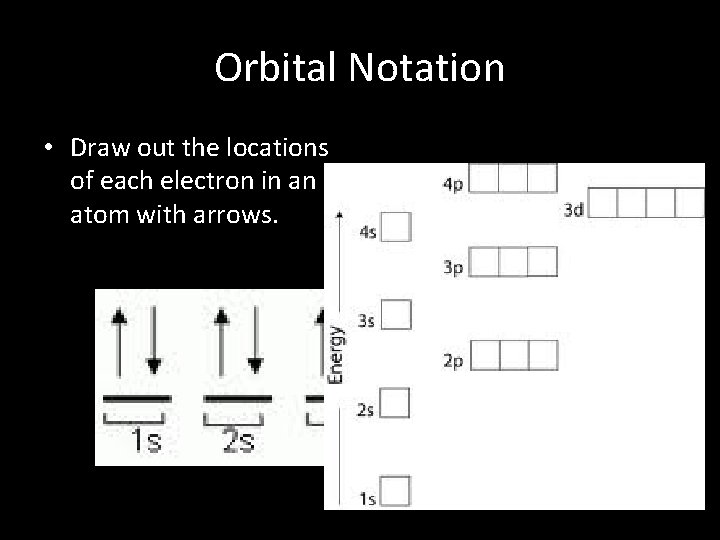
Orbital Notation • Draw out the locations of each electron in an atom with arrows.

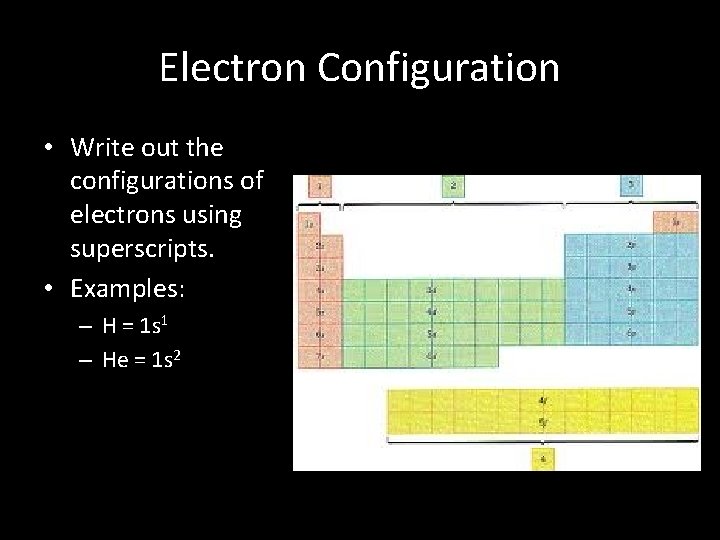
Electron Configuration • Write out the configurations of electrons using superscripts. • Examples: – H = 1 s 1 – He = 1 s 2

Electron Configurations • Noble Gas Shorthand – Write the Noble Gas just before the element. – Add the remainder of the configuration.

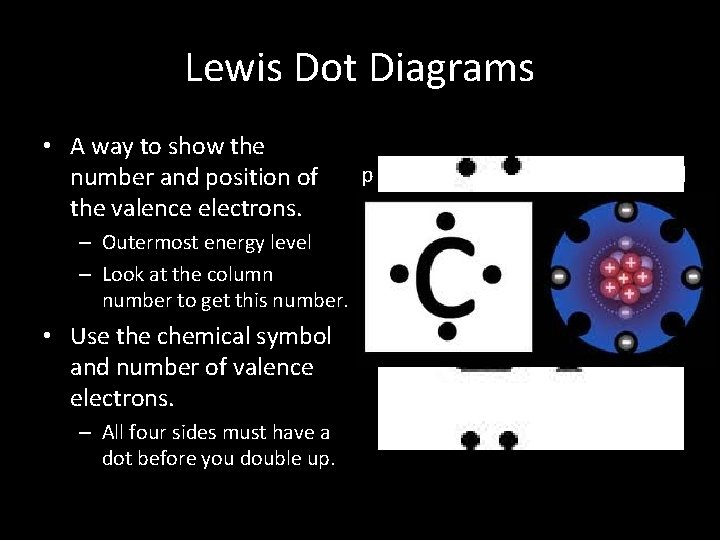
Lewis Dot Diagrams • A way to show the number and position of the valence electrons. – Outermost energy level – Look at the column number to get this number. • Use the chemical symbol and number of valence electrons. – All four sides must have a dot before you double up. p orbitals s orbital p 1 p 2 X p 3 s