Corrosion and Its control Dr Harikrishna Erothu Associate









- Slides: 33

Corrosion and Its control Dr Harikrishna Erothu Associate Professor Center for Advanced Energy Studies K L University 1

Natural Abundance of Metals In the form of oxides, carbonates, chlorides, silicates etc. Dr Harikrishna Erothu 2

Corrosion: The destructive and unintentional degradation of metallic material, through an unwanted chemical or electrochemical attack by its environment • Dry or Chemical Corrosion Types: • Wet or Electrochemical Corrosion Dr Harikrishna Erothu 3

Dry or Chemical Corrosion 1. Oxidation Corrosion 2. Corrosion by other gases 3. Liquid Metal Corrosion Dr Harikrishna Erothu 4

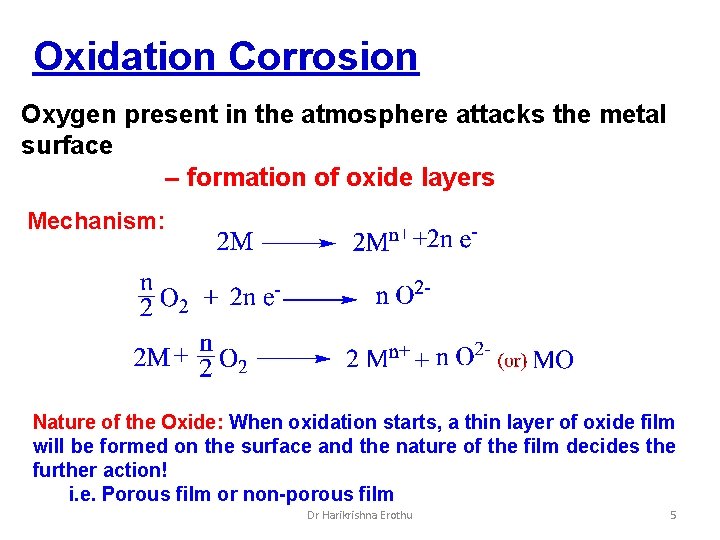
Oxidation Corrosion Oxygen present in the atmosphere attacks the metal surface – formation of oxide layers Mechanism: Nature of the Oxide: When oxidation starts, a thin layer of oxide film will be formed on the surface and the nature of the film decides the further action! i. e. Porous film or non-porous film Dr Harikrishna Erothu 5

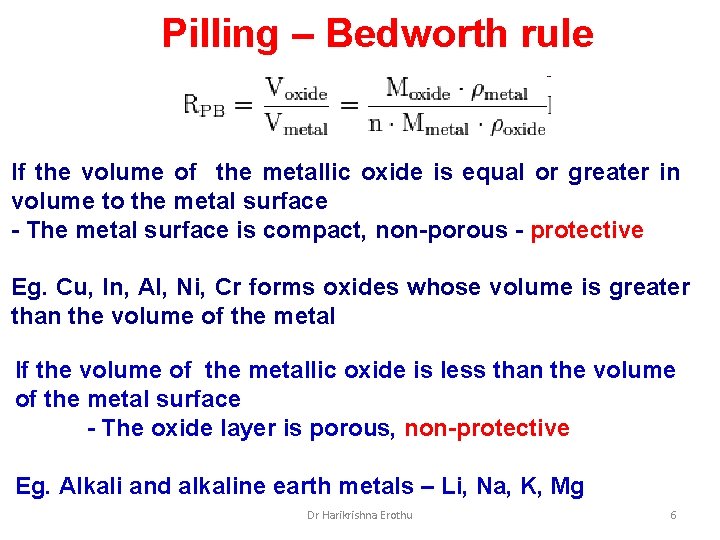
Pilling – Bedworth rule If the volume of the metallic oxide is equal or greater in volume to the metal surface - The metal surface is compact, non-porous - protective Eg. Cu, In, Al, Ni, Cr forms oxides whose volume is greater than the volume of the metal If the volume of the metallic oxide is less than the volume of the metal surface - The oxide layer is porous, non-protective Eg. Alkali and alkaline earth metals – Li, Na, K, Mg Dr Harikrishna Erothu 6

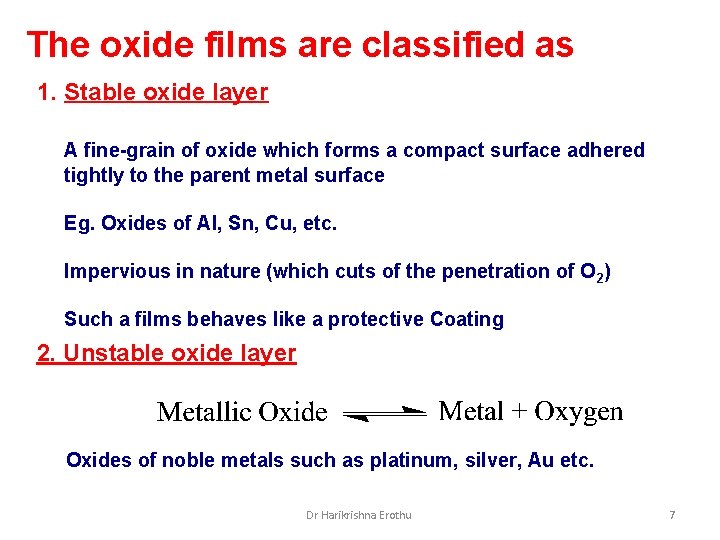
The oxide films are classified as 1. Stable oxide layer A fine-grain of oxide which forms a compact surface adhered tightly to the parent metal surface Eg. Oxides of Al, Sn, Cu, etc. Impervious in nature (which cuts of the penetration of O 2) Such a films behaves like a protective Coating 2. Unstable oxide layer Oxides of noble metals such as platinum, silver, Au etc. Dr Harikrishna Erothu 7

3. Volatile layer Oxide layers volatilize as soon as they are formed Excessive corrosion Molybdenum oxide (Mo. O 3) 4. Porous Oxide layers with minute pores Volume of the oxide layer is ………. . than metal Corrosion ……………. . Dr Harikrishna Erothu 8

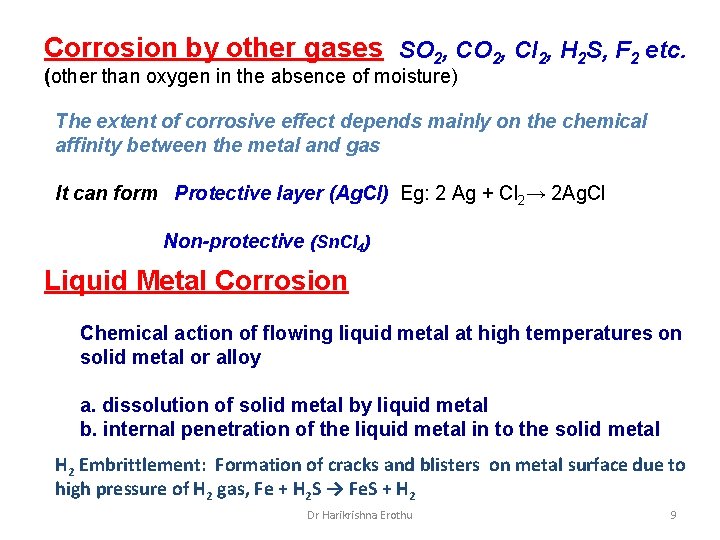
Corrosion by other gases SO 2, Cl 2, H 2 S, F 2 etc. (other than oxygen in the absence of moisture) The extent of corrosive effect depends mainly on the chemical affinity between the metal and gas It can form Protective layer (Ag. Cl) Eg: 2 Ag + Cl 2→ 2 Ag. Cl Non-protective (Sn. Cl 4) Liquid Metal Corrosion Chemical action of flowing liquid metal at high temperatures on solid metal or alloy a. dissolution of solid metal by liquid metal b. internal penetration of the liquid metal in to the solid metal H 2 Embrittlement: Formation of cracks and blisters on metal surface due to high pressure of H 2 gas, Fe + H 2 S → Fe. S + H 2 Dr Harikrishna Erothu 9

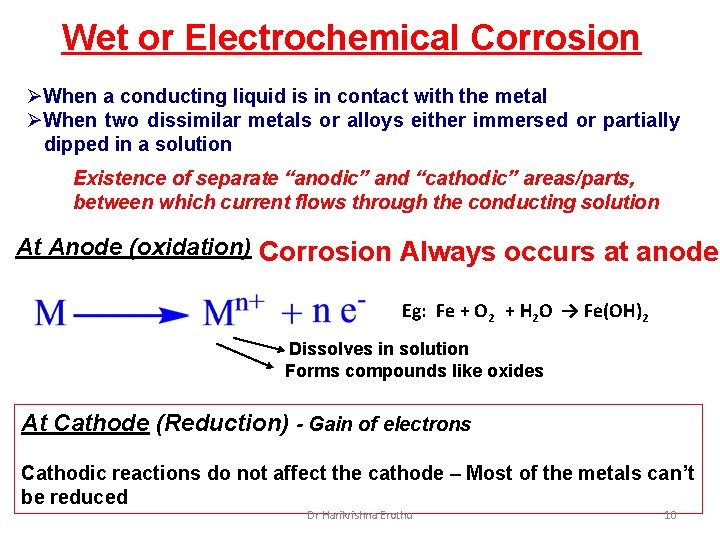
Wet or Electrochemical Corrosion ØWhen a conducting liquid is in contact with the metal ØWhen two dissimilar metals or alloys either immersed or partially dipped in a solution Existence of separate “anodic” and “cathodic” areas/parts, between which current flows through the conducting solution At Anode (oxidation) Corrosion Always occurs at anode Eg: Fe + O 2 + H 2 O → Fe(OH)2 Dissolves in solution Forms compounds like oxides At Cathode (Reduction) - Gain of electrons Cathodic reactions do not affect the cathode – Most of the metals can’t be reduced Dr Harikrishna Erothu 10

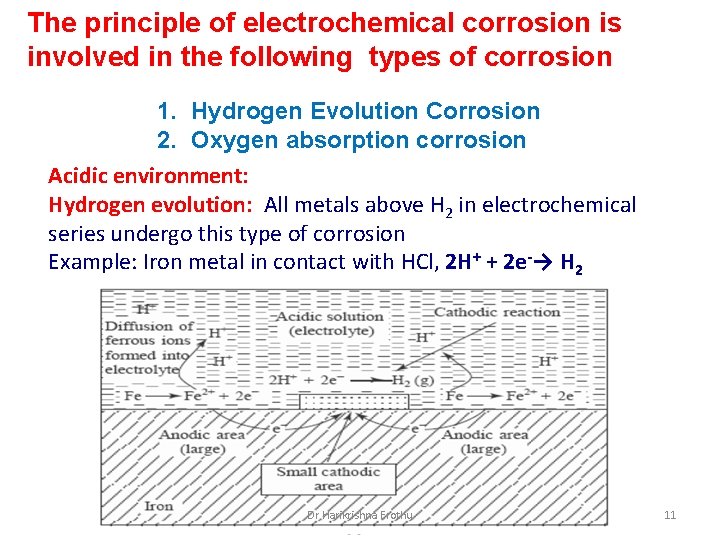
The principle of electrochemical corrosion is involved in the following types of corrosion 1. Hydrogen Evolution Corrosion 2. Oxygen absorption corrosion Acidic environment: Hydrogen evolution: All metals above H 2 in electrochemical series undergo this type of corrosion Example: Iron metal in contact with HCl, 2 H+ + 2 e-→ H 2 Dr Harikrishna Erothu 11

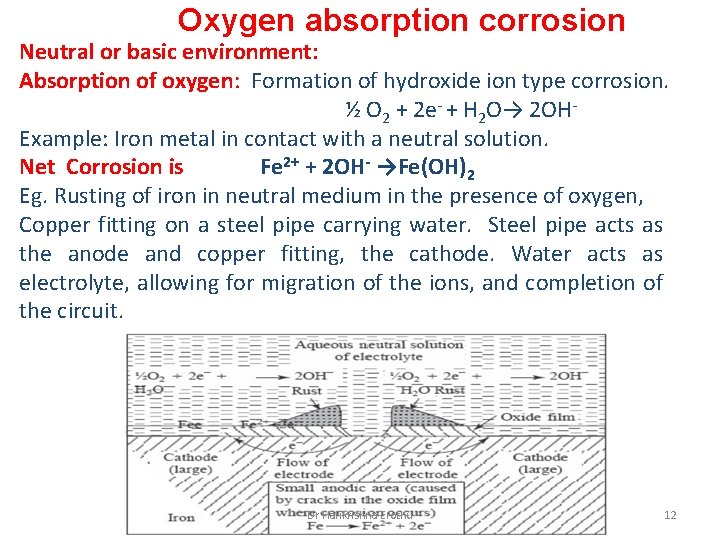
Oxygen absorption corrosion Neutral or basic environment: Absorption of oxygen: Formation of hydroxide ion type corrosion. ½ O 2 + 2 e- + H 2 O→ 2 OHExample: Iron metal in contact with a neutral solution. Net Corrosion is Fe 2+ + 2 OH- →Fe(OH)2 Eg. Rusting of iron in neutral medium in the presence of oxygen, Copper fitting on a steel pipe carrying water. Steel pipe acts as the anode and copper fitting, the cathode. Water acts as electrolyte, allowing for migration of the ions, and completion of the circuit. Dr Harikrishna Erothu 12

Types of Wet Corrosion There are different types of corrosions based on the reactions and physical states. (a) Galvanic Corrosion (b) Pitting Corrosion (c) Stress Corrosion (d) Water-line Corrosion (e) Differential aeration Corrosion (f) Inter granular corrosion (g) Crevice Corrosion Dr Harikrishna Erothu 13

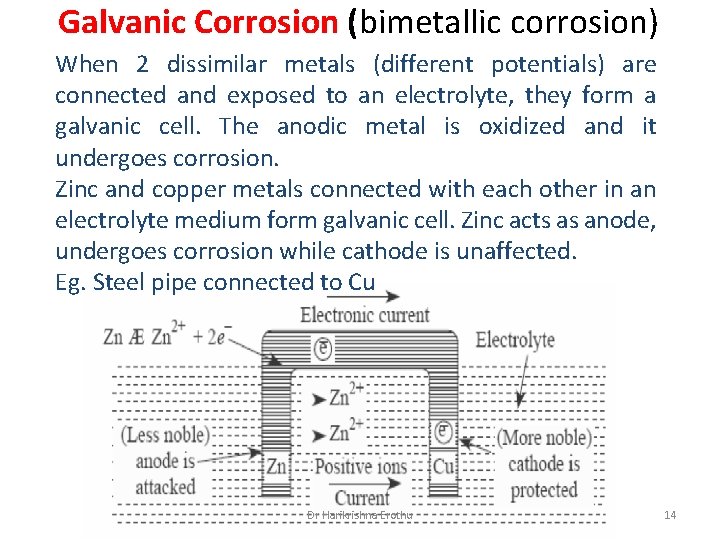
Galvanic Corrosion (bimetallic corrosion) When 2 dissimilar metals (different potentials) are connected and exposed to an electrolyte, they form a galvanic cell. The anodic metal is oxidized and it undergoes corrosion. Zinc and copper metals connected with each other in an electrolyte medium form galvanic cell. Zinc acts as anode, undergoes corrosion while cathode is unaffected. Eg. Steel pipe connected to Cu Dr Harikrishna Erothu 14

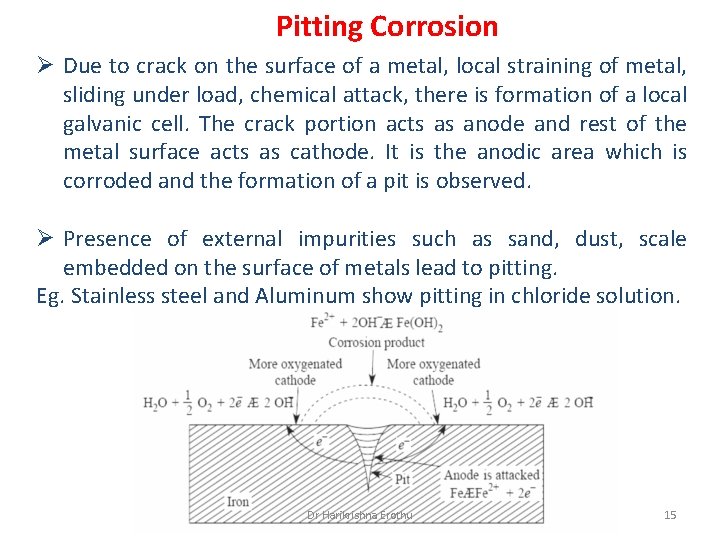
Pitting Corrosion Ø Due to crack on the surface of a metal, local straining of metal, sliding under load, chemical attack, there is formation of a local galvanic cell. The crack portion acts as anode and rest of the metal surface acts as cathode. It is the anodic area which is corroded and the formation of a pit is observed. Ø Presence of external impurities such as sand, dust, scale embedded on the surface of metals lead to pitting. Eg. Stainless steel and Aluminum show pitting in chloride solution. Dr Harikrishna Erothu 15

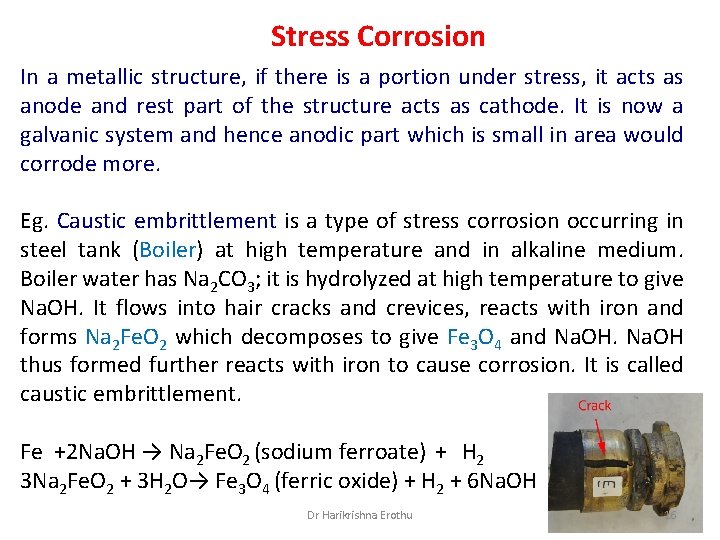
Stress Corrosion In a metallic structure, if there is a portion under stress, it acts as anode and rest part of the structure acts as cathode. It is now a galvanic system and hence anodic part which is small in area would corrode more. Eg. Caustic embrittlement is a type of stress corrosion occurring in steel tank (Boiler) at high temperature and in alkaline medium. Boiler water has Na 2 CO 3; it is hydrolyzed at high temperature to give Na. OH. It flows into hair cracks and crevices, reacts with iron and forms Na 2 Fe. O 2 which decomposes to give Fe 3 O 4 and Na. OH thus formed further reacts with iron to cause corrosion. It is called caustic embrittlement. Fe +2 Na. OH → Na 2 Fe. O 2 (sodium ferroate) + H 2 3 Na 2 Fe. O 2 + 3 H 2 O→ Fe 3 O 4 (ferric oxide) + H 2 + 6 Na. OH Dr Harikrishna Erothu 16

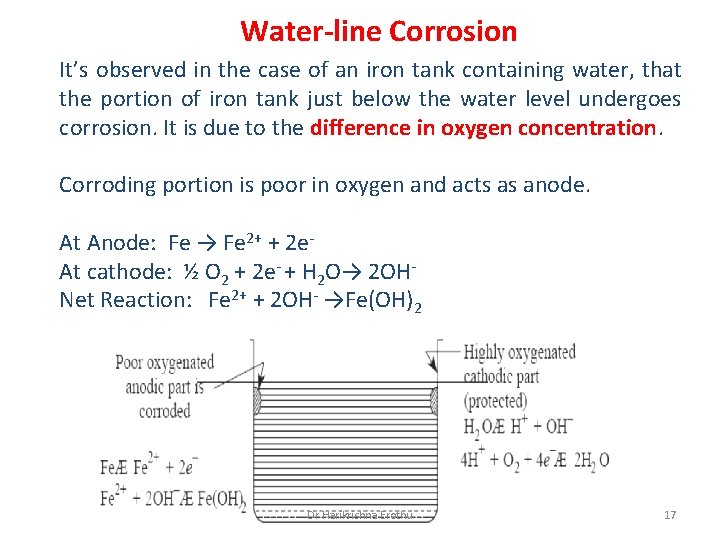
Water-line Corrosion It’s observed in the case of an iron tank containing water, that the portion of iron tank just below the water level undergoes corrosion. It is due to the difference in oxygen concentration. Corroding portion is poor in oxygen and acts as anode. At Anode: Fe → Fe 2+ + 2 e. At cathode: ½ O 2 + 2 e- + H 2 O→ 2 OHNet Reaction: Fe 2+ + 2 OH- →Fe(OH)2 Dr Harikrishna Erothu 17

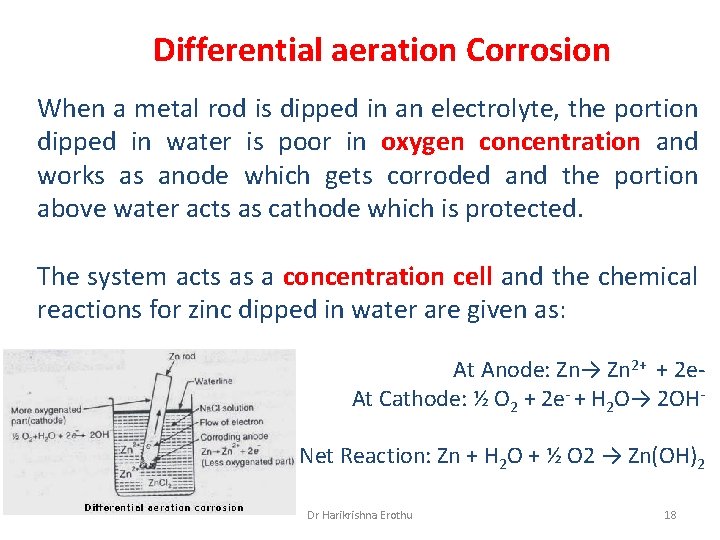
Differential aeration Corrosion When a metal rod is dipped in an electrolyte, the portion dipped in water is poor in oxygen concentration and works as anode which gets corroded and the portion above water acts as cathode which is protected. The system acts as a concentration cell and the chemical reactions for zinc dipped in water are given as: At Anode: Zn→ Zn 2+ + 2 e. At Cathode: ½ O 2 + 2 e- + H 2 O→ 2 OHNet Reaction: Zn + H 2 O + ½ O 2 → Zn(OH)2 Dr Harikrishna Erothu 18

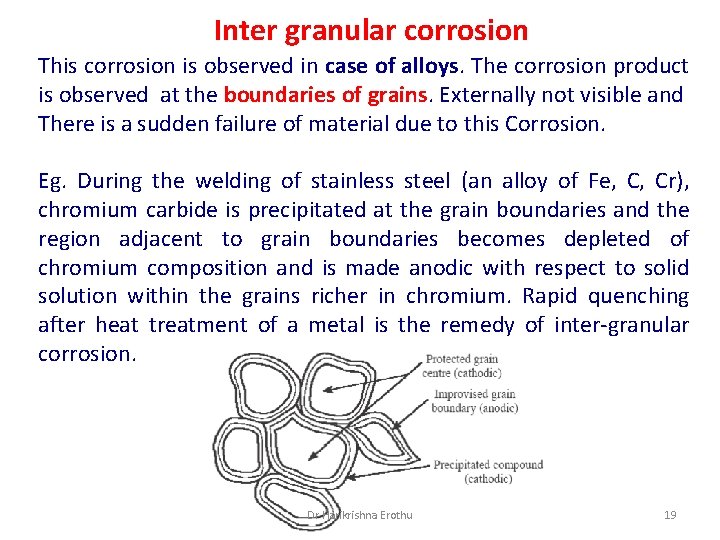
Inter granular corrosion This corrosion is observed in case of alloys. The corrosion product is observed at the boundaries of grains. Externally not visible and There is a sudden failure of material due to this Corrosion. Eg. During the welding of stainless steel (an alloy of Fe, C, Cr), chromium carbide is precipitated at the grain boundaries and the region adjacent to grain boundaries becomes depleted of chromium composition and is made anodic with respect to solid solution within the grains richer in chromium. Rapid quenching after heat treatment of a metal is the remedy of inter-granular corrosion. Dr Harikrishna Erothu 19

Crevice Corrosion Crevice corrosion occurs when two components are joined close together to form a crevice. Corrosion occurs as the crevice accumulates water. If the crevice is small enough a differential oxygen concentration in the water can form. When this happens the base of the crevice becomes anodic to the upper region. Crevice corrosion often occurs under bolts and rivet heads as well as in shielded areas and under dirt or sand deposits. Dr Harikrishna Erothu 20

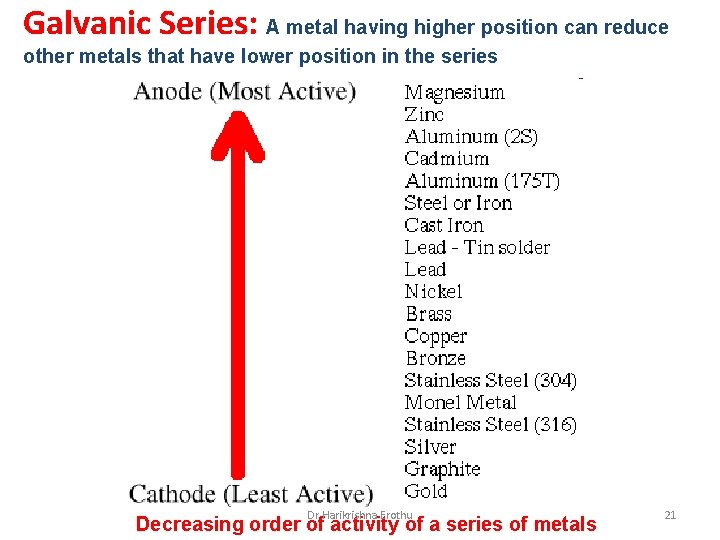
Galvanic Series: A metal having higher position can reduce other metals that have lower position in the series Dr Harikrishna Erothu Decreasing order of activity of a series of metals 21

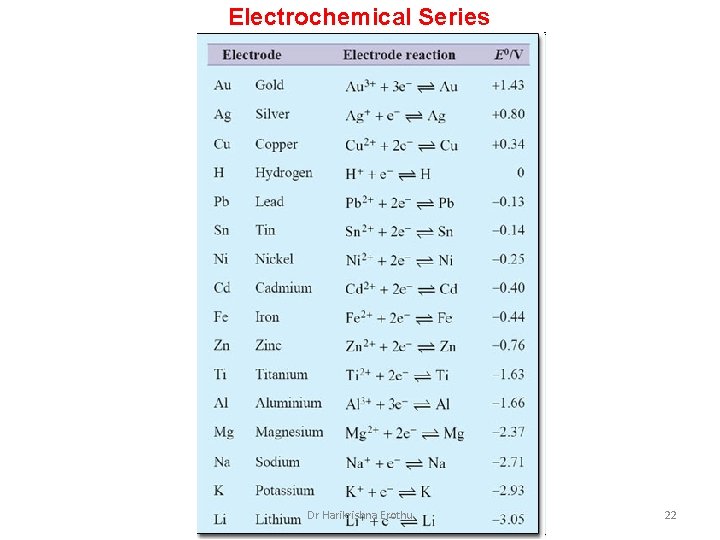
Electrochemical Series Dr Harikrishna Erothu 22

Factors Influencing Corrosion Effect of Environment Nature of the Metal Position of metal in galvanic series Temperature Purity of the metal Humidity Relative areas of anode and cathode Physical state of the metal Nature of the oxide film Impurities in the atmosphere p. H value O 2 concentration and Formation of O 2 concentration cell Volatility and Solubility of Corrosion products Dr Harikrishna Erothu 23

Corrosion Control 1. Proper Designing i. Avoid contact of dissimilar metals ii. Anodic material should be large iii. Dissimilar metals should be as close as possible in galvanic series iv. Insulating fitting between dissimilar metals v. Anodic material should not be painted vi. Prevent inhomogenities vii. No sharp edges or corners & crevices in joints viii. Free circulation of air ix. Uniform flow of liquids x. Prevent some areas of structure to stress Dr Harikrishna Erothu 24

Corrosion Control 2. Using pure metal (impurity causes corrosion) 3. Using metal alloys (should be homogeneous) 4. Modifying the environment i. De-aeration (removes O 2) ii. Deactivation (Na 2 SO 3, N 2 H 4 absorb oxygen) iii. Dehumidification (adding alumina or silica gel) iv. Alkaline neutralization (CO 2, H 2 S, SO 2 & HCl give acidic medium responsible for corrosion and are neutralised by NH 3 or Na. OH or lime) Dr Harikrishna Erothu 25

Corrosion Control 5. Corrosion Inhibitors – Reduce the rate of corrosion ü Two types - anodic and cathodic Inhibitors Anodic ü Inhibitors form sparingly - soluble products which are adsorbed on the surface of metal, protect from corrosion Eg. Phosphate, chromate and tungstate protect anode ü Cathodic Inhibitors - organic amines, mercaptans, thiourea and substituted urea, delay reduction reaction occurs at cathode 6. Cathodic protection (Electrochemical method) i. Sacrificial anodic protection ii. Impressed current cathodic protection Dr Harikrishna Erothu 26

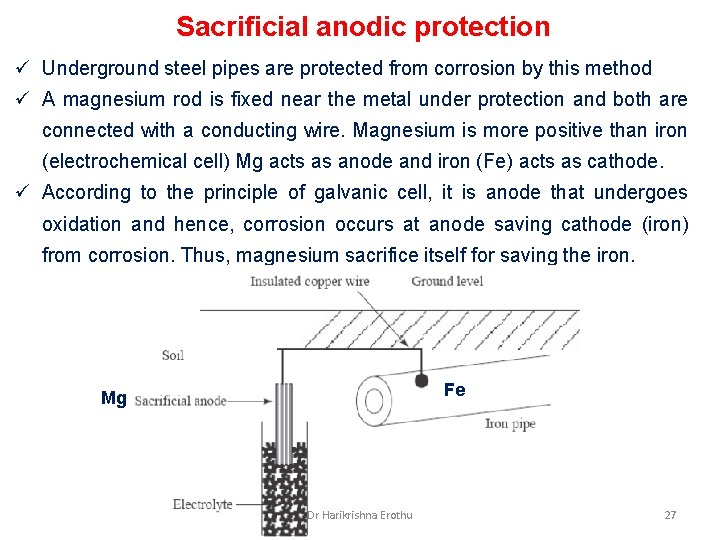
Sacrificial anodic protection ü Underground steel pipes are protected from corrosion by this method ü A magnesium rod is fixed near the metal under protection and both are connected with a conducting wire. Magnesium is more positive than iron (electrochemical cell) Mg acts as anode and iron (Fe) acts as cathode. ü According to the principle of galvanic cell, it is anode that undergoes oxidation and hence, corrosion occurs at anode saving cathode (iron) from corrosion. Thus, magnesium sacrifice itself for saving the iron. Fe Mg Dr Harikrishna Erothu 27

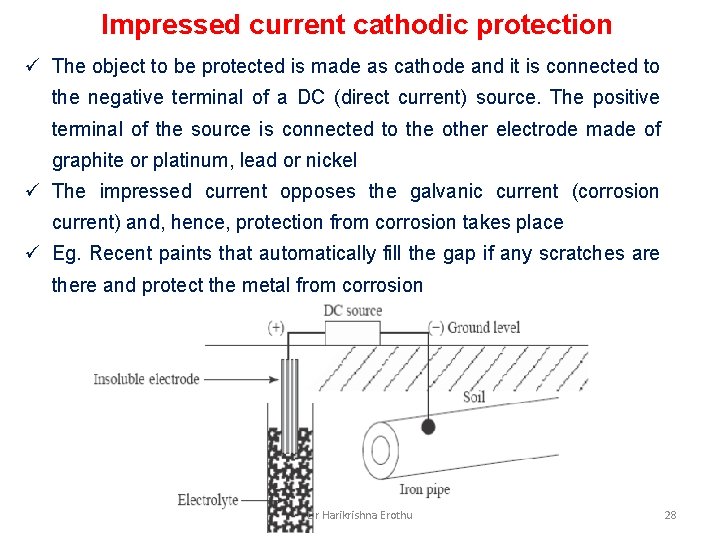
Impressed current cathodic protection ü The object to be protected is made as cathode and it is connected to the negative terminal of a DC (direct current) source. The positive terminal of the source is connected to the other electrode made of graphite or platinum, lead or nickel ü The impressed current opposes the galvanic current (corrosion current) and, hence, protection from corrosion takes place ü Eg. Recent paints that automatically fill the gap if any scratches are there and protect the metal from corrosion Dr Harikrishna Erothu 28

Electroplating - organic surface coatings ü An electrochemical process in which a base metal is coated by Zn, Ag, Cr, Au, Sn etc. to protect it from corrosion and also to make it shine and decorative. The base metal is made cathode, dipped in a suitable electrolyte, and the metal to be deposited is made an anode. ü They not only protect from corrosion but also give a good look to the metal. ü Organic coats must have chemical inertness, good surface adhesiveness and non-effectiveness towards inorganic chemicals and water. Dr Harikrishna Erothu 29

Electroplating - Purification Before subjecting to electroplating process, it is essential to clean the surface of object. The following methods are used to clean the metal surface. 1. Solvent cleaning: Organic solvents such as Trichloro ethylene and methylene chloride are used to remove organic matter and grease on the surface of cathode. Trichloro ethylene is used to remove paint varnishes, films, resins and Per chloro ethylene is used to remove high melt waxes and cleaning printed circuit board (PCB). 2. Alkali cleaning: After solvent cleaning, the metal is cleaned with alkali solutions such as soaps, detergents, sodium carbonate, sodium hydroxide, sodium phosphate etc. 3. Mechanical cleaning: The object is subjected for mechanical cleaning to remove oxide scales, rust and other impurities on the metal surface. This method involves cleaning with bristle brushes, mechanical polishing, grinding using polishing machines and sand blasting. 4. Pickling: In this method the oxides scales are removed by dipping the base metal in a dilute acids. Pickling of steel involves dipping in dilute HCl or dilute H 2 SO 4 to remove rust and other oxide scales. 5. Rinsing with water: After surface cleaning process, the metallic surface is thoroughly rinsed with water, dried and subjected for electroplating process. Dr Harikrishna Erothu 30

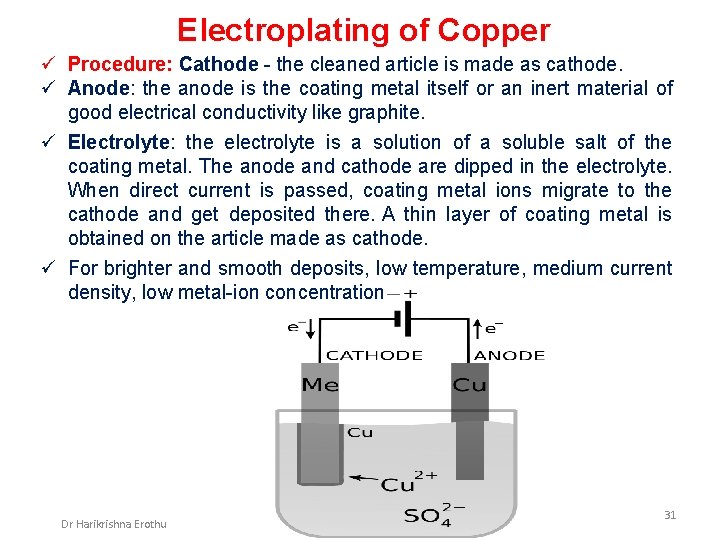
Electroplating of Copper ü Procedure: Cathode - the cleaned article is made as cathode. ü Anode: the anode is the coating metal itself or an inert material of good electrical conductivity like graphite. ü Electrolyte: the electrolyte is a solution of a soluble salt of the coating metal. The anode and cathode are dipped in the electrolyte. When direct current is passed, coating metal ions migrate to the cathode and get deposited there. A thin layer of coating metal is obtained on the article made as cathode. ü For brighter and smooth deposits, low temperature, medium current density, low metal-ion concentration Dr Harikrishna Erothu 31

Electroplating of Copper Process in steps: ü Step 1: The metal to be electroplated is first treated with organic solvents like CCl 4 (carbon tetra chloride) to remove oils, greases etc. , ü Step 2: The metal to be coated i. e. the base metal after step-1 is cleaned with dil. HCl to remove scales or the oxide layer. ü Step 3: Anode - pure copper, Cathode - base metal ü Electrolyte is Cu. SO 4 solution taken in an electrolytic tank. Anode and Cathode are immersed in the electrolyte. Reactions: At anode: Cu (s) Cu 2+ (aq) + 2 e- (Oxidation) Copper is dissolved At cathode: Cu 2+ (aq) + 2 e- Cu (s) (Reduction) Copper is deposited Dr Harikrishna Erothu 32

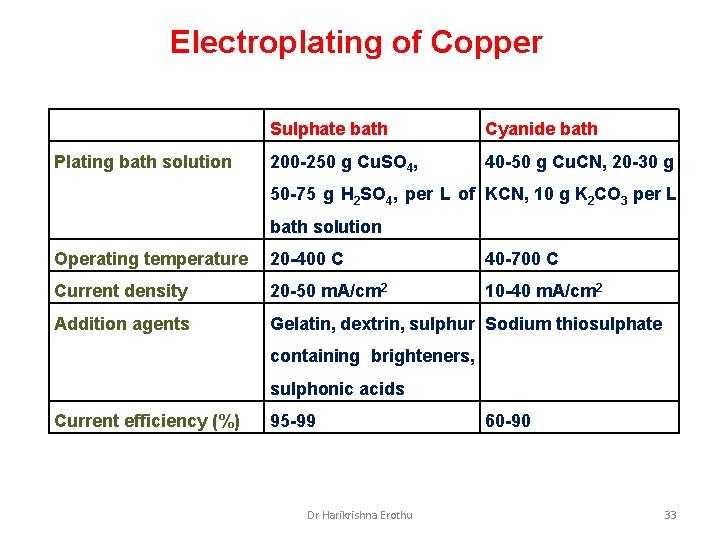
Electroplating of Copper Plating bath solution Sulphate bath Cyanide bath 200 -250 g Cu. SO 4, 40 -50 g Cu. CN, 20 -30 g 50 -75 g H 2 SO 4, per L of KCN, 10 g K 2 CO 3 per L bath solution Operating temperature 20 -400 C 40 -700 C Current density 20 -50 m. A/cm 2 10 -40 m. A/cm 2 Addition agents Gelatin, dextrin, sulphur Sodium thiosulphate containing brighteners, sulphonic acids Current efficiency (%) 95 -99 Dr Harikrishna Erothu 60 -90 33