UN Prequalification Programme Milan Smid WHO Prequalification of



















- Slides: 28

UN Prequalification Programme Milan Smid WHO Prequalification of Medicines Programme WHO Prequalification Programme, Beijing, P. R. China, DIA, May, 2011

UN Prequalification Programme for Priority Essential Medicines Action plan of UN from 2001 for expanding access to selected priority medicines Objective: • To ensure quality, efficacy and safety of medicines procured using international funds (e. g. GFTAM, UNITAID) to serve patients in developing countries Components: • Evaluation of Quality, Safety and Efficacy of prioritised Essential medicines (FPPs and APIs), inspections of manufacturers and monitoring of the products after their prequalification. • Prequalification of quality control laboratories. • Building capacity of regulators, manufacturers and quality control laboratories. 2 WHO Prequalification Programme, Beijing, P. R. China, DIA, May, 2011

Potential benefits for manufacturers • Participation in tender procedures organized by international procurers and financial profit • Recognition as being WHO listed company • Facilitated registration in some recipient countries • Reduction of inspections from recipient countries • Possibility to be assisted by expert consultants (GMP, dossier) • Learning process improving company's chance to succeed with submissions to SRAs • Recognition of prequalified APIs 3 WHO Prequalification Programme, Beijing, P. R. China, DIA, May, 2011

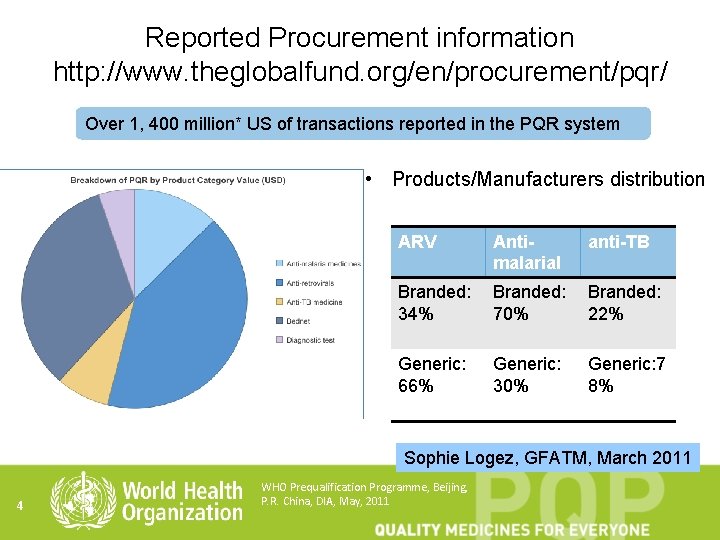
Reported Procurement information http: //www. theglobalfund. org/en/procurement/pqr/ Over 1, 400 million* US of transactions reported in the PQR system • Products/Manufacturers distribution ARV Antimalarial anti-TB Branded: 34% Branded: 70% Branded: 22% Generic: 66% Generic: 30% Generic: 7 8% Sophie Logez, GFATM, March 2011 4 WHO Prequalification Programme, Beijing, P. R. China, DIA, May, 2011

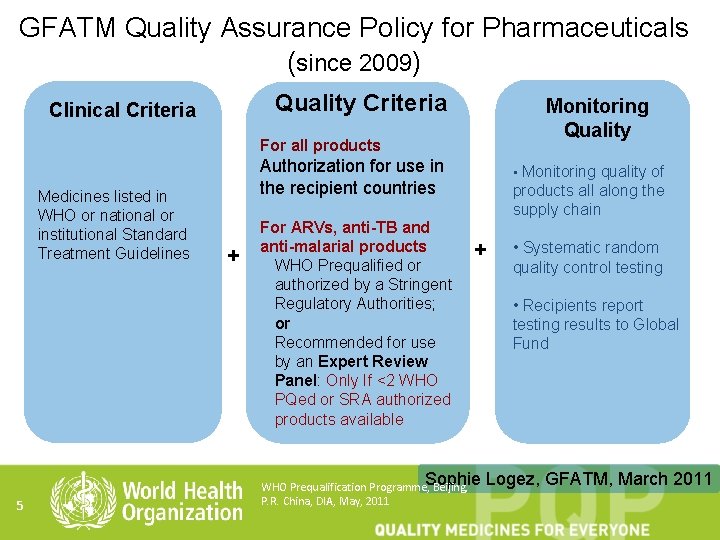
GFATM Quality Assurance Policy for Pharmaceuticals (since 2009) Quality Criteria Clinical Criteria Monitoring Quality For all products Medicines listed in WHO or national or institutional Standard Treatment Guidelines 5 Authorization for use in the recipient countries + For ARVs, anti-TB and anti-malarial products WHO Prequalified or authorized by a Stringent Regulatory Authorities; or Recommended for use by an Expert Review Panel: Only If <2 WHO PQed or SRA authorized products available • Monitoring quality of products all along the supply chain + WHO Prequalification Programme, Sophie Beijing, P. R. China, DIA, May, 2011 • Systematic random quality control testing • Recipients report testing results to Global Fund Logez, GFATM, March 2011

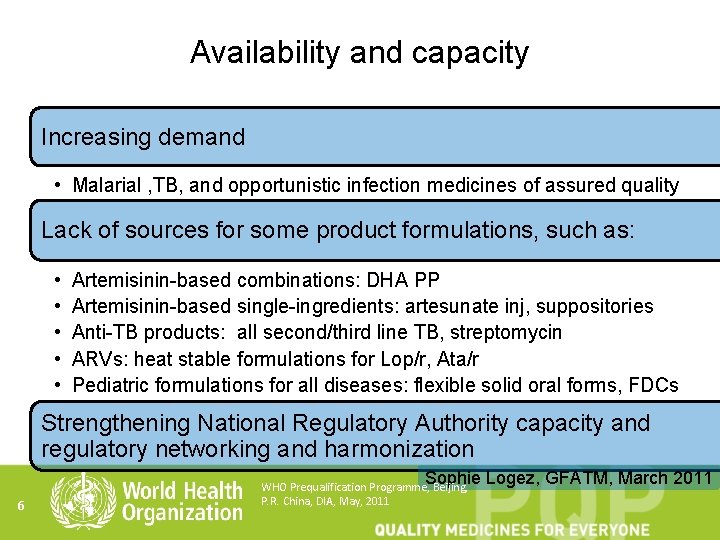
Availability and capacity Increasing demand • Malarial , TB, and opportunistic infection medicines of assured quality Lack of sources for some product formulations, such as: • • • Artemisinin-based combinations: DHA PP Artemisinin-based single-ingredients: artesunate inj, suppositories Anti-TB products: all second/third line TB, streptomycin ARVs: heat stable formulations for Lop/r, Ata/r Pediatric formulations for all diseases: flexible solid oral forms, FDCs Strengthening National Regulatory Authority capacity and regulatory networking and harmonization Sophie Logez, GFATM, March 2011 6 WHO Prequalification Programme, Beijing, P. R. China, DIA, May, 2011

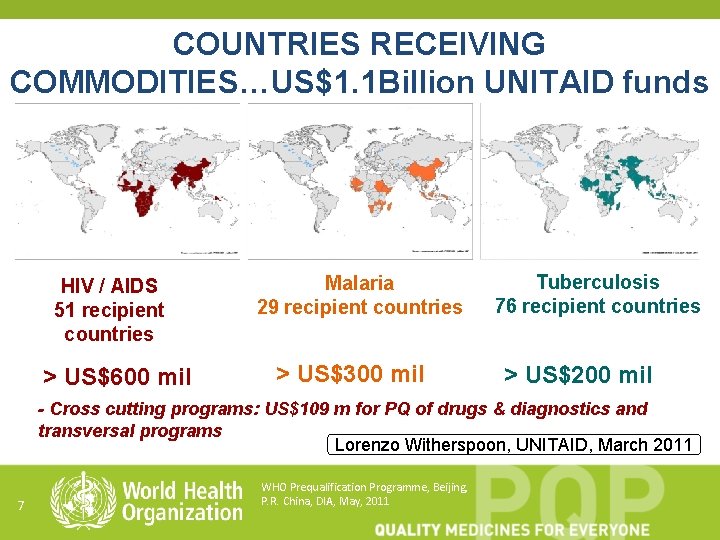
COUNTRIES RECEIVING COMMODITIES…US$1. 1 Billion UNITAID funds HIV / AIDS 51 recipient countries > US$600 mil Malaria 29 recipient countries > US$300 mil Tuberculosis 76 recipient countries > US$200 mil - Cross cutting programs: US$109 m for PQ of drugs & diagnostics and transversal programs Lorenzo Witherspoon, UNITAID, March 2011 7 WHO Prequalification Programme, Beijing, P. R. China, DIA, May, 2011

Categories of medicines invited • Primary categories of medicines: – HIV/AIDS – Malaria – Tuberculosis • Later added: – Reproductive health – Influenza – Acute diarrhoea • Potentially other categories of products, if there is the need (neglected diseases) • Prequalification also applicable for APIs • Published in invitations for Expression of Interest (EOI) on Prequalification website 8 WHO Prequalification Programme, Beijing, P. R. China, DIA, May, 2011

How prequalification is organized? q WHO manages and organizes the programme on behalf of the United Nations: • provides technical and scientific support to assessment, inspections (GMP, GCP, GLP) and quality control (GPCL) • involvement of qualified assessors and inspectors mostly from NRAs of ICH and associated countries and PIC/S inspectorates • guarantees that international norms and standards are applied all through the process • supports capacity of NRA in developing countries to evaluate, inspect and control the quality of medicines • involvement of qualified assessors and inspectors from NRAs in developing countries • by involvement of manufacturers from developing countries into the project supports their capacity to produce according to international norms and standards 9 WHO Prequalification Programme, Beijing, P. R. China, DIA, May, 2011

Essential steps of PQ evaluation procedure • • 10 Need is specified and agreed by WHO treatment programmes Invitation for Expression of Interest (EOI) is published Interested parties submit dossiers Dossiers receive initial screening Full dossiers are assessed Inspections are conducted at manufacturing sites and at CROs Samples are tested, if needed If outcome is positive, pharmaceutical product is listed on the website, including product information (SPC, PIL), assessment report (WHOPAR) and inspection report (WHOPIR) WHO Prequalification Programme, Beijing, P. R. China, DIA, May, 2011

Essential steps of monitoring of PQ product • Variations to the dossier of prequalified product • Sampling and Testing • Reinspections • Requalification • Management of complaints • De-listing or suspension (if and when appropriate) 11 WHO Prequalification Programme, Beijing, P. R. China, DIA, May, 2011

Steps in WHO prequalification Expression of Interest I Product dossier SMF Assessment Additional information and data Corrective actions Compliance Prequalification Maintenance and monitoring 12 Inspections WHO Prequalification Programme, Beijing, P. R. China, DIA, May, 2011

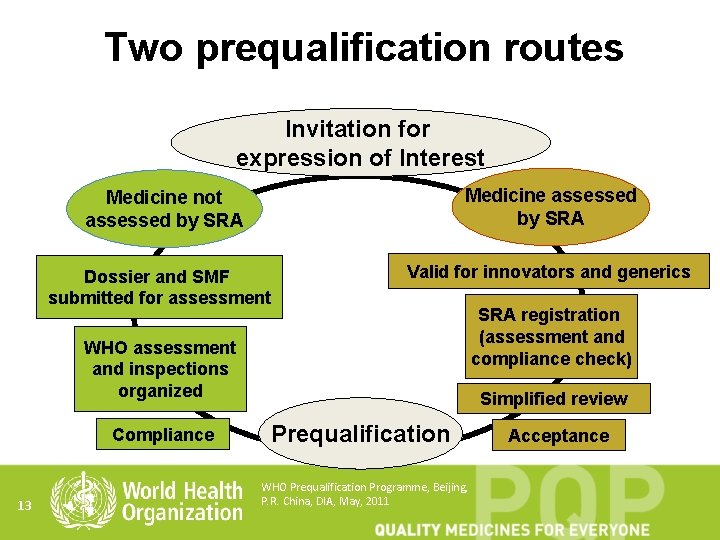
Two prequalification routes Invitation for expression of Interest Medicine not assessed by SRA Medicine assessed by SRA Dossier and SMF submitted for assessment Valid for innovators and generics WHO assessment and inspections organized Compliance 13 SRA registration (assessment and compliance check) Simplified review Prequalification WHO Prequalification Programme, Beijing, P. R. China, DIA, May, 2011 Acceptance

Evaluation procedure q Assessment of product dossiers (Quality specifications, pharmaceutical development, production, control, stability, bioequivalence etc). q Teams of professionals from national Drug Regulatory Authorities (DRA): Including Brazil, China, Canada, Denmark, Estonia, Finland, France, Germany, Hungary, Indonesia, Malaysia, Philippines, Spain, South -Africa, Sweden, Switzerland, Tanzania, Uganda, UK, Zimbabwe. . . q • • 14 Copenhagen assessment week 8 to 20 assessors together during one week at least every two months at UNICEF in Denmark Every dossier is assessed by at least four assessors. An assessment report is issued - signed by assessors Letter summarizing the findings and asking for clarification and additional data if necessary is sent first by e-mail to the applicant followed by surface mail WHO Prequalification Programme, Beijing, P. R. China, DIA, May, 2011

Inspections • Team of inspectors for each inspection • WHO PQ inspector plus PIC/S member country plus local country inspector (observer) • Some cases – capacity building (recipient country) • Preparation includes SMF, product information, inspection reports, complaints etc • Inspections are product oriented • APIs and Bioequivalence studies inspected based on risk assessment 15 WHO Prequalification Programme, Beijing, P. R. China, DIA, May, 2011

Standards • WHO standards as defined in WHO guidelines and International Pharmacopoeia are applied in prequalification process • If these not exist, ICH guidelines are applied • In case of need, guidelines of stringent regulatory authorities, which are involved in ICH process 16 WHO Prequalification Programme, Beijing, P. R. China, DIA, May, 2011

Outcomes of PQ procedure Information in public domain: http: //www. who. int/prequal/ • • • Lists of PQ medicinal products WHOPAR (SPC, PIL, labelling) WHOPIR (both FPP and API) Notices of Concern and Suspensions Information on progress of assessment procedure and inspections • Supportive documents: WHO guidelines, description of PQ procedure, training materials 17 WHO Prequalification Programme, Beijing, P. R. China, DIA, May, 2011

www. who. int/preq ual/ 18 WHO Prequalification Programme, Beijing, P. R. China, DIA, May, 2011

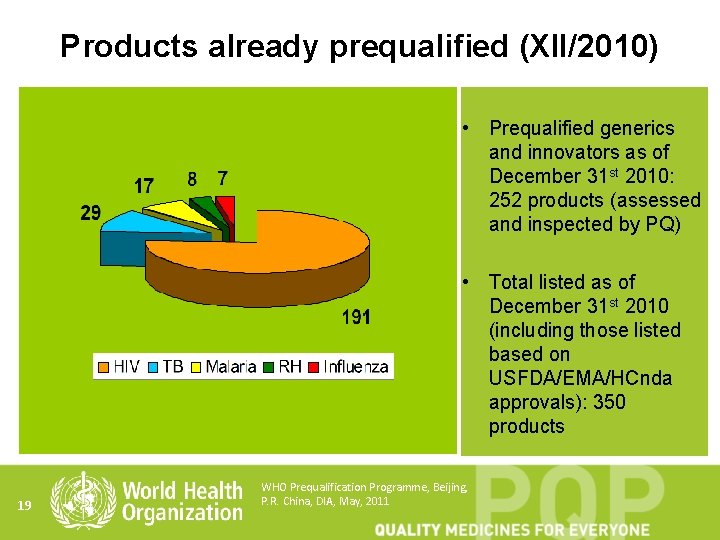
Products already prequalified (XII/2010) • Prequalified generics and innovators as of December 31 st 2010: 252 products (assessed and inspected by PQ) • Total listed as of December 31 st 2010 (including those listed based on USFDA/EMA/HCnda approvals): 350 products 19 WHO Prequalification Programme, Beijing, P. R. China, DIA, May, 2011

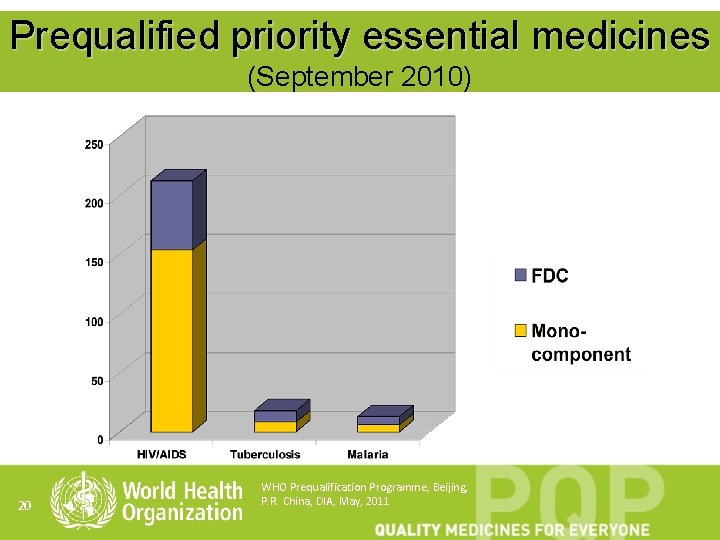
Prequalified priority essential medicines (September 2010) 20 WHO Prequalification Programme, Beijing, P. R. China, DIA, May, 2011

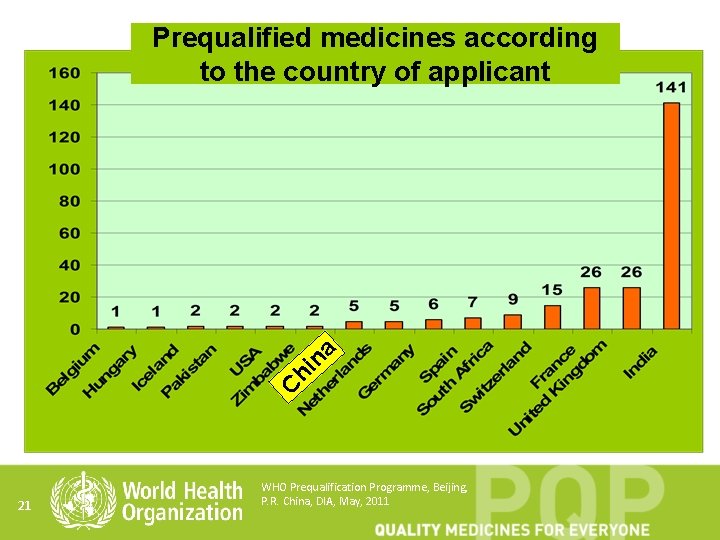
C hi na Prequalified medicines according to the country of applicant 21 WHO Prequalification Programme, Beijing, P. R. China, DIA, May, 2011

Contribution of PQ to capacity building • Organization of trainings – general and problem specific (HIV/AIDS, TB and antimalarial products, pediatric dosage forms, BE/BCS, GMP) – Trainings of NRA staff and manufacturers frequently combined • Involvement of assessors from NRAs into PQ assessment • Involvement of inspectors from NRAs into PQ inspections • 3 months rotations of experts from NRAs in WHO HQ – PQT 22 WHO Prequalification Programme, Beijing, P. R. China, DIA, May, 2011

23 WHO Prequalification Programme, Beijing, P. R. China, DIA, May, 2011

24 WHO Prequalification Programme, Beijing, P. R. China, DIA, May, 2011

Technical Assistance • Provision of expert consultants to – Manufacturers – Quality control laboratories – Regulators • Assistance focuses on – GMP, GCP or GLP compliance – Dossier development • Assistance is separated from the assessment / inspections and may be followed by specific trainings 25 WHO Prequalification Programme, Beijing, P. R. China, DIA, May, 2011

Conditions for provision of technical assistance Manufacturers: • Participation in the prequalification programme, • Found to be capable and willing to improve • Location in a developing country Products: • Inclusion in the list of expression of interest • High value for Public Health purpose • Poor representation on the Prequalification list. 26 WHO Prequalification Programme, Beijing, P. R. China, DIA, May, 2011

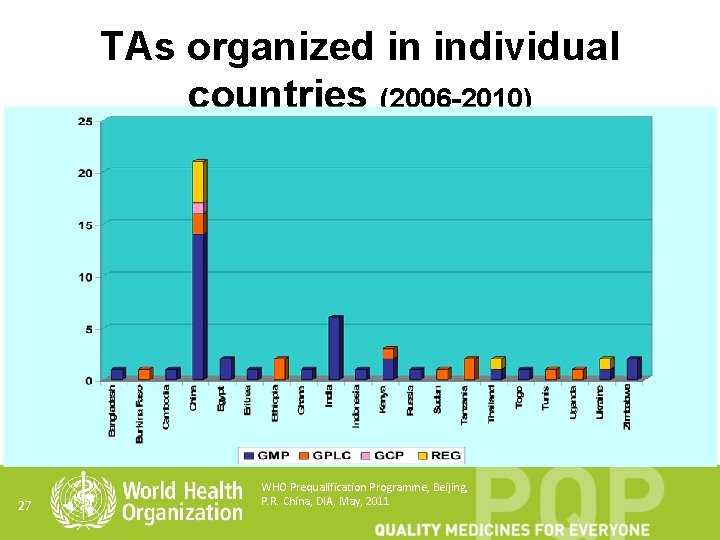
TAs organized in individual countries (2006 -2010) 27 WHO Prequalification Programme, Beijing, P. R. China, DIA, May, 2011

Thank you for the attention smidm@who. int 28 WHO Prequalification Programme, Beijing, P. R. China, DIA, May, 2011