DRUGS ACTING ON RAAS DR SHREETAL RAJAN NAIR














































![Trial data – aldosterone antagonists �Aldosterone antagonists (or mineralocorticoid receptor antagonists [MRAs]) are guideline-recommended Trial data – aldosterone antagonists �Aldosterone antagonists (or mineralocorticoid receptor antagonists [MRAs]) are guideline-recommended](https://slidetodoc.com/presentation_image/2438cc53e57bb8453633ac2fe82e28cd/image-81.jpg)




















- Slides: 110

DRUGS ACTING ON RAAS DR SHREETAL RAJAN NAIR

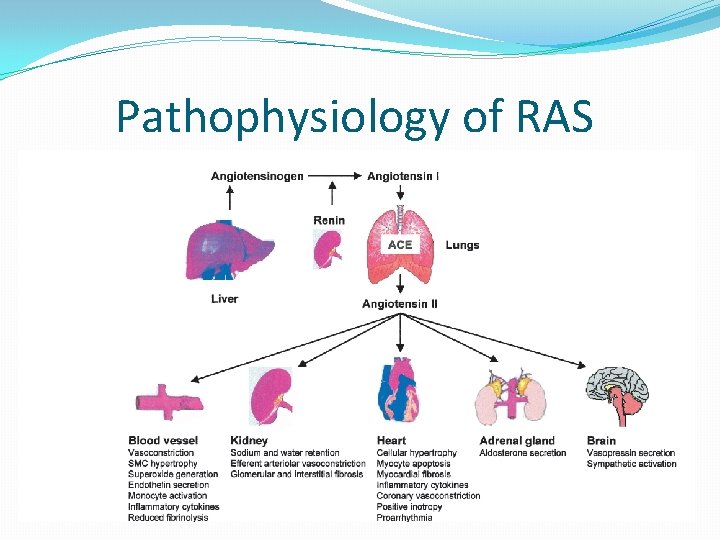
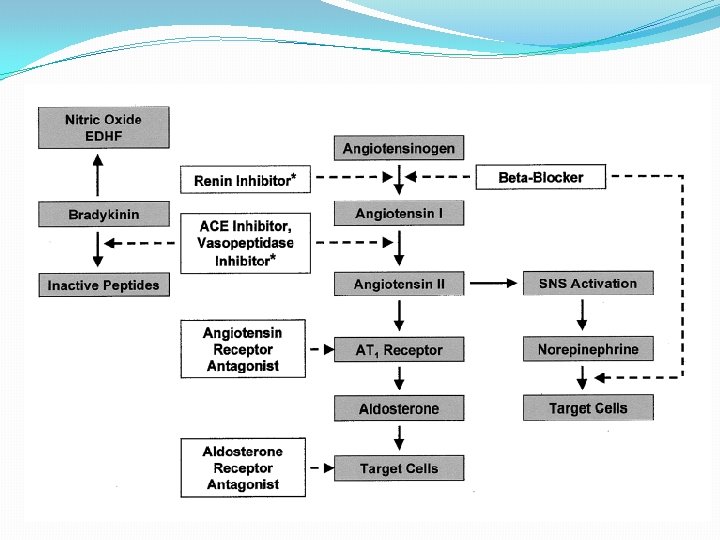
The Renin Angiotensin Aldosterone System (RAAS) �Most important neurohormonal system that maintains vascular tone and fluid-electrolyte balance in our body �Is involved in the pathophysiology of most cardiovascular diseases and hence its importance �Works through a negative feedback loop in our body

Pathophysiology of RAS


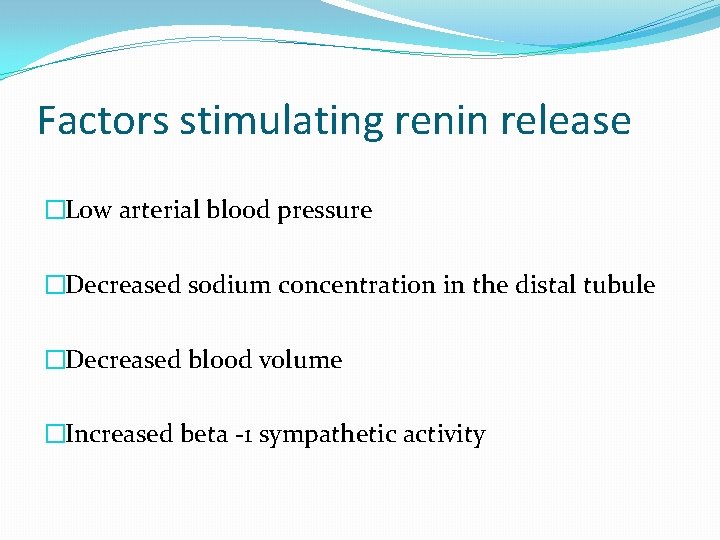
Factors stimulating renin release �Low arterial blood pressure �Decreased sodium concentration in the distal tubule �Decreased blood volume �Increased beta -1 sympathetic activity


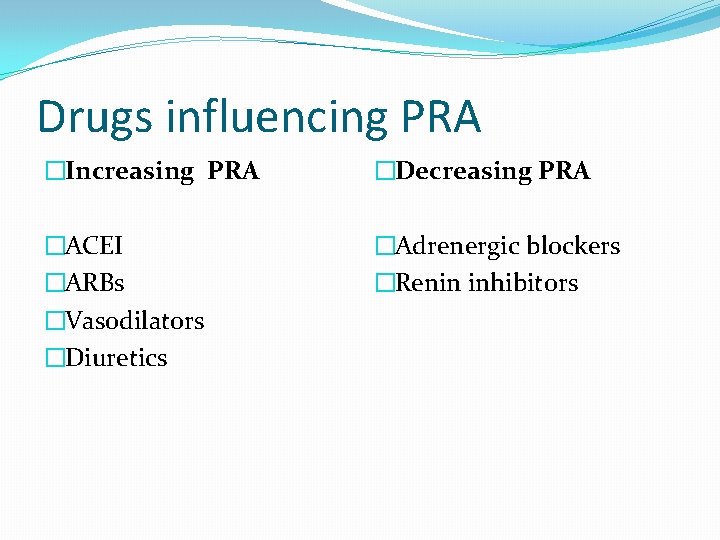
Drugs influencing PRA �Increasing PRA �Decreasing PRA �ACEI �ARBs �Vasodilators �Diuretics �Adrenergic blockers �Renin inhibitors



DRUGS ACTING ON RAAS �ACE Inhibitors �Angiotensin receptor blockers �Aldosterone antagonists �Renin inhibitors �New therapeutic pathways

ACE Inhibitors

Historical aspects �ACE was initially discovered from the venom of pit viper and named bradykinin potentiating factor and later it was found that this kininase and ACE were the same. �Teprotide was the first ACEI to be synthezised �But it had limitations �Later , captopril was developed as the first ACEI in 1977

ACEI �ACE inhibitors differ 1. in the chemical structure of their active moieties, 2. in potency, 3. in bioavailability, 4. in plasma half-life, 5. in route of elimination, 6. in their distribution and affinity for tissue-bound ACE, and 7. in whether they are administered as prodrugs.

ACE Inhibitors CLASSIFICATION � Class I : Containing a sulfhydryl group - Captopril ( proline derivative) � Class II : Prodrugs � Class III : Water soluble - Lisinopril ( Lysine derivative )

ACEI �Captopril is the prototype of the sulfhydryl-containing ACE inhibitors; others are fentiapril, pivalopril, zofenopril, and alacepril. �In vitro studies suggest that the presence of the sulfhydryl group may confer properties other than ACE inhibition to these drugs, such as free-radical scavenging and effects on prostaglandins �In vivo no much benefit has been found

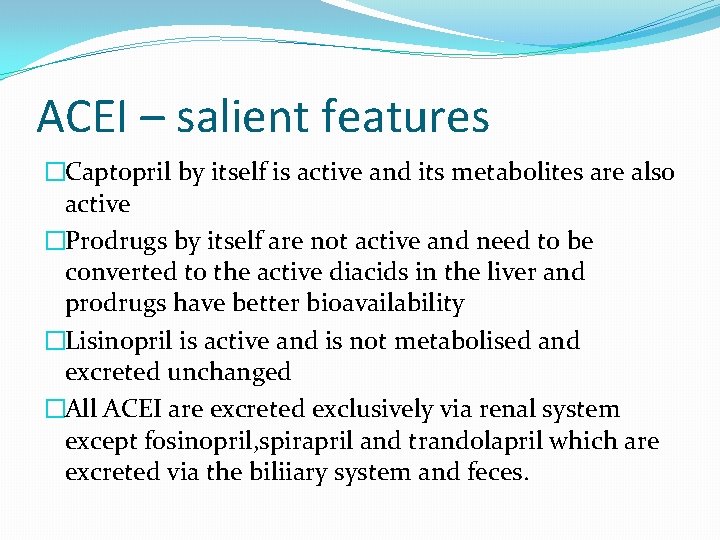
ACEI – salient features �Captopril by itself is active and its metabolites are also active �Prodrugs by itself are not active and need to be converted to the active diacids in the liver and prodrugs have better bioavailability �Lisinopril is active and is not metabolised and excreted unchanged �All ACEI are excreted exclusively via renal system except fosinopril, spirapril and trandolapril which are excreted via the biliiary system and feces.


ACEI – salient features �Bioavailability : highest – captopril; least – perindopril �Most prodrugs are carboxyl derivatives except fosinopril �Time to peak action – fastest captopril ( 1 hr) �Elimination t ½ - longest with ramipril (8 -48 hrs) �ACEI with duration of action > 24 hrs : enalapril, lisinopril, ramipril and perindopril �Captopril is the only ACEI to cross BBB but its clinical significance is unknown

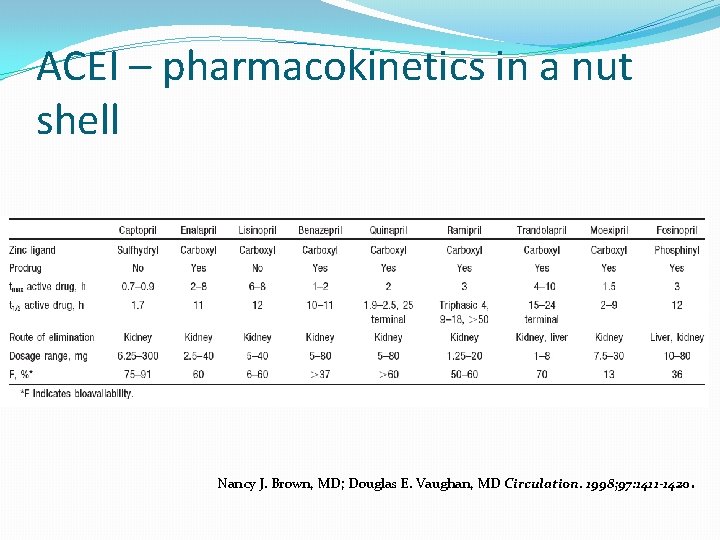
ACEI – pharmacokinetics in a nut shell Nancy J. Brown, MD; Douglas E. Vaughan, MD Circulation. 1998; 97: 1411 -1420.


ACEI- hemodynamic effects �ACEI are beneficial in many ways �Prevents generation of angiotensin II �Useful in conditions in which the renin angiotensin system is dysregulated like essential hypertension and renovascular hypertension �Decreases the peripheral vascular resistance �Fall in systolic and diastolic BP �No effect on cardiac output

INDICATIONS OF ACEI 1. Heart failure � Chronic heart failure due to any cause 2. AMI � Early phase � AMI with HF 3. Hypertension 4. Chronic Renal Disease 5. Diabetic nephropathy 6. Risk prevention

ACEI – other indications Non – cardiovascular 1. Diabetic and non- diabetic nephropathy 2. Scleroderma crisis ( captopril test to diagnose renovascular hypertension )


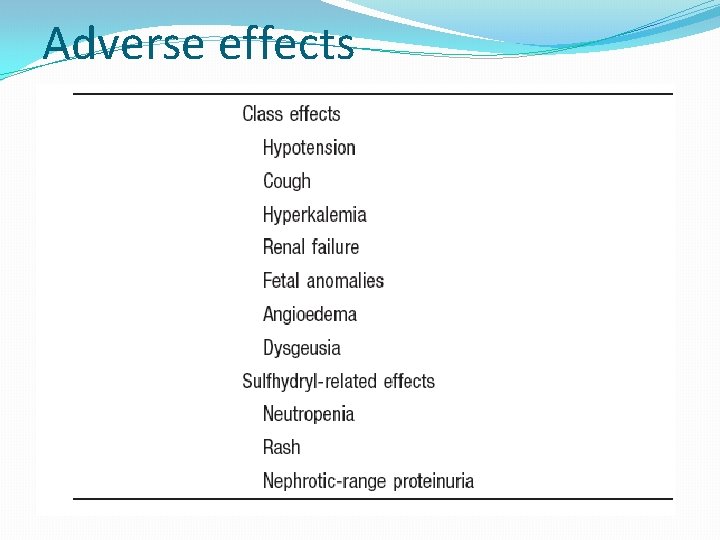
Adverse effects

Adverse effects �First dose hypotension more in diuretic treated patients �Hyperkalemia more in patients with impaired renal function and those taking potassium sparing diuretics and NSAIDs �Cough : occurs in patients within 1 -8 weeks ; subsides in 7 -21 days after discontinuation ; not dose related- earlier it was believed to be due to inhibition of bradykinin degradation; now it is believed to be mediated by substance P as kininase II is also believed to be degrade substance P. More in women

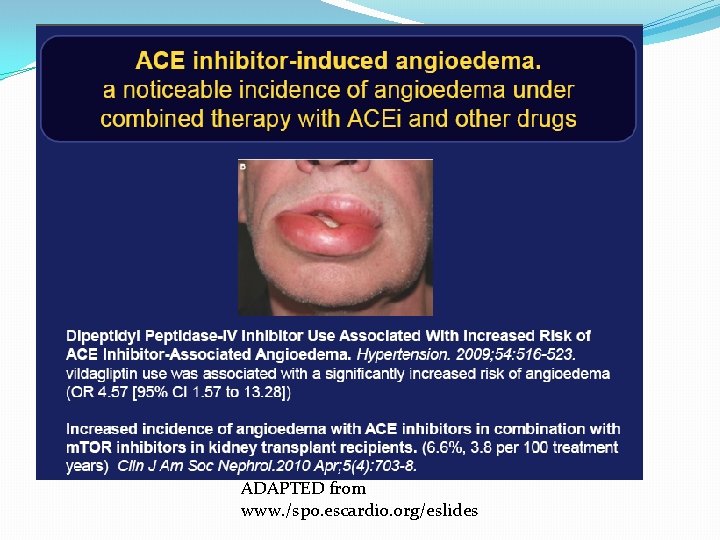
Adverse effects �Angioedema: the pathogenesis is found to be similar to the cough; cough and angioedema found in higher incidence in those concomitantly using DPP IV inhibitors as DPP is also responsible for substance P breakdown. �Rashes, urticaria: do not need drug stoppage �Dysguesia is a reversible alteration in the taste sensation – found in captopril treated patients and believed to be due to the sulfhydryl moiety

ADAPTED from www. /spo. escardio. org/eslides

Adverse effects �Granulocytopenia and proteinuria very rare but warrant withdrawal �Headache, dizziness , nausea and bowel upset- in 1 -4 % �ARF in bilateral RAS due to dilatation of efferent arterioles and fall in GFR and hence contraindicated �Fetopathic effects- 1 st trimester produces cardiovascular malformations ( PDA ) and 2 nd and 3 rd trimester responsible for oligohydramnios, fetal calvarial hypoplasia, fetal pulmonary hypoplasia, fetal growth retardation, fetal death, neonatal anuria, and neonatal death and hence contraindicated in pregnancy.

Interactions �NSAIDs especially in elderly, taking diuretics and ACEI �Potassium sparing diuretics/ on K+ supplements �Antacids decrease absorption �Reduce Lithium clearance and predispose to toxicity in those taking lithium �Caution in Impaired renal function, hypovolemia or dehydration

Contraindications �Bilateral RAS �Pregnancy �Hyperkalemia �Known allergy or hypersensitivity �Serum creatinine (>2. 5 – 3. 0 mg/dl ) arbitrary cut off in patients with heart failure

Pharmacogenomics �Found to be less effective in young and elderly blacks because are found to have less PRA. �Adding drugs which increase PRA like diuretics have found to increase response to therapy �An insertion (I)/deletion (D) polymorphism in the ACE gene that correlates with ACE activity such that ACE levels are highest in patients who are homozygous for the ACE D allele, lowest in patients homozygous for the ACE I allele, and intermediate in those who are heterozygous. Persons with the D/D phenotype had subdued response to the ACEI �Angioedema more in whites.

Some new concepts in ACE inhibition �ACE inhibitors have also been shown to cause a central enhancement of parasympathetic nervous system activity in healthy volunteers and patients with heart failure. This action may reduce the prevalence of malignant cardiac arrhythmias, and the reduction in sudden death. �The ACE inhibitor enalapril has also been shown to reduce cardiac cachexia in patients with chronic heart failure. Cachexia is a poor prognostic sign in patients with chronic heart failure

Some new concepts in ACE inhibition �ACE inhibitors are under early investigation for the treatment of frailty and muscle wasting (sarcopenia) in elderly patients without heart failure �The lactotripeptides Val-Pro and Ile-Pro produced by the probiotic Lactobacillus helveticus or derived from casein have been shown to have ACEinhibiting and antihypertensive functions ( discovered in Japan IN 1991)


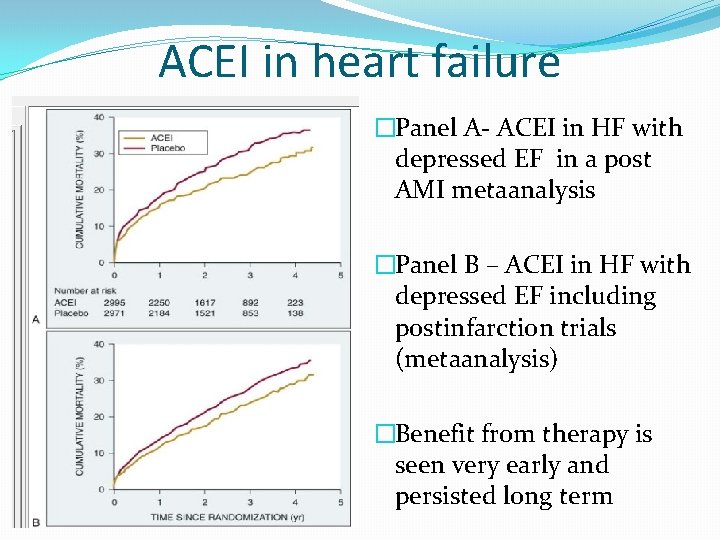
ACEI in heart failure �Panel A- ACEI in HF with depressed EF in a post AMI metaanalysis �Panel B – ACEI in HF with depressed EF including postinfarction trials (metaanalysis) �Benefit from therapy is seen very early and persisted long term

Omapatrilat �Omapatrilat was a novel antihypertensive agent that inhibits both neutral endopeptidase (NEP) and angiotensin converting enzyme (ACE). � NEP inhibition results in elevated natriureticpeptide levels, promoting natriuresis, diuresis, vasodilation, and reductions in preload and ventricular remodeling. �This was being promoted for CHF but was not approved by the FDA due to angioedema safety concerns

Omapatrilat - trials �The OVERTURE (Omapatrilat Versus Enalapril Randomized Trial of Utility in Reducing Events) study: Omapatrilat was as good as enalapril but not better �In the OCTAVE (Omapatrilat Cardiovascular Treatment Assessment Versus Enalapril) study 25, 267 hypertensives were randomised to Omapatrilat or enalapril and a difference of approximately 3 mm. Hg in favour of Omapatrilat was seen. �Significantly more cases of angioedema were seen with Omapatrilat in both trials.

IMPRESS randomised trial �Comparison of vasopeptidase inhibitor, omapatrilat, and lisinopril on exercise tolerance and morbidity in patients with heart failure �Showed a trend in favour of omapatrilat

Angiotensin receptor blockers (ARBs)

ARBs �The ARBs act on the next step in RAAS and they block the angiotensin II receptor through which angiotensin II exerts its effects �Why the need of ARBs arose after ACEI ? 1. Clinical and experimental studies showed the initial suppression of angiotensin II after the administration of angiotensin-converting enzyme (ACE) inhibitors is later reversed and returns almost to pretreatment levels. �The ESCAPE phenomenon was hypothesized which was strengthened by the discovery that angiotensin II can also be generated through non-ACEs 2. Increased incidence of adverse effects with ACEI therapy

Advantages of ARBs over ACEI �Do not interfere with degradation of bradykinin and other ACE substrates �More complete inhibition of AT 1 receptor activation �Indirect activation of AT 2 receptor �Other molecular effects apart from the receptor action

�Losartan was the first ARB to be synthezised and it was a imidazole derivative �All ARBs expect for losartan are highly selective for the AT 1 receptor. In fact, ARBs show 10, 000– 30, 000 times greater affinity for the AT 1 receptor than for the AT 2 receptor �The majority of ARBs produce insurmountable antagonism ( non competitive inhibition )

What is the advantage of AT 1 receptor specificity ? �AT 2 receptor may be exposed to a higher concentration of Ang II �It increases the Ang II-induced AT 2 receptor stimulation which may cause anti-cell proliferation and vasodilation

Other effects of ARBs �Inverse agonism of AT 1 receptor �Anti platelet effects �Anti – inflammatory effects �Reduction in serum uric acid levels


Benefits of Inverse agonism 1. Sometimes AT 1 receptors are mutated and have constitutive activity which means the receptors can get activated in the absence of its ligand �Constitutive has also been found in wild type receptors �Losartan, valsartan, olmesartan and candesartan have significant inverse agonism

Benefits of inverse agonism 2. AT 1 receptor m. RNA levels are upregulated by myocyte stretching over time �Studies have demonstrated that the AT 1 receptor is activated by the mechanical stretching of cultured rat myocytes and constriction of the transverse aorta in angiotensinogen knockout mice without the involvement of Ang II, and these adverse effects were suppressed by an inverse agonist

Anti platelet effects �Losartan has some degree of antagonistic action on the thromboxane A 2 receptor which is responsible for the platelet antiaggregatory effects

Anti inflammatory effects �Ang II induces inflammation in vasculature and vascular remodeling, and subsequently promotes atherosclerosis. Ang II stimulates monocyte chemoattractant protein-1 (MCP-1), interleukin (IL)-8, tumor necrosis factor-a and IL-6 production �Decrease in MCP-1 levels seen with irbesartan and losartan �Increase in adiponectin expression seen with irbesartan, losartan, candesartan and telmisartan

Anti inflammatory effects �PPAR gamma activation with eprosartan – may be one reason of preventing New Onset Diabetes(NOD), the other being increased adiponectin levels �Irbesartan and olmesartan act as antagonists of chemokine receptors Actions are independent of actions on the AT 1 and AT 2 receptors and suggest a molecular level of action for the ARBs

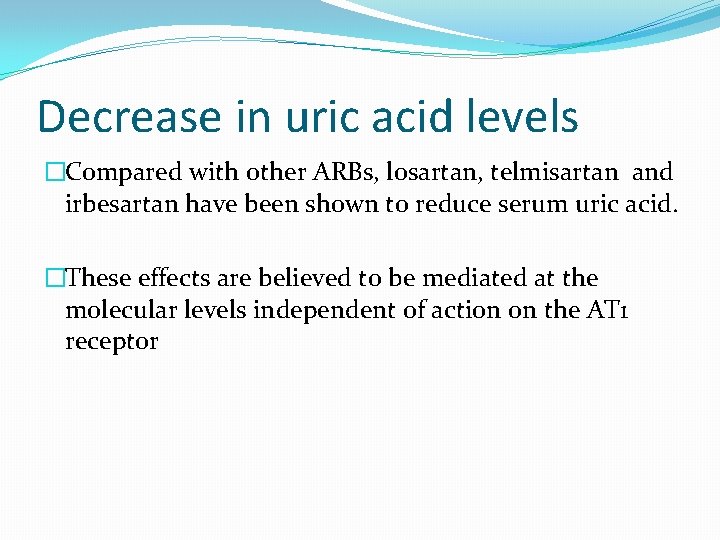
Decrease in uric acid levels �Compared with other ARBs, losartan, telmisartan and irbesartan have been shown to reduce serum uric acid. �These effects are believed to be mediated at the molecular levels independent of action on the AT 1 receptor

In a nut shell Angiotensin II type 1 receptor blockers: class effects versus molecular effects Shin-ichiro Miura, Sadashiva S. Karnik and Keijiro Saku -Journal ofthe Renin-Angiotensin-Aldosterone System (Including other Peptidergic systems) March 2011 Volume 12 Number 1

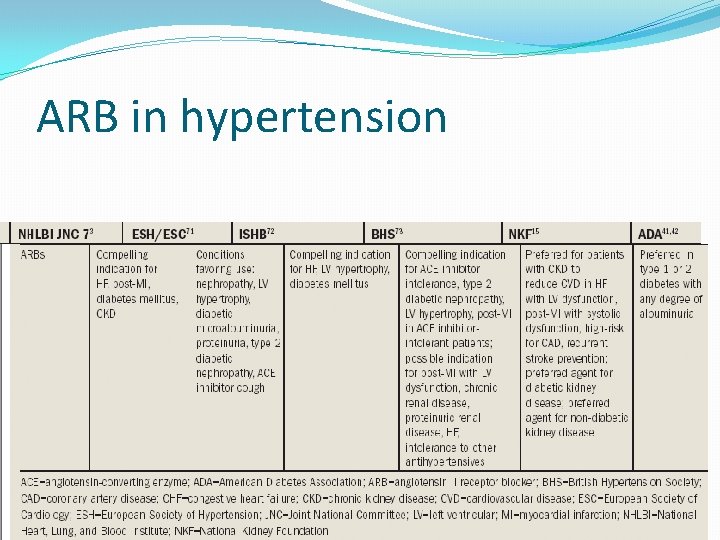
ARB in hypertension


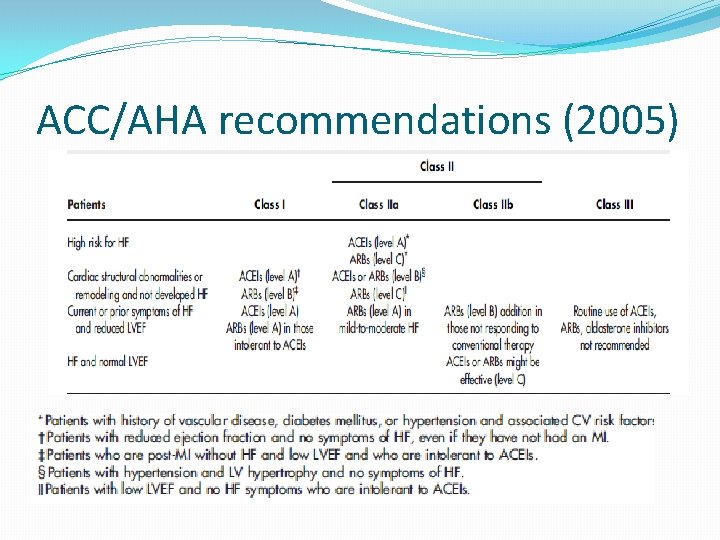
ACC/AHA recommendations (2005)


The concept of dual RAAS blockade with ACEI and ARB �Arose because of the phenomenon of escape phenomenon with ACEI �To achieve complete and more effective blockade of Angotensin action �It was combining ACEI and ARB would be of benefit but studies did not give promising results �Now dual ACEI and ARB therapy not recommended except in non- diabetic renal disease (COOPERATE, 2003 trial showed progression of non diabetic renal disease retarded to a greater extent than with monotherapy.

Some salient features of ARBs �Highest affinity for AT 1 receptor – candesartan �Longest duration of action - telmisartan �All ARBs need dose reduction in liver diseases

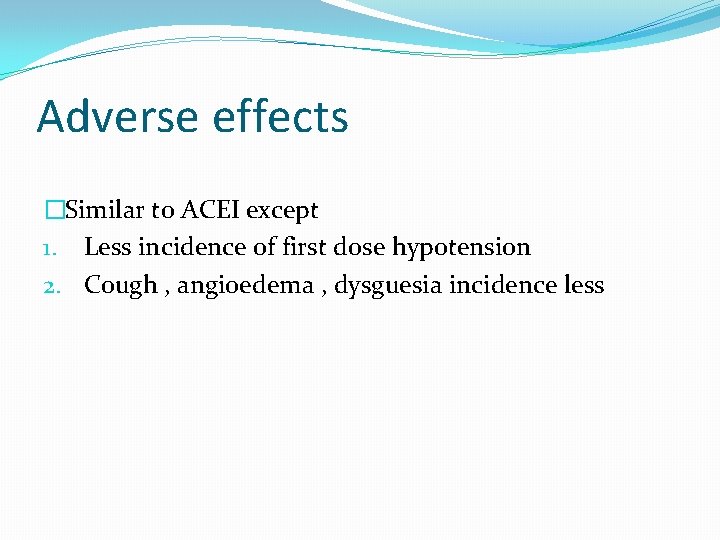
Adverse effects �Similar to ACEI except 1. Less incidence of first dose hypotension 2. Cough , angioedema , dysguesia incidence less

Interactions �Similar to that with ACEI

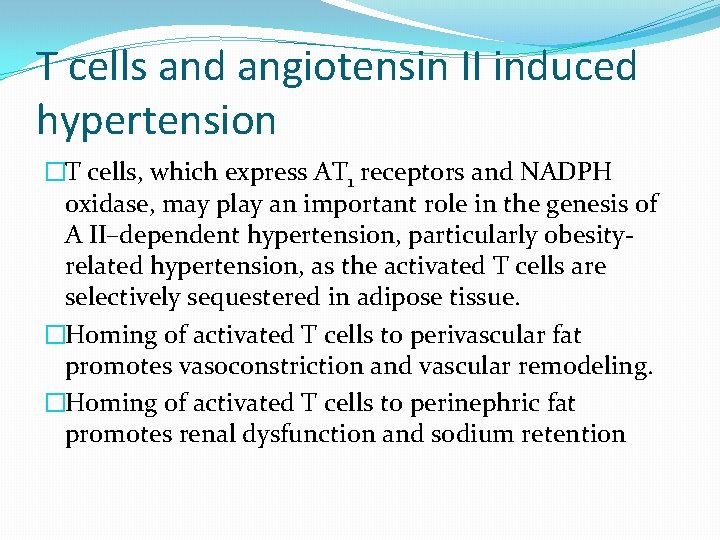
T cells and angiotensin II induced hypertension �T cells, which express AT 1 receptors and NADPH oxidase, may play an important role in the genesis of A II–dependent hypertension, particularly obesityrelated hypertension, as the activated T cells are selectively sequestered in adipose tissue. �Homing of activated T cells to perivascular fat promotes vasoconstriction and vascular remodeling. �Homing of activated T cells to perinephric fat promotes renal dysfunction and sodium retention

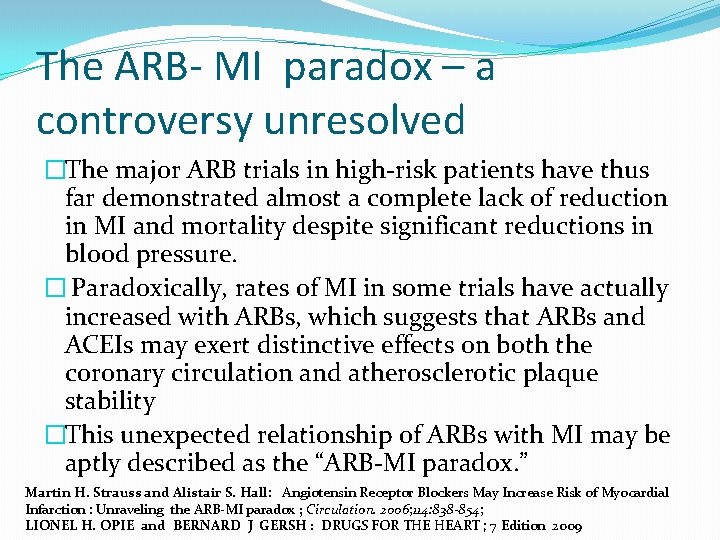
The ARB- MI paradox – a controversy unresolved �The major ARB trials in high-risk patients have thus far demonstrated almost a complete lack of reduction in MI and mortality despite significant reductions in blood pressure. � Paradoxically, rates of MI in some trials have actually increased with ARBs, which suggests that ARBs and ACEIs may exert distinctive effects on both the coronary circulation and atherosclerotic plaque stability �This unexpected relationship of ARBs with MI may be aptly described as the “ARB-MI paradox. ” Martin H. Strauss and Alistair S. Hall: Angiotensin Receptor Blockers May Increase Risk of Myocardial Infarction : Unraveling the ARB-MI paradox ; Circulation. 2006; 114: 838 -854; LIONEL H. OPIE and BERNARD J GERSH : DRUGS FOR THE HEART ; 7 Edition 2009

Aldosterone antagonists

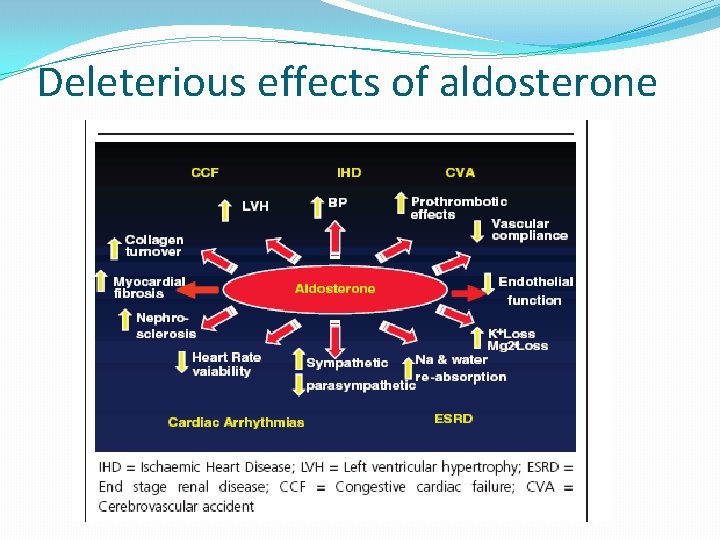
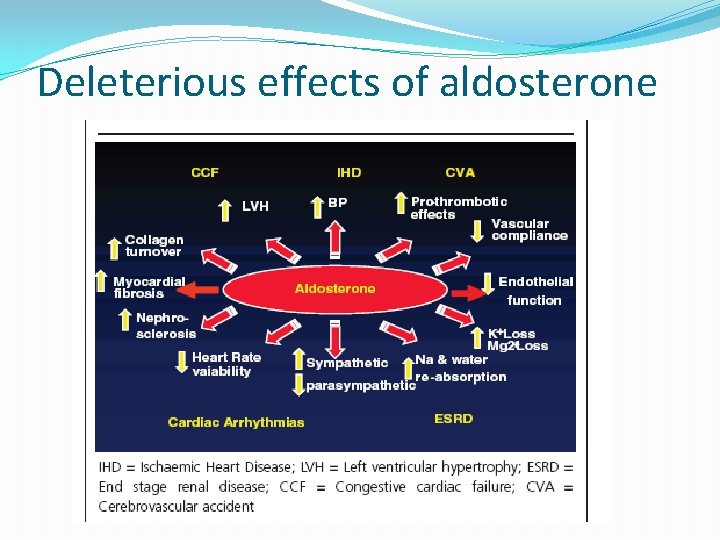
Role of aldosterone in CV disease �Direct correlation of aldosterone levels and mortality in heart failure �Increase in myocardial fibrosis �Inhibition of the release of NO �Increased incidence of arrhythmias �Increased response to vasoconstrictor doses of angiotensin I �Critical mediator of early angiotensin II induced experimental myocardial injury

Deleterious effects of aldosterone

Aldosterone antagonists �Spironolactone �Eplerenone �Canrenone ( available only in europe)

What do they do? �Decrease extracellular markers of fibrosis �Decreases the release of cardiac norepinephrine �Vasodilator effects

Spironolactone- mechanism of action �It is a steroid chemically related to aldosterone �Competitive inhibition of the mineralocorticoid receptor from the interstitial side of the tubular cell �Inhibits formation of (Aldosterone induced proteins) AIPs


Spironolactone �Spironolactone acts as an antagonist and/or agonist at the following sites: �Antagonism Mineralocorticoid receptor Androgen receptor �Agonism Progesterone receptor Glucocorticoid receptor

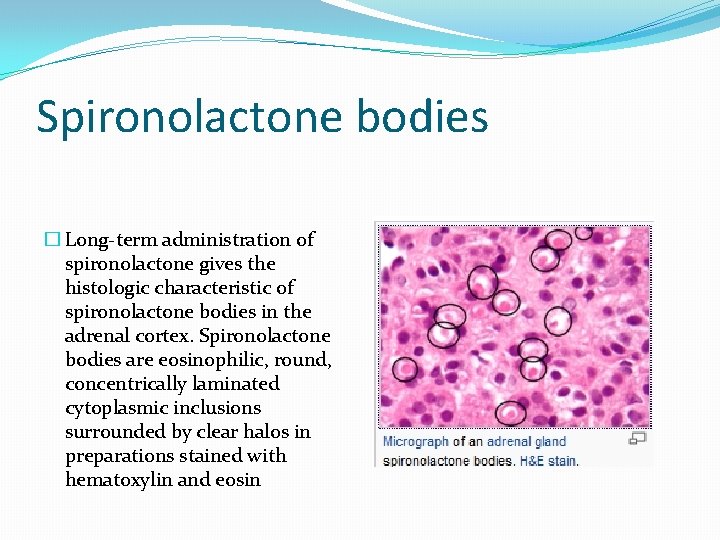
Spironolactone bodies � Long-term administration of spironolactone gives the histologic characteristic of spironolactone bodies in the adrenal cortex. Spironolactone bodies are eosinophilic, round, concentrically laminated cytoplasmic inclusions surrounded by clear halos in preparations stained with hematoxylin and eosin

Contraindications �Hyperkalemic states �Pregnancy - high risk of feminisation of female fetuses

Pharmacokinetics � 75% oral bioavailability �Highly bound to plasma proteins �Completely metabolised in the liver �Active metabolite canrenone ( ½ - 2/3 rd of action in vivo is due to this metabolite) �Half life of spironolactone is 1 -2 hours; canrenone is 18 hours

Indications �Hypertension �To improve survival of stable patients with LV systolic dysfunction ( EF </= 40% ) �Clinical evidence of CHF after AMI �Refractory edema in cirrhosis and renal disease – helps to breakdown resistance to thiazide diuretics due to secondary hyperaldosteronism �To counteract K+ loss due to thiazide and loop diuretics

Interactions �Dangerous hyperkalemia can occur if given with K+ supplements or drugs causing hyperkalemia ( ACEI or ARBs) �Aspirin blocks action of spironolactone by inhibiting tubular secretion of canrenone �Spironolactone increases plasma digoxin concentration

Adverse effects �Drowsiness , confusion and abdominal discomfort �Hirsutism, gynecomastia, impotence and menstrual irregularities �Hyperkalemia especially in renal disease �Risk of acidosis in cirrhotics

Eplerenone �More selective aldosterone antagonist �Less likely to cause hormonal disturbances like gynecomastia, impotence and menstrual irregularities

EPLERENONE �Starting dose 25 mg daily increased to 50 mg daily if serum potassium < 5. 0 m. Eq/L �If serum K+ is > 5. 5 m. Eq/L then the dose must be decreased or discontinued �Specific warning in T 2 DM with hypertension and microalbuminuria because of the risk of hyperkalemia �Dose for hypertension ; 50 -100 mg once daily �Equally effective in white and black patients.

![Trial data aldosterone antagonists Aldosterone antagonists or mineralocorticoid receptor antagonists MRAs are guidelinerecommended Trial data – aldosterone antagonists �Aldosterone antagonists (or mineralocorticoid receptor antagonists [MRAs]) are guideline-recommended](https://slidetodoc.com/presentation_image/2438cc53e57bb8453633ac2fe82e28cd/image-81.jpg)
Trial data – aldosterone antagonists �Aldosterone antagonists (or mineralocorticoid receptor antagonists [MRAs]) are guideline-recommended therapy for patients with moderate to severe heart failure (HF) symptoms and reduced left ventricular ejection fraction (LVEF), and in postmyocardial infarction patients with HF.

Trial data �RALES, TOPCAT ( spironolactone) �EPHESUS ; EMPHASIS-HF ; 4 E ( eplerenone) �AREA IN CHF ( canrenone )

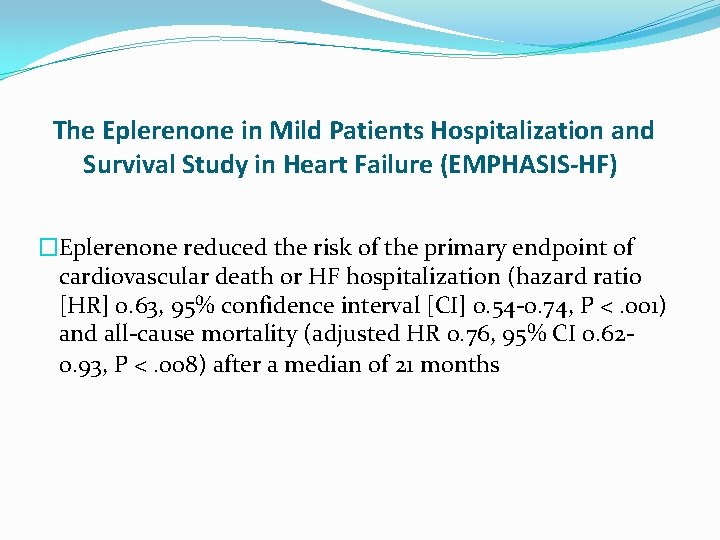
The Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF) �Eplerenone reduced the risk of the primary endpoint of cardiovascular death or HF hospitalization (hazard ratio [HR] 0. 63, 95% confidence interval [CI] 0. 54 -0. 74, P <. 001) and all-cause mortality (adjusted HR 0. 76, 95% CI 0. 620. 93, P <. 008) after a median of 21 months

� The Randomized Aldactone Evaluation Study(RALES) Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) established that spironolactone and eplerenone, respectively, increased survival in patients with severe CHF symptoms from LV systolic dysfunction occurring with minimal exertion or at rest (New York Heart Association [NYHA] class III or IV) or CHF after an acute myocardial infarction

THE 4 E STUDY �The 4 E Study (Eplerenone, Enalapril, and Eplerenone/Enalapril Combination Therapy in Patients with Left Ventricular Hypertrophy) compared the effects of 9 -month treatment with eplerenone 200 mg/d (n=64), enalapril 40 mg/d (n=71), or eplerenone 200 mg/d plus enalapril 10 mg/d (n= 67) on LV mass, systolic and diastolic blood pressures, and urinary albumin-creatinine ratio (UACR) in patients with mild-to-moderate hypertension and echocardiographic evidence of LVH. �Combination therapy with eplerenone and enalapril significantly reduced LV mass

Ongoing trial �TOPCAT study (Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist)- is ongoing �At this time, however, there are insufficient clinical data to recommend the use of aldosterone antagonist therapy for the treatment of diastolic dysfunction.

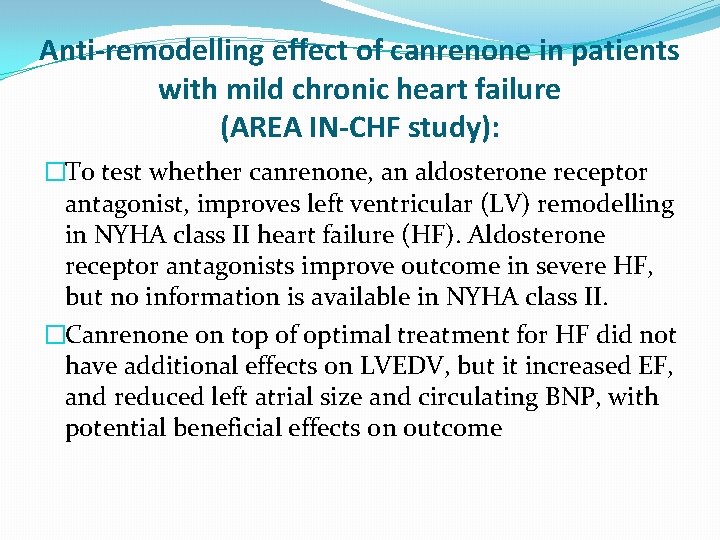
Anti-remodelling effect of canrenone in patients with mild chronic heart failure (AREA IN-CHF study): �To test whether canrenone, an aldosterone receptor antagonist, improves left ventricular (LV) remodelling in NYHA class II heart failure (HF). Aldosterone receptor antagonists improve outcome in severe HF, but no information is available in NYHA class II. �Canrenone on top of optimal treatment for HF did not have additional effects on LVEDV, but it increased EF, and reduced left atrial size and circulating BNP, with potential beneficial effects on outcome

Direct renin inhibitors

DRI- Historical aspects �The concept of blocking the RAAS at its origin by inhibiting renin has existed for at least 50 years. �The first synthetic renin inhibitor was pepstatin, which was followed by first-generation agents that were active but required parenteral administration �Oral agents that were subsequently developed, such as enalkiren, remikiren, and zankiren, had limited clinical use because they demonstrated poor bioavailability (< 2%), short half-lives, and weak antihypertensive activity

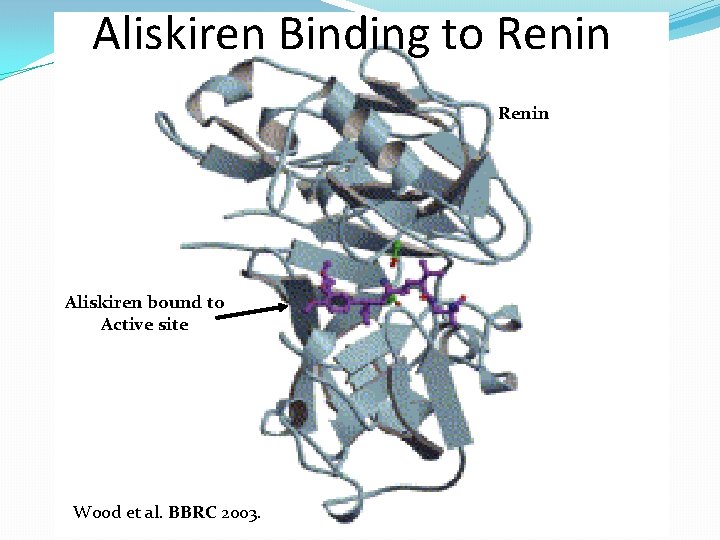
Aliskiren �Aliskiren is the first synthetic non peptide direct renin inhibitor (DRI) to be approved by the U. S. Food and Drug Administration and the European Medicines Agency for treating hypertension in 2007. �Aliskiren binds to the active site (S 1/S 3 hydrophobic binding pocket) of renin, preventing the conversion of angiotensinogen to angiotensin I

Aliskiren Binding to Renin Aliskiren bound to Active site Wood et al. BBRC 2003.

ALISKIREN named after ALICE HUXLEY

Aliskiren - pharmacokinetics �Direct renin inhibitor � 50 – 80 % decrease plasma renin activity �Pharmacokinetics �Accumulation Half-life of ~ 24 hours �Oral bioavailability of 2. 6% � 7 – 8 days to achieve steady state levels �Elimination Half-life of ~ 48 hours � 25 % excreted by kidneys �Metabolized by CYP 450 -3 A 4 �Does not induce or suppress CYP 450 �No effect on QT interval

Aliskiren – pharmacokinetics �Pathway of elimination for aliskiren is via biliary excretion as unmetabolized drug. �Less than 1% of an orally administered dose is excreted in urine. �Not metabolized by, and does not induce or inhibit, cytochrome P 450 enzymes and shows no clinically relevant pharmacokinetic interactions with warfarin, lovastatin, atenolol, celecoxib, cimetidine, amlodipine, valsartan, hydroc hlorothiazide (HCTZ), or ramipril. �The pharmacokinetics of aliskiren remain unaffected by ethnicity, age, gender, hepatic impairment, renal impairment, and diabetes.

Drug interactions �It reduces furosemide blood concentration. �Atorvastatin may increase blood concentration, but no dose adjustment is needed. �Due to possible interaction with ciclosporin, the concomitant use of ciclosporin and aliskiren is contraindicated. �Aliskiren is a minor substrate of CYP 3 A 4 and, more important, P-glycoprotein �Caution should be exercised when aliskiren is administered with ketoconazole or other moderate P-gp inhibitors (itraconazole, clarithromycin, telithromycin, erythromycin, or amiodarone). �Doctors should stop prescribing aliskiren-containing medicines to patients with diabetes (type 1 or type 2) or with moderate to severe kidney impairment who are also taking an ACE inhibitor or ARB, and should consider alternative antihypertensive treatment as necessary

Adverse effects �Angioedema �Hyperkalemia (particularly when used with ACE inhibitors in diabetic patients) �Hypotension (particularly in volume-depleted patients) �Diarrhea and other GI symptoms �Headache, Dizziness �Cough �Rash �Elevated uric acid, gout, and renal stones

Potential therapeutic roles of aliskiren �Monotherapy for hypertension �Component of combination therapy for hypertension, with a diuretic, a CCB, an ACEI, and/or an ARB �Alternative to ACEIs or ARBs in the management of hypertension and the prevention of organ damage �Alternative to ACEIs in patients with diabetic nephropathy or cardiovascular disease �Use in patients with diabetic nephropathy or in African American hypertensive patients, in whom intrarenal angiotensin II formation occurs via ACE or non–ACEdependent pathways

ali

ATMOSPHERE (Efficacy and Safety of Aliskiren and Aliskiren/Enalapril Combination on Morbi-mortality in Patients With Chronic Heart Failure) study �Aliskiren is currently being evaluated in a phase III study that will evaluate the efficacy and safety of both aliskiren monotherapy and aliskiren-enalapril combination therapy as compared with enalapril monotherapy in regard to cardiovascular death and heart failure hospitalizations in NYHA Classes II to IV HF patients


Aldosterone antagonists

Role of aldosterone in CV disease �Direct correlation of aldosterone levels and mortality in heart failure �Increase in myocardial fibrosis �Inhibition of the release of NO �Increased incidence of arrhythmias �Increased response to vasoconstrictor doses of angiotensin I �Critical mediator of early angiotensin II induced experimental myocardial injury

Deleterious effects of aldosterone

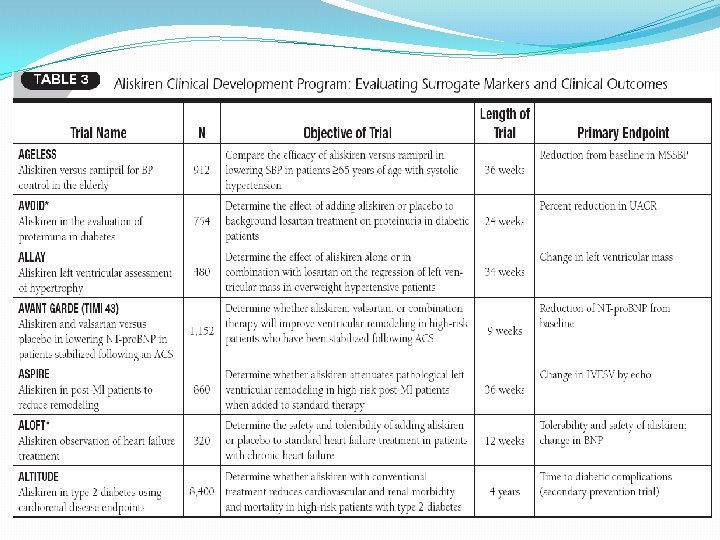
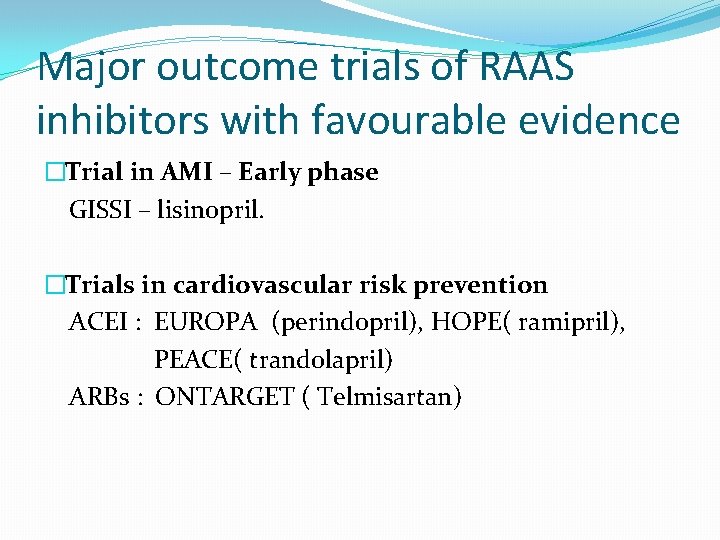
Major outcome trials of RAAS inhibitors with favourable evidence Trials in post MI HF �ACEI – SAVE (Captopril), AIRE (Ramipril ), TRACE (Trandolapril) �ARB – VALIANT (Valsartan ) �Aldosterone antagonists ( EPHESUS)

Major outcome trials of RAAS inhibitors with favourable evidence �Trial in AMI – Early phase GISSI – lisinopril. �Trials in cardiovascular risk prevention ACEI : EUROPA (perindopril), HOPE( ramipril), PEACE( trandolapril) ARBs : ONTARGET ( Telmisartan)

New therapeutic pathways in ACE inhibition

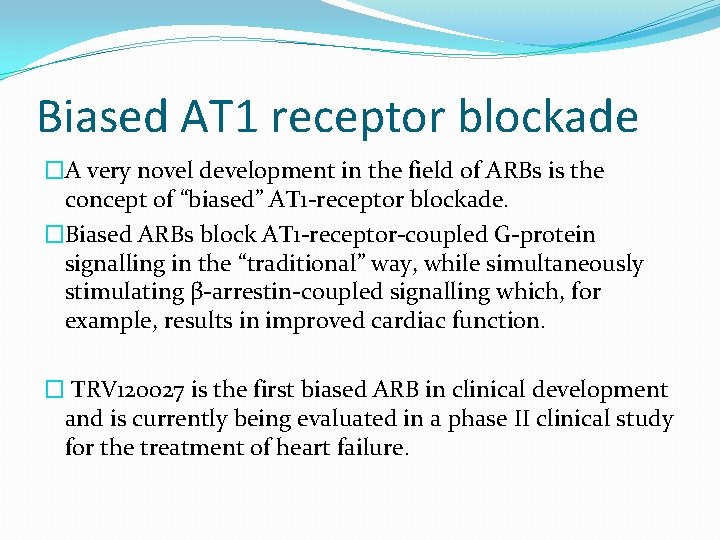
Biased AT 1 receptor blockade �A very novel development in the field of ARBs is the concept of “biased” AT 1 -receptor blockade. �Biased ARBs block AT 1 -receptor-coupled G-protein signalling in the “traditional” way, while simultaneously stimulating β-arrestin-coupled signalling which, for example, results in improved cardiac function. � TRV 120027 is the first biased ARB in clinical development and is currently being evaluated in a phase II clinical study for the treatment of heart failure.

AT 1 -receptor blockade combined with neutral endopeptidase inhibition �NEP is responsible for the degradation of atrial and brain natriuretic factor, which both have cardioprotective properties. Consequently, inhibition of NEP increases plasma levels of these protective molecules. �LCZ 696, which may become the first in class ARNI (AT 1 receptor and NEP inhibitor) lowered blood-pressure more effectively than valsartan monotherapy in a phase II clinical trial. �Phase III clinical trials are currently ongoing to test LCZ 696 for the treatment of heart failure. �Outcomes of these studies and data about long-term safety (potential risks for obesity, Alzheimer’s disease and angioedema have been discussed) have to be awaited

Novel therapies �AT 1 -receptor blockade combined with endothelin A receptor blockade RE-021 is a dual AT 1 -receptor and endothelin-A receptor (ETA) antagonist which was successfully taken through a phase IIb study in patients in hypertension. It has potential for use in FSGS �AT 1 -receptor blockade combined with nitric oxide (NO) release is in the pipeline

Novel therapies �AT 2 RECEPTOR AGONISTS potential use in post-myocardial infarction (MI) cardiac function, hypertension-induced vascular remodelling, pulmonary hypertension), neurological (e. g. stroke, spinal cord injury, Alzheimer’s disease) and immunological (e. g. multiple sclerosis, rheumatoid arthritis) diseases �AT 2 RECEPTOR ANTAGONISTS in post herpetic pain �ANGIOTENSIN 1 -7 analogues and special formulations in hypertension, post-MI cardiac failure, metabolic syndrome, diabetes, renal disease and RA �Recombinant ACE 2 and ACE 2 formulations
 Dr. rajan joshi
Dr. rajan joshi Rajan dermatology
Rajan dermatology Sandeep rajan md
Sandeep rajan md Rajan mahadevan
Rajan mahadevan Rajan kapur
Rajan kapur Causes of hyperkalemia
Causes of hyperkalemia Guyton
Guyton Raas mechanism
Raas mechanism Raas
Raas Albuminouria
Albuminouria Raas system
Raas system Raas
Raas Macula densa
Macula densa Naav raas
Naav raas Fortecor
Fortecor Impulse pneumatic circuit
Impulse pneumatic circuit Double acting cylinder with 5/2 way valve
Double acting cylinder with 5/2 way valve Difference between single acting and double acting
Difference between single acting and double acting Method acting vs natural acting
Method acting vs natural acting Indirect acting cholinergic drugs
Indirect acting cholinergic drugs Irreversible indirect acting cholinergic agonist
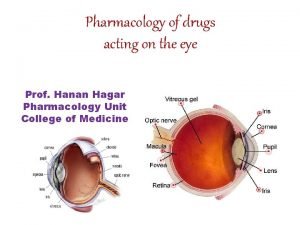
Irreversible indirect acting cholinergic agonist Pharmacology of drugs acting on respiratory system
Pharmacology of drugs acting on respiratory system Centrally acting sympathoplegic drugs
Centrally acting sympathoplegic drugs Rema nair
Rema nair Dr ajay nair
Dr ajay nair Ram nair
Ram nair Photomath qualification test
Photomath qualification test Vincent nair
Vincent nair Gopakumar nair associates
Gopakumar nair associates Ajai nair
Ajai nair Dr dinesh nair
Dr dinesh nair Bhavana nair
Bhavana nair When was nair invented
When was nair invented Bolshevik nair
Bolshevik nair Aakash r nair
Aakash r nair Dr gopakumar nair
Dr gopakumar nair Impact of tidal energy on environment
Impact of tidal energy on environment Dr bipin nair
Dr bipin nair Dyuthi nair
Dyuthi nair Basic propeller principles
Basic propeller principles What forces are acting on you right now?
What forces are acting on you right now? What are the basic principles of pantomime
What are the basic principles of pantomime Joaquin phoenix your honor
Joaquin phoenix your honor What is the only force acting on the projectile
What is the only force acting on the projectile Head race and tail race
Head race and tail race V effect brecht
V effect brecht The main force acting on the body is the
The main force acting on the body is the Nepa cil acting as agent
Nepa cil acting as agent Tubokurarin
Tubokurarin Dominant on
Dominant on Audition script
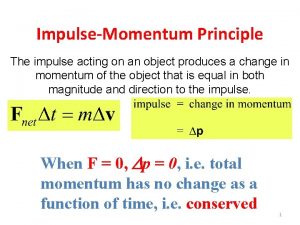
Audition script Principle of impulse
Principle of impulse Acting the first six lessons summary
Acting the first six lessons summary Pneumatic system symbol
Pneumatic system symbol Gravity return single acting cylinder
Gravity return single acting cylinder The position of the acting area in relation to the audience
The position of the acting area in relation to the audience Forces acting on earthen dam
Forces acting on earthen dam Pantomime is without words.
Pantomime is without words. Elizabethan acting companies
Elizabethan acting companies Acting on feedback
Acting on feedback Strainer symbol
Strainer symbol The only force acting in a vertical projectile motion is
The only force acting in a vertical projectile motion is Double acting reciprocating compressor
Double acting reciprocating compressor Rapid acting prandial insulin
Rapid acting prandial insulin Aircraft hydraulic system
Aircraft hydraulic system The discharge through a single acting reciprocating pump is
The discharge through a single acting reciprocating pump is Soft news
Soft news Is an object upon which the only force acting is gravity.
Is an object upon which the only force acting is gravity. Forces acting at a point in statics
Forces acting at a point in statics Elliptical clause
Elliptical clause Basic acting skills
Basic acting skills What is the buoyant force acting on the block?
What is the buoyant force acting on the block? Ciliary muscle contraction
Ciliary muscle contraction Hydraulic cylinder cushions
Hydraulic cylinder cushions The system stanislavski
The system stanislavski Whats gote
Whats gote Contoh deep acting
Contoh deep acting Adjective phrases
Adjective phrases Ngs acting
Ngs acting Acting as if adlerian therapy
Acting as if adlerian therapy Centrally acting skeletal muscle relaxants
Centrally acting skeletal muscle relaxants What were elizabethan theatres like
What were elizabethan theatres like Verb acting as a noun examples
Verb acting as a noun examples Example of reverse acting controller
Example of reverse acting controller For every girl who is tired of acting weak
For every girl who is tired of acting weak Gambar actuator silinder double action
Gambar actuator silinder double action How are quick breads classified
How are quick breads classified Globe theatre flags
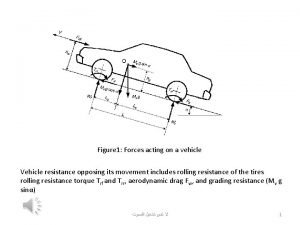
Globe theatre flags Forces acting on a vehicle
Forces acting on a vehicle Wordless acting
Wordless acting Indian dramas
Indian dramas Turing test
Turing test Projectile motion fbd
Projectile motion fbd Naturalism in drama
Naturalism in drama Short acting adalah
Short acting adalah Acting out cycle
Acting out cycle Realism and naturalism in modern drama
Realism and naturalism in modern drama What is acting
What is acting Peripherally acting muscle relaxant
Peripherally acting muscle relaxant Stage rules
Stage rules Alternate rebellion dbt
Alternate rebellion dbt An individual characteristic pattern of thinking
An individual characteristic pattern of thinking Acting out cycle
Acting out cycle Acting
Acting Boleslavsky the first six lessons
Boleslavsky the first six lessons Charlie chaplin coding
Charlie chaplin coding Verbals and verbal phrases
Verbals and verbal phrases Fast acting circuit breaker
Fast acting circuit breaker Acting queue
Acting queue Humiliating
Humiliating Supply air throttling system
Supply air throttling system