Drug Regulatory Overview Milan Smid MD Ph D













































- Slides: 58

Drug Regulatory Overview Milan Smid, MD, Ph. D Technical Officer Quality Assurance and Safety: Medicines Essential Medicines and Pharmaceutical Policies, World Health Organization smidm@who. int

l Principles of drug regulation l WHO regulatory support activities – Harmonization and worksharing – Assessment of regulatory functions – Trainings l Discussion 2| Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008

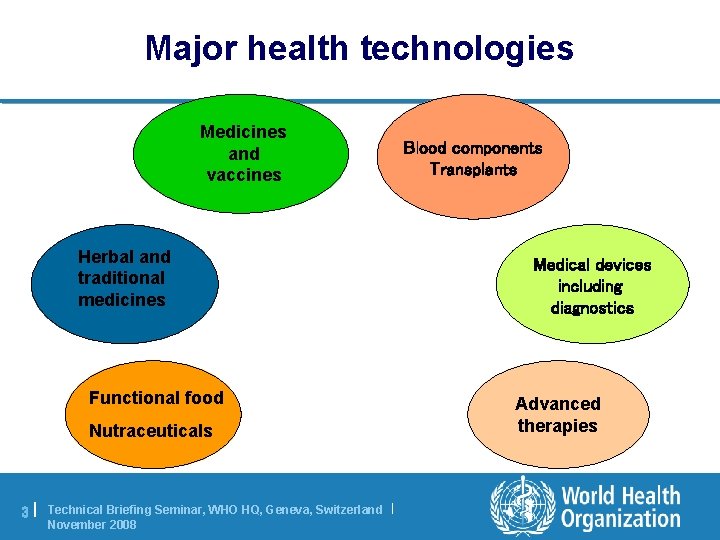
Major health technologies Medicines and vaccines Herbal and traditional medicines Functional food Nutraceuticals 3| Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008 Blood components Transplants Medical devices including diagnostics Advanced therapies

Common features of health technologies l Used to prevent, diagnose or treat disease l Benefits from use must overweight the risks in order to improve health l To evaluate risk-benefit is frequently highly scientifically demanding l Not all health technologies bear comparable risks l Health technologies are subject of commerce and profit oriented behaviour 4| Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008

Protection of public health l To achieve acceptable level of risk related to use of health technologies and maximize their benefit, governments have to intervene l Governmental interventions interfere with rights of persons and companies and with business practices l Regulatory systems and scope of regulation differ worldwide l Health products are split into different categories l Regulation of medicines is currently one of most sophisticated and complicated regulatory functions globally 5| Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008

Medicines regulation § Medicines regulation is the totality of all measures - legal, administrative and technical - which governments take to ensure the safety, efficacy and quality of medicines, as well as the relevance and accuracy of product information § Medicines are special goods for which also prices are frequently regulated by governments to achieve affordability for patients § Medicines are needed and different government incentives are used to develop and produce them 6| Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008

Medicines regulation Models for regulation of medicines are determined by: § Public health needs § Organization of the state and state administration § Size of the pharmaceutical market § Presence and type of pharmaceutical industry § Availability of resources (human, scientific, financial) § Maturity of stakeholders § Participation in regulatory networks 7| Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008

Rationale for Government role Consequences of weak drug regulatory capacity n n 8| substandard, counterfeit, harmful, useless medicines on the market biased information about medicines circulates substandard practices in clinical testing, manufacture and supply of medicines irrational prescription and consumption Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008

Rationale for Government role Consequences of over- or improper regulation n 9| lack of needed medicines or delayed access increased costs of medicines due to cost of regulatory system lack of regulatory flexibility Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008

Regulatory basics Regulation should n n n n 10 | Keep patient in its centre Be evidence based Be risk oriented Bring added value Respect interests of stakeholders and real possibilities Be transparent but respect confidentiality Be effective and flexible Be part of broader drug policy Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008

Parties involved in regulatory system l Regulated subjects – – Investigators and sponsors, members of ethics commitees Manufacturers and importers Wholesalers and distributors Health professionals l Regulators – Regulatory authorities – Governments and political representations l Interested parties – General public and patients groups – Academia – Media 11 | Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008

Development of drug regulation l Quality l Safety l Efficacy l Information l Environment l Risk management and risk minimization 12 | Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008

Essential regulatory activities l R&D – data quality and ethics (GLP, GCP, bioethics) l Manufacture, import and supply – quality (GMP, GDP) l Market entry and existence on the market – Q, S, E, I, Env authorization/registration, maintenance, renewals l Special access schemes l Dispensation – safe and indicated use (GPh. P) l Use – pharmacovigilance (GPh. VP) l Promotion – unbiased, valid and understandable information (Conduct codes) l Environment – premarket review and environment monitoring l Regulatory and scientific advice 13 | Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008

Regulatory toolkit l Legislation l Standards (GXPs, Pharmacopoeias, Q, S, E guidelines) l Data assessment – Submitted data – Collected data l Inspections l Licensing (authorizations / registrations, products and operations) l Certification l Laboratory control l Provision of information l Regulatory intelligence and co-operation 14 | Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008

Incentives to develop, manufacture and supply needy medicines l Patents and supplementary patent protection l Data exclusivities l Market exclusivities l Limited data requirements l Regulatory advice l Regulatory fast tracks l Waivers of regulatory fees l Public-private partnership, stimulating price, guaranteed demand 15 | Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008

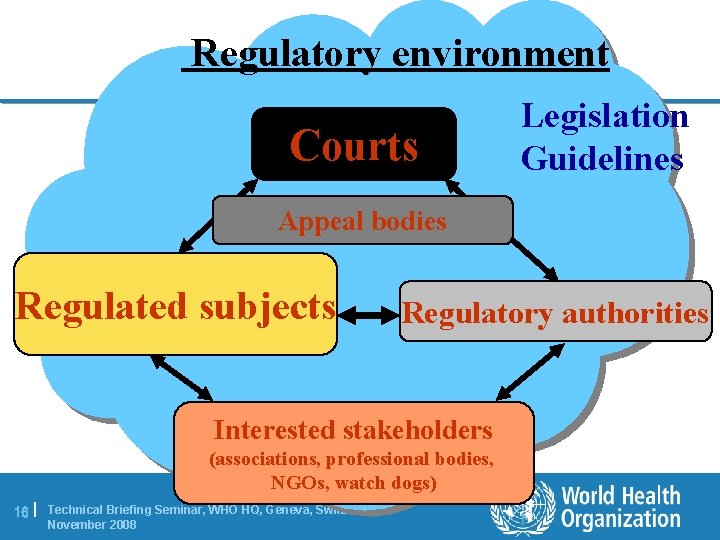
Regulatory environment Courts Legislation Guidelines Appeal bodies Regulated subjects Regulatory authorities Interested stakeholders (associations, professional bodies, NGOs, watch dogs) 16 | Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008

Responsibilities of parties involved in regulatory system Legislated l Investigators and sponsors l Manufacturers and importers l Wholesalers and distributors l Health professionals l Regulatory authorities Non-legislated l Governments and political representations l Patients l Media 17 | Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008

Registration Line extensions PSUR Variations Renewals PSUR Drug Regulatory Cycle PSUR 18 | Postregistration commitments Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008 PSUR Emergency and crisis management

Principles applied in regulatory approvals l Benefits prevail the risks at a time of regulatory approval and nothing indicates that benefits will not prevail also during use of product in normal medical practice – Available data about quality (Dossier) – Available data about efficacy and safety or interchangeability (Dossier) – Available data are credible and were eticaly obtained • Good practices (GLP, GCP, GPh. VP, …) – Existing reassurance about production in stable quality and quality assurance mechanisms • GMP – Way of use of medicine characterized for physicians and patients • Data sheets, SPCs, PILs, package labeling – Lack of knowledge is properly managed • Pharmacovigilance, risk management programmes, commitments 19 | Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008

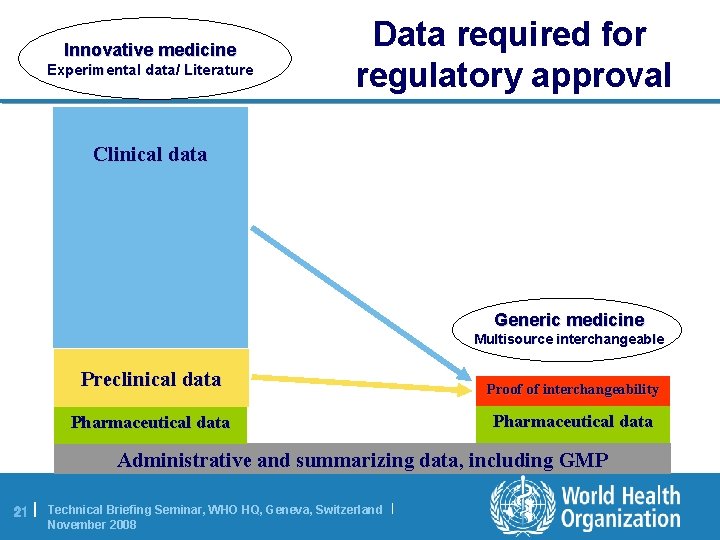
Regulation of innovatory products and generics l For innovator products proof of QUALITY, SAFETY and EFFICACY is needed. Newly also plan of prospective risk-management. l For multisource products QUALITY, safety and efficacy data is referred to the originator, providing only evidence of INTERCHANGEABILITY (bioequivalence, clinical testing, in certain cases dissolution data). 20 | Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008

Innovative medicine Experimental data/ Literature Data required for regulatory approval Clinical data Generic medicine Multisource interchangeable Preclinical data Proof of interchangeability Pharmaceutical data Administrative and summarizing data, including GMP 21 | Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008

Scientific and regulatory complexity concerning medicinal products is growing l l l New technologies New therapeutic approaches Evidence based medicine Globalisation of commerce Telematic instruments Information and transparency requirements l Risk aversion in society l New tasks l Speed of change l Regulatory resources rarely reflect expectations of society and regulators themselves Paradigms of drug regulation are evolving 22 | Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008

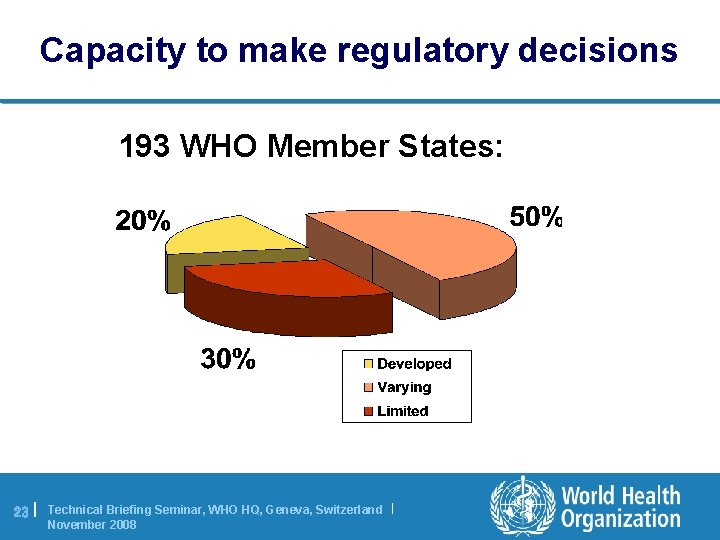
Capacity to make regulatory decisions 193 WHO Member States: 23 | Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008

Challenges § Regulatory delays and backlogs § Lack of regulatory tools and guidelines § Lack of expertise § Lack of management skills § Regulatory pendulum 24 | Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008

Strategies used to cope with increasing demands? l Concentration on priority issues most relevant for public health l Co-operation with partners in order to increase regulatory capacity by elimination of duplicated activities – Facilitated by comparable standards and administrative requirements l Increased effectivity of internal operations – Quality systems, international benchmarking 25 | Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008

Sharing of expertise vs. recognition of decision l Acceptance of expertise is not equal to acceptance of decision l Acceptance of decision – is formal legal act, frequently requiring international treaties – may modify liabilities of involved parties and requires legal specification of acceptance and non-acceptance l Acceptance of expertise – is sovereign and complex regulatory decision of NRA based on scientific arguments and confidence – may be applied case to case – is followed by formal independent decision according to national legislation and mandate of NRA 26 | Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008

WHO approaches for consideration § Stimulating / initiating collaboration between regulators from various developing countries on various regulatory activities; § Employing internationally accepted guidelines adapted to suit local needs/circumstances; § Facilitating joint assessments between regulators through sub-regional approaches. 27 | Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008

13 th ICDRA 16 -19 September 2008, Bern l Concentration on priority issues most relevant for public health – Risk-based approaches: regulation of clinical trials, GMP, product licensing, safety monitoring, market oversight l Increased effectivity and efficiency of internal operations – assessment of practices and performance by common toolkits 28 | Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008

13 th ICDRA 16 -19 September 2008, Bern l Co-operation with partners in order to increase regulatory capacity by elimination of duplicated work investments and optimising use of experts – Harmonization: standards: terminology, guidelines, good practices, pharmacopoeias, format and data content of applications, safety monitoring practices, – Collaboration and networking: inspections, market surveillance, counterfeits, – Information sharing and common data sets, information availability: trial registries, inspection outcomes and assessment reports, licensing outcomes, pharmacovigilance – Building mutual trust – New phenomenon: Major regulatory authorities (FDA, EMEA) recognize need of collaboration with NRA in exporting countries concerning oversight of manufacturers and information exchange 29 | Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008

Different initiatives to increase regulatory capacity and assemble needed scientific / inspection resources WHO – standards, international expert teams, pharmacovigilance UMC ICH – common standards for developed markets, involvement of regulators as well as industry experts EMEA - co-ordination of expertise available to EU/EEA Member States Australia and New Zealand – merger of NRAs (currently frozen) Monaco, Liechtenstein – recognition of regulatory decisions of France resp. Switzerland Canada, Switzerland, Japan, EU, Australia etc. – MRA on mutual acceptance of GMP standards FDA, EMEA, WHO, TGA … etc. – Mo. U on sharing of information PIC/S – share of standards and expertise on GMP, building confidence Regional and subregional initiatives (ASEAN, GCC, EAC) – different extent of co-operation National initiatives – involvement of external experts to support NRAs, recognition of expertise or decisions, co-operation with industry and learned societies 30 | Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008

l Not to share expertise - If trustful expertise is available, not to consider it may be unjustified waste of resources needed elsewhere l Accepting the expertise of other regulators is complex regulatory and scientific decision, depending on – – legislation, mandate capacities and competence available for given issue scientific arguments related to benefit/risk for public health priorities for protection of public health l Understanding to limitations of transfer of expertise and its proper management are needed when considering expertise of others l Targeted, risk-based approach may be appropriate in some situations 31 | Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008

Expertise in support of developing countries l WHO PQP - HIV/AIDS, TB, malaria, RH, paediatric therapy l US PEPFAR - HIV/AIDS l EU Article 58 – assessment of products for non-EU teritories l Canada's Access to Medicines Regime – assessment of products according to WHO Model List of Essential Medicines l Other - orphans, paediatric therapy 32 | Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008

Faster access to medicines through sharing of regulatory information § WHO is working with regulators to find out how best to build confidence in regulatory decisions taken by other regulators, including -- how to facilitate exchange of consolidated information about assessments. 33 | Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008

WHO registration package Purpose: § For less-resourced agencies – in decision making process, to benefit from technical information developed by well-resourced ("mature") regulatory authorities; § To facilitate the "transfer of expertise"; § To create a format for exchange of regulatory information. 34 | Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008

Different initiatives to increase regulatory capacity WHO proposed a framework based on what is publicly available: – Summary Basis for Decision (Health Canada) – European Public Assessment Report (EMEA and EC) – Common Technical Document (US FDA) – WHO Public Assessment Report (WHOPAR) and Public Inspection Report (WHOPIR) 35 | Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008

WHO Pilot Project § Supported by European Commission; § With participation of 5 (7) countries: Ghana, Kenya, Nigeria, South Africa, Uganda, United Republic of Tanzania and Zimbabwe 36 | Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008

WHO Pilot Project Purpose: § To field-test a model technical registration package; § To finalize the content of the package and the guidance for its effective implementation in countries; § To ensure that the package is developed and implemented through a wide consultation with existing and potential stakeholders; 37 | Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008

WHO Pilot Project Field testing exercise § In order to have a maximum benefit from the field-test exercise, it was decided to provide all participating DRAs with the same drug application; § To ask them to develop their own registration package, based on the provided draft template; § One of the recently developed pharmaceutical products has served as a model application for the field-testing of the WHO model registration package. 38 | Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008

WHO Pilot Project § The same application was submitted to EMEA experts for their opinion; § In the end of the project a follow up WHO consultative meeting was conducted; § The aim was to consider: ─ the results and lessons learnt from the field-testing exercise, ─ to finalize the contents of the model technical regulatory package, ─ to provide guidance for adoption of the package by national MRAs. 39 | Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008

Conclusions (1) § The main objective for DRAs should be to get the relevant information for the country, while avoiding collection of large amounts of the country-specific data that does not add value; § Sharing of information can help with best use of available resources, reduce workload and improve overall regulatory performance; § Available tools can be packaged for ease of reference and information exchange; 40 | Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008

Conclusions (2) § Procedures can be streamlined and processes can be improved (e. g. , Fast Track or Priority Review for those products that address unmet medical needs); § Expert knowledge can be pooled and resources directed to functions that can improve public health and facilitate access to essential medicines. 41 | Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008

Regulatory support core functions l Developing evidence on the situation of Medicines Regulatory Systems worldwide l Providing Country support for strengthening medicines regulation l Providing Regional support for strengthening medicines regulation l Facilitate communication and promote harmonization among medicines regulatory authorities l Develop and continuously improve internal supporting tools, mechanism and capacities 42 | Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008

Developing evidence l To develop and maintain a comprehensive database on DRAs l To assess of medicine regulatory systems – Perform assessment to identify needs – Provide evidence for various supporting activities (Financial, Training, Consultancy, Equipment) – Develop institutional plan l To promote self-assessment as a tool for analysis and continuous improvement – Two training events performed for DRAs l Tool for harmonization purposes – 5 Pacific Island Countries / WPRO – 3 EAC Countries / AFRO 43 | Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008

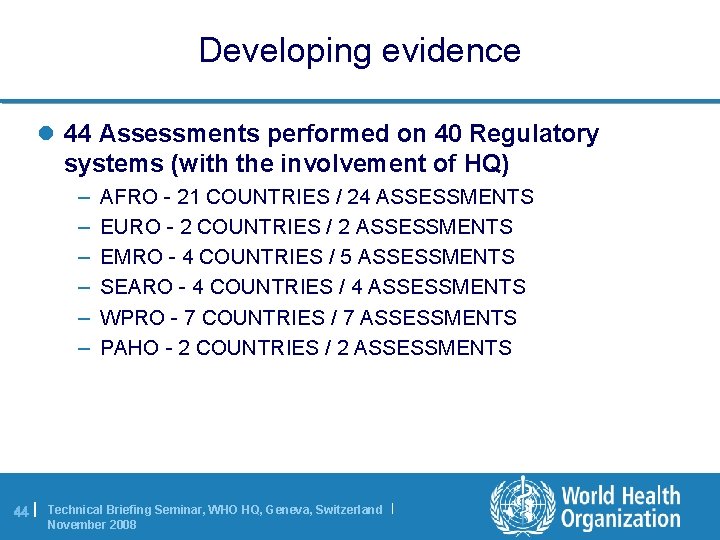
Developing evidence l 44 Assessments performed on 40 Regulatory systems (with the involvement of HQ) – – – 44 | AFRO - 21 COUNTRIES / 24 ASSESSMENTS EURO - 2 COUNTRIES / 2 ASSESSMENTS EMRO - 4 COUNTRIES / 5 ASSESSMENTS SEARO - 4 COUNTRIES / 4 ASSESSMENTS WPRO - 7 COUNTRIES / 7 ASSESSMENTS PAHO - 2 COUNTRIES / 2 ASSESSMENTS Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008

Developing evidence 2008 2007 2006 2004 2003 No 45 | Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008

Country support l Develop and organize training opportunities – – Strengthen information management capacity - SIAMED Strengthen inspection capacity Strengthen QC laboratory capacity Strengthen marketing authorization capacities l Promoting good regulatory practices by providing guidelines, tools and technical assistance l In close collaboration with capacity building team within the PQ Program l In cooperation with IVB Vaccines on clinical trials 46 | Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008

Regional support l Provision of technical assistance to harmonization initiative and supporting participation of regulators – SADC, EAC, PIC, CARICOM, … l Financial support for technical secretariat (EAC, SADC and UEMOA) l WHO/EAC joined assessment of DRAs l Promoting and facilitating networking – Network of DRAS (EAC, SADC) 47 | Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008

Introduction to the grading model l Preliminary work made by Eshetu Wondemagegnehu – Possible approaches to developing drug regulation in: Effective Drug Regulation: what can countries do? l Based on Capability Maturity Model (CMM) – first described by W. Humphrey in Managing the Software Process l Maturity model for quality management system – introduced by P. Crosby in his book 'Quality is Free'. Generic approach of maturity of organization as a learning curve applied to medicines regulatory processes Not to give a mark 48 | Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008

For what purposes can it be used? l At country level – – – To build a strategy To visualize the stage of development/maturity To visualize and demonstrate progress To assist process of harmonization To identify areas of priority support l At sub-regional level – To organize sharing and exchange of expertise – To develop process of harmonization, cooperation or collaboration l At international level – To map regulatory systems – To build strategy/approach – To document results and planned activities 49 | Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008

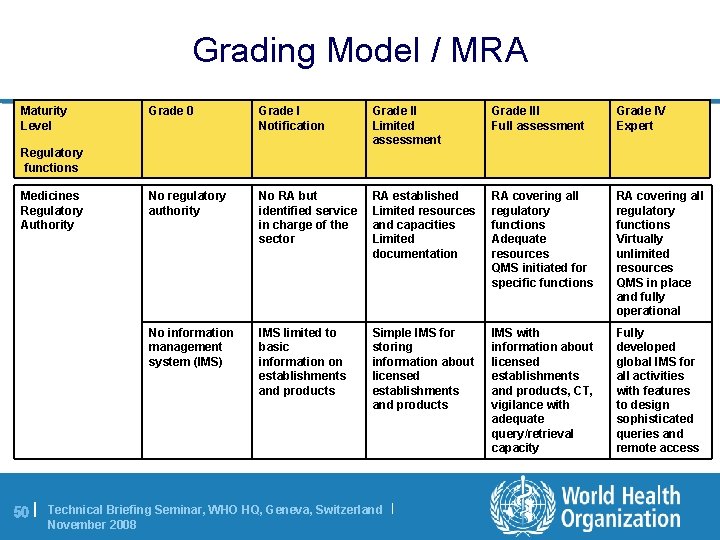
Grading Model / MRA Maturity Level Grade 0 Grade I Notification Grade II Limited assessment Grade III Full assessment Grade IV Expert No regulatory authority No RA but identified service in charge of the sector RA established Limited resources and capacities Limited documentation RA covering all regulatory functions Adequate resources QMS initiated for specific functions RA covering all regulatory functions Virtually unlimited resources QMS in place and fully operational No information management system (IMS) IMS limited to basic information on establishments and products Simple IMS for storing information about licensed establishments and products IMS with information about licensed establishments and products, CT, vigilance with adequate query/retrieval capacity Fully developed global IMS for all activities with features to design sophisticated queries and remote access Regulatory functions Medicines Regulatory Authority 50 | Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008

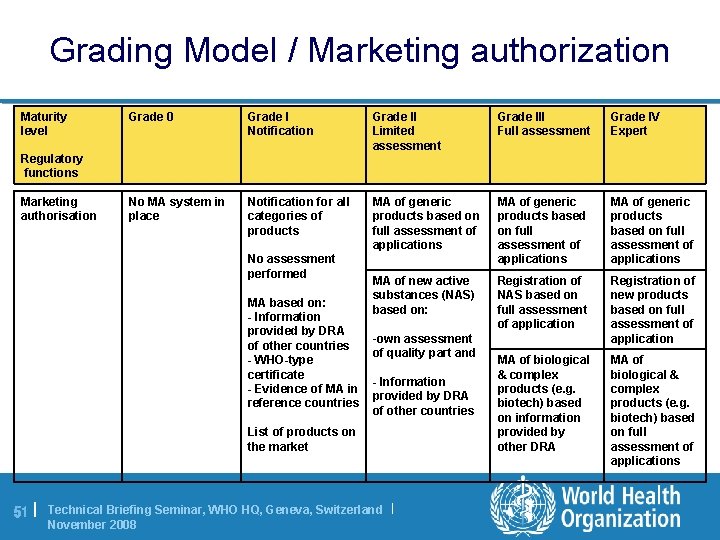
Grading Model / Marketing authorization Maturity level Grade 0 Grade I Notification Grade II Limited assessment Grade III Full assessment Grade IV Expert No MA system in place Notification for all categories of products MA of generic products based on full assessment of applications MA of new active substances (NAS) based on: Registration of NAS based on full assessment of application Registration of new products based on full assessment of application MA of biological & complex products (e. g. biotech) based on information provided by other DRA MA of biological & complex products (e. g. biotech) based on full assessment of applications Regulatory functions Marketing authorisation No assessment performed MA based on: - Information provided by DRA of other countries - WHO-type certificate - Evidence of MA in reference countries -own assessment of quality part and - Information provided by DRA of other countries List of products on the market 51 | Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008

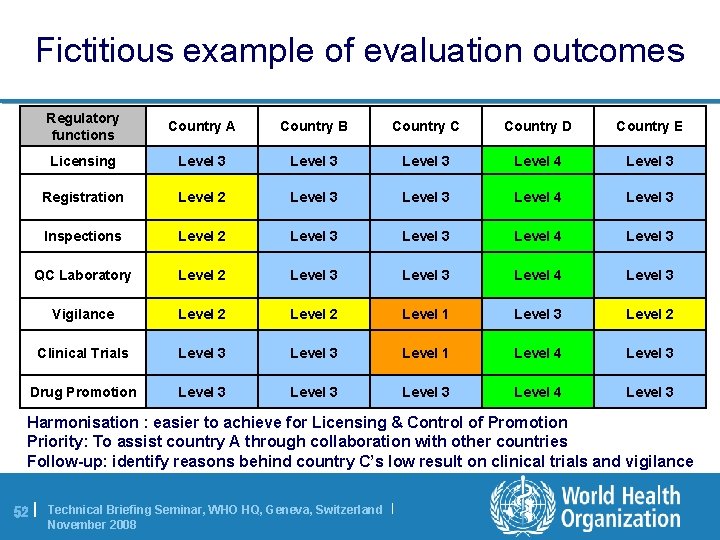
Fictitious example of evaluation outcomes Regulatory functions Country A Country B Country C Country D Country E Licensing Level 3 Level 4 Level 3 Registration Level 2 Level 3 Level 4 Level 3 Inspections Level 2 Level 3 Level 4 Level 3 QC Laboratory Level 2 Level 3 Level 4 Level 3 Vigilance Level 2 Level 1 Level 3 Level 2 Clinical Trials Level 3 Level 1 Level 4 Level 3 Drug Promotion Level 3 Level 4 Level 3 Harmonisation : easier to achieve for Licensing & Control of Promotion Priority: To assist country A through collaboration with other countries Follow-up: identify reasons behind country C’s low result on clinical trials and vigilance 52 | Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008

Contribution of PQ to capacity building l Organization of trainings – general and problem specific (HIV/AIDS, TB and antimalarial products, pediatric dosage forms, BE/BCS) – Trainings of NRA staff and manufacturers frequently combined l Involvement of assessors from NRAs into PQ assessment l Involvement of inspectors from NRAs into PQ inspections l 3 months rotations of experts from NRAs in WHO HQ – PQT l Technical Briefing Seminars 53 | Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008

54 | Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008

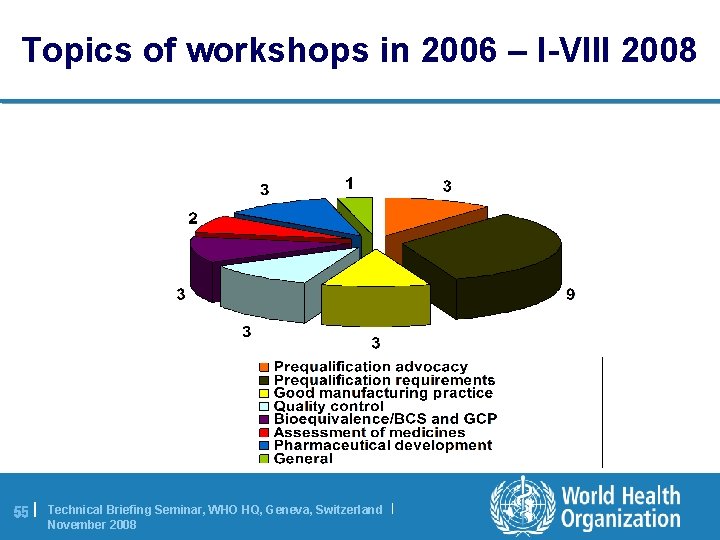
Topics of workshops in 2006 – I-VIII 2008 55 | Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008

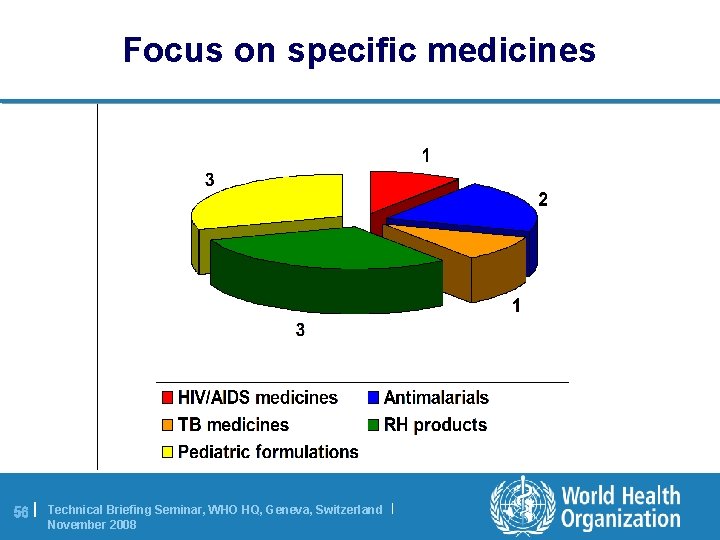
Focus on specific medicines 56 | Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008

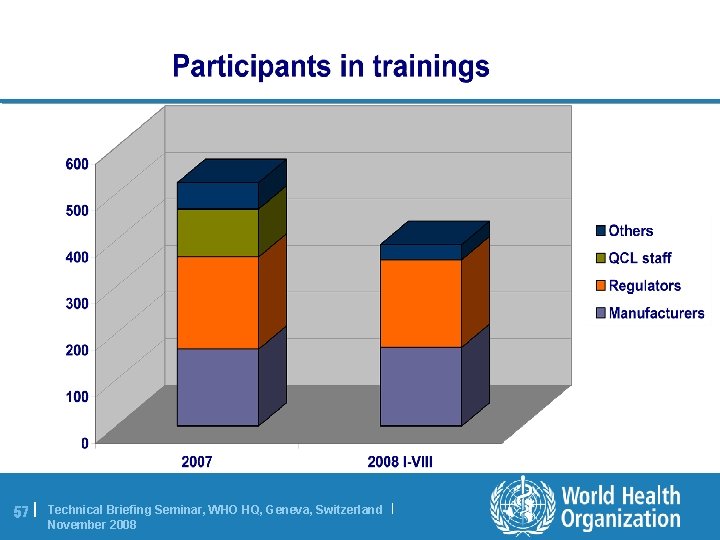
57 | Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008

Thank you for the attention 58 | Technical Briefing Seminar, WHO HQ, Geneva, Switzerland | November 2008
 Smid
Smid An example of crude drug adulterated with exhausted drug
An example of crude drug adulterated with exhausted drug Milan neděla
Milan neděla Milan rufus basne o mame
Milan rufus basne o mame Audit gcp
Audit gcp Milan infotech pvt ltd
Milan infotech pvt ltd Milan kubiatko
Milan kubiatko La moda shoes
La moda shoes Milan petronijevic rukomet
Milan petronijevic rukomet La pinacoteca di brera
La pinacoteca di brera Arth 232
Arth 232 Milan macura bridge
Milan macura bridge Milan ambrož
Milan ambrož Vyskumny problem
Vyskumny problem Milan radojicic fon
Milan radojicic fon Základy umelej inteligencie milan ftáčnik
Základy umelej inteligencie milan ftáčnik 313 date
313 date Ciclo vital geyman
Ciclo vital geyman Milan njegomir
Milan njegomir Milan hlavačka
Milan hlavačka Milan m cirkovic
Milan m cirkovic Erlay milan nicolarde
Erlay milan nicolarde Milan herman
Milan herman Moribundus adlatus
Moribundus adlatus Milan miljevic
Milan miljevic Milan kozlevčar geton
Milan kozlevčar geton Trnera
Trnera Jukov milan
Jukov milan Milan radojicic fon
Milan radojicic fon Schmidke grafiky
Schmidke grafiky Paolo vinci avvocato
Paolo vinci avvocato Dr milan mrdak iskustva
Dr milan mrdak iskustva Milan pilát
Milan pilát Constantine was the emperor who ______.
Constantine was the emperor who ______. Okupaciona podela jugoslavije
Okupaciona podela jugoslavije Milan šimek
Milan šimek Milan miljevic
Milan miljevic Essee4
Essee4 Milan miljevic
Milan miljevic Milan kozlevčar geton
Milan kozlevčar geton Milan cathedral altar
Milan cathedral altar Dr milan mijovic
Dr milan mijovic Milan jansen
Milan jansen Milan kubiatko
Milan kubiatko Zvony detstva
Zvony detstva Rightful duke of milan
Rightful duke of milan Milan glasinčanin
Milan glasinčanin Dr ljiljana trkulja kardiolog
Dr ljiljana trkulja kardiolog Dr sipka banja luka
Dr sipka banja luka Milan remiš
Milan remiš Paisajes de milan
Paisajes de milan Milan r iz bara
Milan r iz bara Regulatory motifs
Regulatory motifs Pharmaceutical regulatory and compliance congress
Pharmaceutical regulatory and compliance congress Sign shapes meaning
Sign shapes meaning Dispositional framework vs regulatory framework
Dispositional framework vs regulatory framework Traffic signs purpose
Traffic signs purpose Regulatory reform fire safety order 2005 summary
Regulatory reform fire safety order 2005 summary Film regulatory bodies
Film regulatory bodies