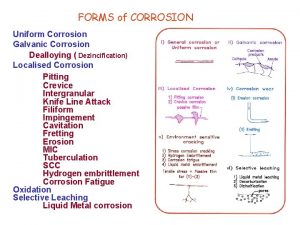
CORROSION AND PROTECTIVE COATINGS PROTECTION FROM CORROSION 1











































- Slides: 58

CORROSION AND PROTECTIVE COATINGS

PROTECTION FROM CORROSION 1. Proper selection and designing 2. Cathodic and anodic protection 3. Protective coating (a ) Metal Coating (b) Inorganic coating (c) Organic coating 4. Corrosion Inhibitors

1. Proper selection and designing Design an equipment by avoiding contact between two dissimilar metals. Maintaining a larger anodic area of the metal. Metals should be close in the galvanic series.

2. Cathodic Protection In this method, the corroding metal is forced to behave like a cathode. There are two types of cathodic protection

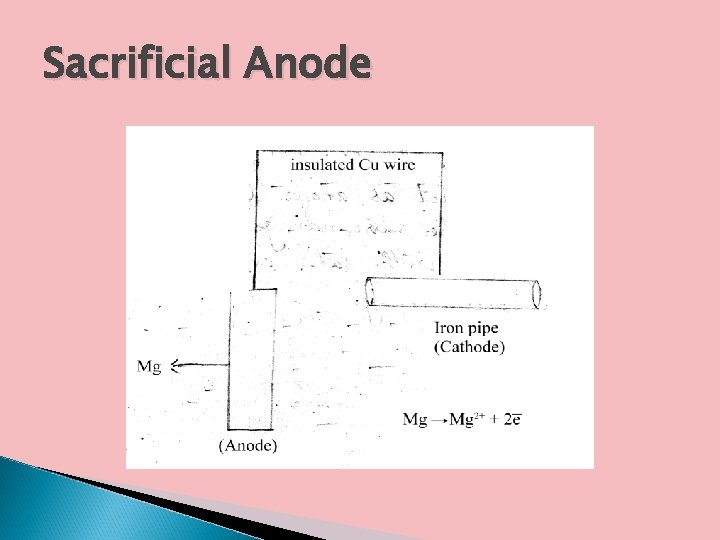
a. Sacrificial Anodic protection In this method, the metallic structure which is to be protected from corrosion is connected to a more anodic metal by a wire so that the entire corrosion is concentrated on this more active metal. The more active metal loses and get corroded and this metal is called sacrificial anode. Metals commonly employed as sacrificial anode are Mg, Zn, Al and their alloys.

Sacrificial Anode

Applications Important applications of sacrificial anodic method include protection of buried pipe lines, underground cables, marine structures etc.

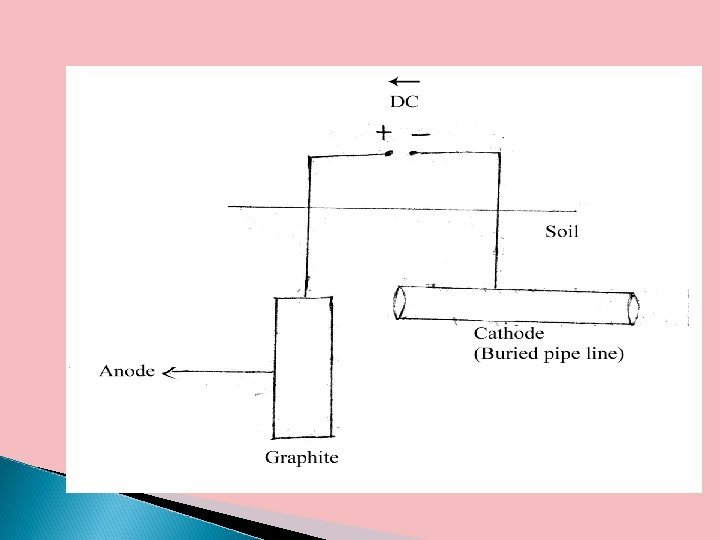
b. Impressed current cathodic protection In this method, an impressed current is applied in the opposite direction to nullify corrosion current so as to convert the corroding metal from anode to cathode. Impressed current can be derived from a direct current source like battery. An inert or insoluble electrode like graphite or silica act as anode to complete the circuit. The surroundings of anode should be filled with salts and carbon to increased the conductivity.


Applications This type of cathodic protection has been applied to water coolers, water tanks, buried oil and water pipes, transmission towers etc. This type of protection is employed when 1. Long term protection is needed 2. Large structures are to be protected 3. There is a cheap source of electrical power.

3. Protective coatings An important method for protecting a metal from corrosion is to apply a protective coating. The protective coatings may be of metal, inorganic or organic. The coated surface isolates the metal from the corroding medium. The coating applied must be chemically inert towards the environment.

Metallic Coatings Metallic coatings are mostly applied on Iron and steel because these are cheap and commonly used construction materials. There are two types of metallic coatings.

i. Anodic coatings The base metal which is to be protected is coated with a more anodic metal for eg. Coatings of Zn, Al and Cd steel are anodic because their electrode potentials are lower than that of the base metal ie. Fe.

ii. Cathodic Coatings It is obtained by coating a more inert metal having higher electrode potential. Than the base metal. Eg. Coating of Sn, Cr, Ni on Fe surface. The coating should be continuous and free from pores and cracks. These coating metals usually have higher corrosion resistance than the base metal.

METHODS OF APPLICATION OF METALLIC COATING

1. Hot Dipping It is used for producing a coating of low melting metal such as Zn, Sn, Ph, Al etc on relatively higher melting metals such as iron, steel, copper etc. This is done by immersing the base metal covered by a layer of molten flux. The flux is used to keep the base metal surface clean and also to prevent oxidation of the molten metal. Most widely used hot dipping methods are : (i) galvanization and (ii) tinning

a. Galvanization It is the process of coating Zn over iron or steel sheet by immersing it in molten Zn. The procedure involves the following stages. The iron or steel article is Ist cleaned by pickling with dil H 2 So 4 for 15 – 20 min. at 60 – 900 C in an acid bath. This treatment also removes any oxide layer present on the surface of the metal. The article is then washed with water in a washing bath & dried in a drying chamber.

It is then dipped in a bath of molten Zn kept at 425 – 4350 C. The Surface of the bath is covered with NH 4 Cl flux to prevent oxide formation. The article gets coated with a thin layer of Zn. It is then passed through a pair of hot rollers to remove excess of Zn and to get uniform thickness for coating. Then it is annealed at about 6500 C & cooled slowly. In the case of Zn coating even if the protecting layer has cracks on it, iron being cathodic does not get corroded.

Applications This method is widely used for protection of Fe from atmospheric corrosion in the form of articles like roofing sheets, wires, pipes, nails, screws, tubes etc. It is to be noted that galvanized utensils should not come in contact with acids.

ii. Tinning It is an eg. For cathodic coatings. It is the process of coating Sn over Fe or steel articles by immersing it in molten Sn. The process consists in Ist treating the iron sheet with dil H 2 So 4 to remove any oxide film. After this it is passed through a bath of Zn. Cl 2 flux which helps the molten Sn to adhere to the metal sheet. Next the sheet passes through palm oil which prevents through a pair of hot rollers to remove excess of Sn & produce uniform thickness for Sn coating.

Applications Tinning is widely used for coating steel, Cu and brass sheets which are used for making containers for storing food studs, oils, kerosene & packing food materials. Tinned Cu sheets are used for making cooking utensils & refrigeration equipments.

2. Metal Cladding In this process, a thick homogeneous layer of coating metal is bonded firmly & permanently to the base metal on one or both the sides. This method cnhanceds corrosion resistance. The choice of cladding material depends on the corrosion resistance required for any particular environment. Nearly all existing corrosion resisting metals like Ni, Cu, Al, Ag, Pt and alloys like stainless steel, Ni alloys, Cu alloys can be used as cladding materials. Cladding can be done by different means. a. Fusing cladding material over the base metal b. Welding c. Rolling sheets of cladding material over base metal

3. Metal spraying In this process, the coating metal in the molten state is sprayed on the previously cleaned base metal with the help of a sprayer. The sprayer coatings are continuous but somewhat porous a sealer – oil is applied on such a coating to provide a smooth surface. However, adhesion strength of metallic spraying is usually lesser that obtained by hot dipping or electroplating. It is therefore essential to have a cleaned metal surface. Spraying can be applied by the following two techniques.

i. Wire – gun method In this method, the coating metal in the form of thin wire is melted by an oxy – acetylene flame and vaporized by a blast of compressed air. The coating metal adheres to the base metal. Al is coated on aircraft steel parts using this techniques.

Powder – metal method In this method, the coating metal is supplied in the form of tine powder which is converted in to a cloud of molten globules by a blower and are adsorbed on the base metal surface.

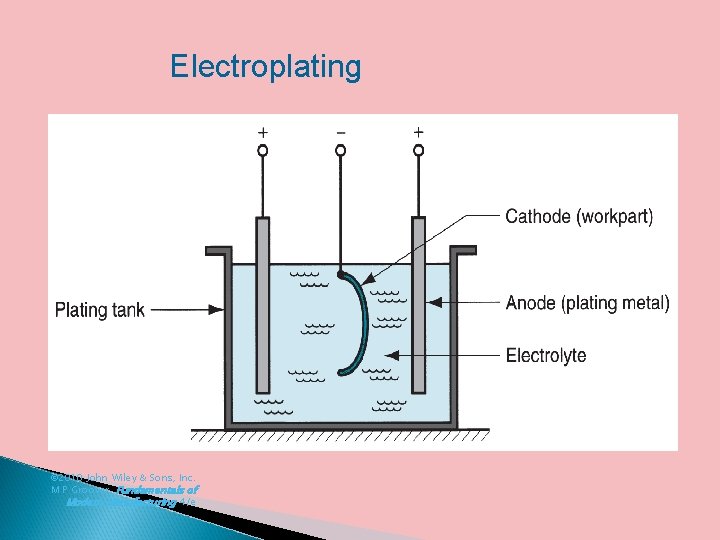
4. Electroplating or Electrodeposition it is probably the most important and most frequently applied industrial method of producing metallic coatings. Electroplating is carried out by a process called electrolysis. Thus in this process, the coating metal is deposited on the base metal by passing direct current through an electrolyte containing the soluble salt of the coating metal. The base metal to be electroplated is made the cathode of the electrolytic cell whereas the anode is either made of the coating metal itself or an inert material of good electrical conductivity like graphic.

Electroplating © 2010 John Wiley & Sons, Inc. M P Groover, Fundamentals of Modern Manufacturing 4/e

For electroplating of Ni, Ni. SO 4 and Ni. Cl 2 are used as the electrolyte. For electroplating of Cr, chromic acid is used as the electrolyte. For Au plating, Au. Cl 3 solution is taken as the electrolyte. For Cu plating Cu. SO 4 solution is used as the electrolyte. In silver plating, Ag. NO 3 solution is used as the electrolyte.

5. Electroless Plating Metallic plating process driven entirely by chemical reactions ‑ no electric current is supplied Deposition onto a part surface occurs in an aqueous solution containing ions of the desired plating metal ◦ Workpart surface acts as a catalyst for the reaction in the presence of reducing agent Metals that can be plated: nickel, copper, and gold Notable application: copper for plating through‑holes of printed circuit boards © 2010 John Wiley & Sons, Inc. M P Groover, Fundamentals of Modern Manufacturing 4/e

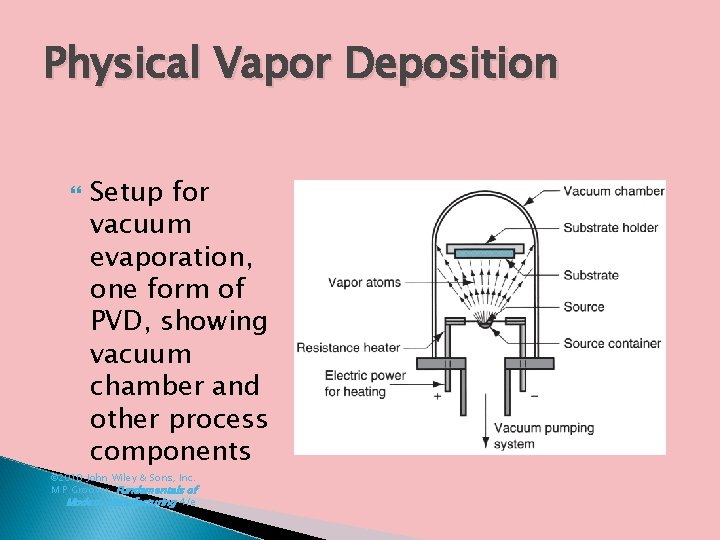
6. Physical Vapor Deposition (PVD) Family of processes in which a material is converted to its vapor phase in a vacuum chamber and condensed onto substrate surface as a very thin film Coating materials: metals, alloys, ceramics and other inorganic compounds, even some polymers Substrates: metals, glass, and plastics Very versatile coating technology ◦ Applicable to an almost unlimited combination of coatings and substrate materials © 2010 John Wiley & Sons, Inc. M P Groover, Fundamentals of Modern Manufacturing 4/e

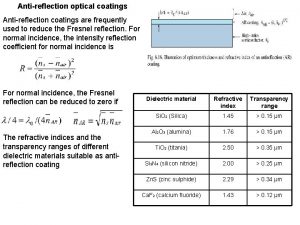
Applications of PVD Decorative coatings on plastic and metal parts such as trophies, toys, pens and pencils, watchcases, and interior trim in automobiles Antireflection coatings of magnesium fluoride (Mg. F 2) onto optical lenses Depositing metal to form electrical connections in integrated circuits Coating titanium nitride (Ti. N) onto cutting tools and plastic injection molds for wear resistance © 2010 John Wiley & Sons, Inc. M P Groover, Fundamentals of Modern Manufacturing 4/e

Processing Steps in PVD All physical vapor deposition processes consist of the following steps: 1. Synthesis of coating vapor 2. Vapor transport to substrate 3. Condensation of vapors onto substrate surface These steps are generally carried out in a vacuum chamber, so evacuation of the chamber must precede PVD process © 2010 John Wiley & Sons, Inc. M P Groover, Fundamentals of Modern Manufacturing 4/e

Physical Vapor Deposition Setup for vacuum evaporation, one form of PVD, showing vacuum chamber and other process components © 2010 John Wiley & Sons, Inc. M P Groover, Fundamentals of Modern Manufacturing 4/e

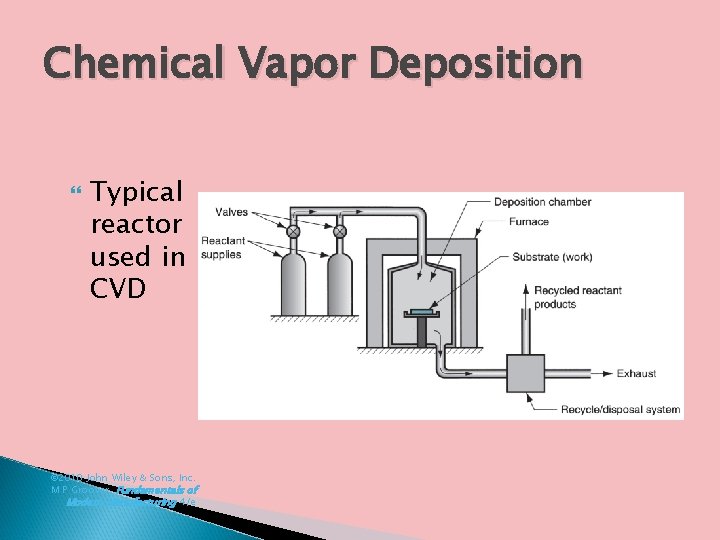
7. Chemical Vapor Deposition (CVD) Involves interaction between a mixture of gases and the surface of a heated substrate, causing chemical decomposition of some of the gas constituents and formation of a solid film on the substrate Reactions occur in enclosed reaction chamber Reaction product nucleates and grows on substrate surface to form the coating Most CVD reactions require heat Variety of coating and substrate materials © 2010 John Wiley & Sons, Inc. M P Groover, Fundamentals of Modern Manufacturing 4/e

Chemical Vapor Deposition Typical reactor used in CVD © 2010 John Wiley & Sons, Inc. M P Groover, Fundamentals of Modern Manufacturing 4/e

Applications of CVD Industrial metallurgical processes ◦ Mond process to reduce nickel from its ore Coated carbide tools Solar cells Refractory metals on jet engine turbine blades Integrated circuit fabrication Other applications for resistance to wear, corrosion, erosion, and thermal shock © 2010 John Wiley & Sons, Inc. M P Groover, Fundamentals of Modern Manufacturing 4/e

8. Ion Implantation Embedding atoms of one (or more) foreign element(s) into a substrate surface using a high ‑energy beam of ionized particles Results in alteration of the chemistry and physical properties of layers near the substrate surface Produces a much thinner altered layer and different concentration profile than diffusion Alternative to diffusion when the latter is not feasible due to high temperatures required

Advantages and Applications of Ion Implantation Advantages ◦ Low temperature processing ◦ Good control and reproducibility of impurity penetration depth ◦ Solubility limits can be exceeded without precipitation of excess atoms Applications ◦ Modifying metal surfaces to improve properties ◦ Fabrication of semiconductor devices © 2010 John Wiley & Sons, Inc. M P Groover, Fundamentals of Modern Manufacturing 4/e

Inorganic coatings The coated surface isolates the metal from the corroding medium. The coating applied must be chemically inert towards the environment. Inorganic coatings are further classified in to chemical conversion coatings and vitreous coatings.

a. CHEMICAL CONVERSATION COATINGS OR SURFACE CONVERSATION COATINGS These coatings are produced on the surface of a metal or alloy by chemical or electrochemical reaction. The metal is immersed in a solution of suitable chemical which reacts with the metal surface producing and adherent coating. These coatings protect the base metal from corrosion. Moreover many of these coatings are particularly useful to serve as excellent bases for the application of paints, enamels and other protective coatings. The most commonly used surface conversion coatings are chromate coatings, phosphate coatings and chemical oxide coatings.

1. Chromate Coatings There are produced by the immersion of the article in a bath of acidic potassium chromate followed by immersion in a bath of neutral chromate solution. The surface film consisting of a mixture of trivals and hexavalent Cr is formed. Chromate coatings possess more corrosion resistance and can also be used as a base for paints. These are applied on Zu, Cd, Mg and Al

II. Phosphate coating These are produced by the chemical reaction of base metal with aqueous solution of phosphoric acid and a phosphate of Fe, Mn or Zn. The reaction results in the formation of a surface film consisting of phosphates of the metal. These coatings are usually applied by immersing or spraying or brushing. These coating do not give complete corrosion resistance but can serve as base for painting. These are applied on metals like Fe, Zn, Cd, Al and Sn.

III. Chemical Oxide Coatings These types of coatings are formed on the surface of metals like Fe, Al, Mg etc by treating the base metal with alkaline oxidizing agents like potassium permanganate. This treatment increases the thickness of the original oxide film on the metal, there by increasing the corrosion resistance. Oxide coatings form a good base for paints. These oxide coatings have got only poor corrosion resistance. However, for better protection the thickness of the oxide film can be increased 100 to 1000 times by electrolytic oxidation or anodisation.

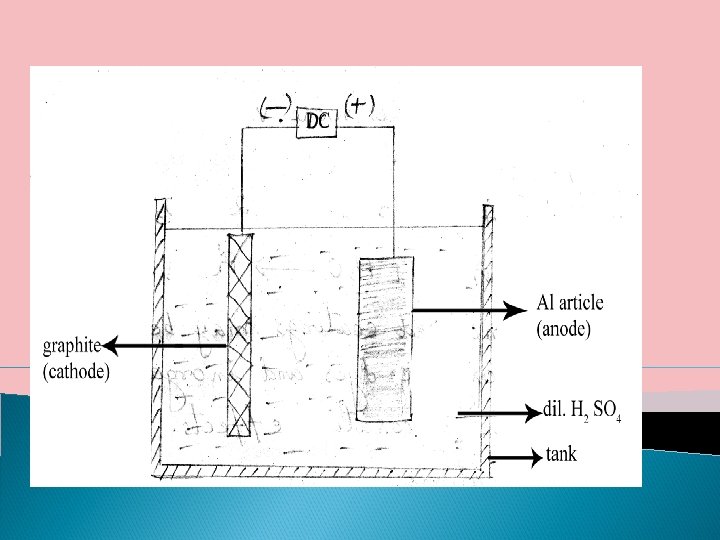
III. A -Anodisation or Anodised Coatings Anodised coatings are generally produced on non – ferrous metals like Al, Zn, Mg and their alloys by anodic oxidation process. In this process, the base metal is made as anode and the cathode is an inert electrode like graphite. The electrolytic bath is usually of H 2 SO 4, chromic acid, boric acid, phosphoric acid, oxalic acid etc The base metal to be anodized is suspended from the anode. The process is carried out by passing a moderate direct current through the electrolytic bath. As the anodized coatings are somewhat thicker than the natural oxide film and they posses improved resistance to corrosion.

Anodizing on Al has gained considerable commercial importance. Al coated surface require oxidation to convert the metal to its inert oxide. Anodising on Al is carried out by an electrolytic process.


The O 2 evolved at the anode oxides the outer layer of Al to the oxide film, Al 2 O 3. the oxide film initially very thin, grows from the metal surface outwards and increases in thickness as oxidation continues at Al anode. The outer part of the oxide film formed is porous and to reduce porosity, the article after electrolysis is kept immersed in a boiling water bath. This treatment changes porous alumina into its monohydrate (Al 2 O 3. H 2 O) which occupies more, volume, thereby the pores are sealed. 4 Al + 3 O 2 Al 2 O 3 + H 2 O Al 2 O 3. H 2 O Anodized coatings may be coloured with organic dyes and inorganic pigments to give decorative effects.

Applications Aircraft parts, refrigerators, reflectors, machine parts etc are anodized by this method Al articles used as doors, windows, showcase panels & household utensils are anodized by this method.

b. VITREOUS COATINGS OR CEREMIC PROTECTIVE COATINGS Ceramic protective coatings can be broadly divided into vitreous enamel coatings and pure ceramic coatings. These coatings have the following advantages. 1. They posses high refractoriness and inertness 2. They are wear resistant & easily be cleaned 3. They are glossy in appearance 4. They are good thermal & electrical insulators

Vitreous enamels are defined as glossy inorganic composition that can adhere to metals by fusion and protect them from corrosion, abrasion, oxidation and high temperature. Vitreous enamel coatings consists of a ceramic mixture of refractories and large proportion of fluxes. These coatings are usually applied on steel and cast iron equipments. The raw materials used for the vitreous coatings are the following.

Vitreous coatings 1. 2. 3. 4. 5. 6. Refractories like quartz (Si. O 2), clay etc. Fluxes like borax (Sodium tetra borate Na 2 B 4 O 7), cryolite (Na 3 Al. F 6) (Sodium alumino fluoride), Soda ash (anhydrous sodium carbonate Na 2 CO 3) etc. Opacifiers like Ti. O 2, Sn. O 2, Al 2 O 3 etc Pigments like metallic oxides organic dyes etc Floating agents like plastic, clay, gum etc Electrolytes like Mg. SO 4, Mg. CO 3, Na 2 Co 3 etc.

Organic Coatings Polymers and resins (natural or synthetic) usually formulated to be applied as liquids that dry or harden as thin surface films on substrate materials Advantages: ◦ ◦ Wide variety of colors and textures available Capacity to protect the substrate surface Low cost Ease with which they can be applied © 2010 John Wiley & Sons, Inc. M P Groover, Fundamentals of Modern Manufacturing 4/e

Ingredients in Organic Coatings 1. 2. 3. 4. Binders - give the coating its properties Dyes or pigments - provide color to the coating Solvents - dissolve the polymers and resins and add proper fluidity to the liquid Additives © 2010 John Wiley & Sons, Inc. M P Groover, Fundamentals of Modern Manufacturing 4/e

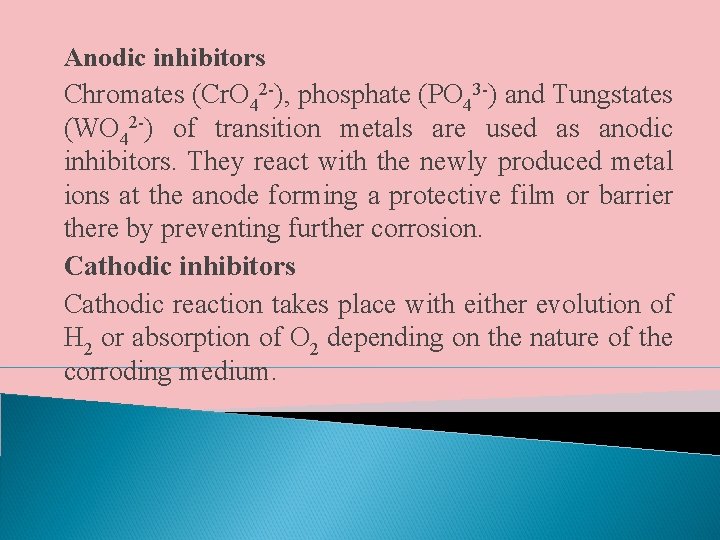
IV. USE OF CORROSION INHIBITORS Chemicals which are added in small quantities to the corroding medium in order to reduce the corrosion rate are called corrosion inhibitors. They reduce corrosion by forming a protective film either at the cathode or anode. Thus there are two types of corrosion inhibitors – anodic inhibitors and cathodic inhibitors

Anodic inhibitors Chromates (Cr. O 42 -), phosphate (PO 43 -) and Tungstates (WO 42 -) of transition metals are used as anodic inhibitors. They react with the newly produced metal ions at the anode forming a protective film or barrier there by preventing further corrosion. Cathodic inhibitors Cathodic reaction takes place with either evolution of H 2 or absorption of O 2 depending on the nature of the corroding medium.

1. Evolution of H 2 in acid medium 2 H+ + 2 e- H 2 (g) Evolution of H 2 can be prevented by slowing down the diffusion of H+ ions to the cathode or by increasing H 2 over voltage. Diffusion of H+ ions can be prevented by adding organic inhibitors such as amines, urea, thiourea etc. These are adsorbed at the surface as a film. Arsenic oxide or antimony oxide is added to increase the H 2 over voltage. These oxides form adherent film of metallic arsenic or antimony at the cathodic areas.

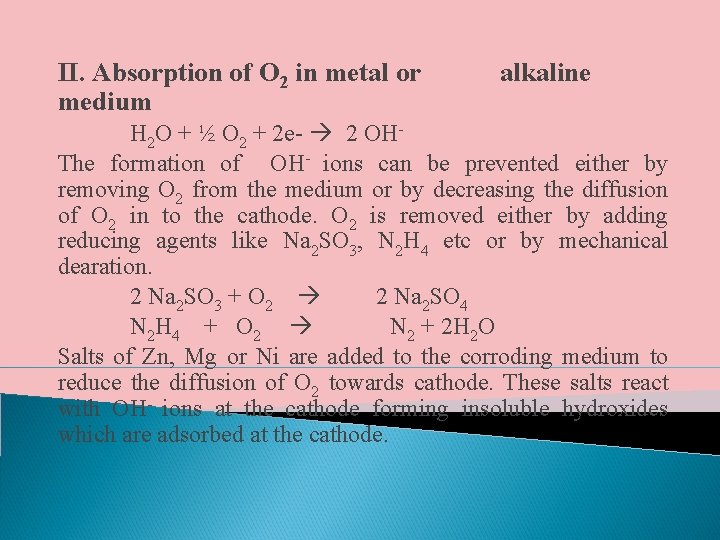
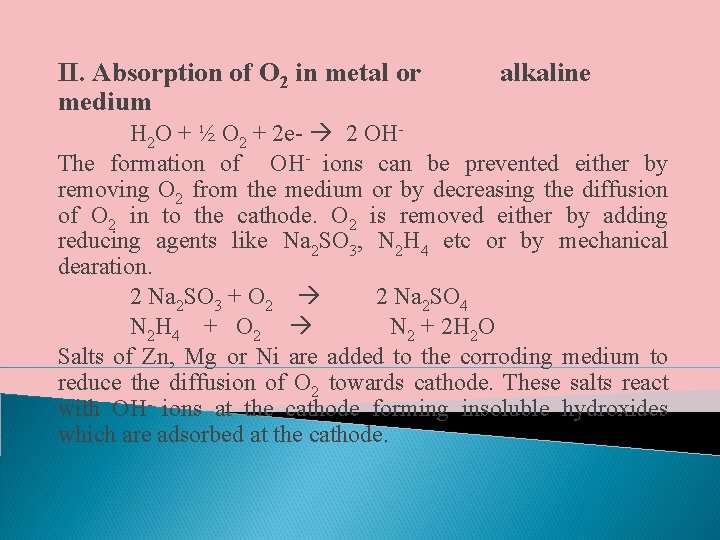
II. Absorption of O 2 in metal or medium alkaline H 2 O + ½ O 2 + 2 e- 2 OHThe formation of OH- ions can be prevented either by removing O 2 from the medium or by decreasing the diffusion of O 2 in to the cathode. O 2 is removed either by adding reducing agents like Na 2 SO 3, N 2 H 4 etc or by mechanical dearation. 2 Na 2 SO 3 + O 2 2 Na 2 SO 4 N 2 H 4 + O 2 N 2 + 2 H 2 O Salts of Zn, Mg or Ni are added to the corroding medium to reduce the diffusion of O 2 towards cathode. These salts react with OH- ions at the cathode forming insoluble hydroxides which are adsorbed at the cathode.

II. Absorption of O 2 in metal or medium alkaline H 2 O + ½ O 2 + 2 e- 2 OHThe formation of OH- ions can be prevented either by removing O 2 from the medium or by decreasing the diffusion of O 2 in to the cathode. O 2 is removed either by adding reducing agents like Na 2 SO 3, N 2 H 4 etc or by mechanical dearation. 2 Na 2 SO 3 + O 2 2 Na 2 SO 4 N 2 H 4 + O 2 N 2 + 2 H 2 O Salts of Zn, Mg or Ni are added to the corroding medium to reduce the diffusion of O 2 towards cathode. These salts react with OH- ions at the cathode forming insoluble hydroxides which are adsorbed at the cathode.
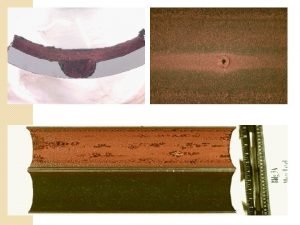
 Dry corrosion and wet corrosion
Dry corrosion and wet corrosion Differentiate between dry corrosion and wet corrosion
Differentiate between dry corrosion and wet corrosion Trenton corrosion protection
Trenton corrosion protection Corrosion intercept
Corrosion intercept Epoxy phenalkamine coatings
Epoxy phenalkamine coatings Plural component spray coating system
Plural component spray coating system Hospital wall coatings
Hospital wall coatings Hts coatings
Hts coatings Pinnacle coatings group
Pinnacle coatings group St powder coatings
St powder coatings Kale group
Kale group St powder coatings
St powder coatings Sherwin williams chemical coatings store
Sherwin williams chemical coatings store Overcurrent protection
Overcurrent protection Protective packaging and materials handling
Protective packaging and materials handling Chapter 36 emergency preparedness and protective practices
Chapter 36 emergency preparedness and protective practices Material handling objectives
Material handling objectives Define topical agent
Define topical agent Chapter 36 emergency preparedness and protective practices
Chapter 36 emergency preparedness and protective practices First aid for slips and falls in the kitchen
First aid for slips and falls in the kitchen What is season
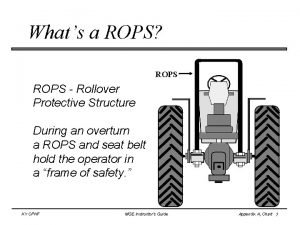
What is season Roll over protective structure (rops)
Roll over protective structure (rops) Adult protective services vancouver wa
Adult protective services vancouver wa Reverse covered call
Reverse covered call Protective ozone layer
Protective ozone layer Protective leaf-like enclosure for the flower bud
Protective leaf-like enclosure for the flower bud Sacramento adult protective services
Sacramento adult protective services Personal protective factor
Personal protective factor Gpg13 protective monitoring
Gpg13 protective monitoring Protective custom choice ul
Protective custom choice ul Ppe for plumbers
Ppe for plumbers Ppe program
Ppe program Special protective equipment used in firefighting except
Special protective equipment used in firefighting except Who is responsible for providing specialized work footwear
Who is responsible for providing specialized work footwear Protective ozone layer
Protective ozone layer Protective ozone layer
Protective ozone layer Broncheols
Broncheols Protective earth connection
Protective earth connection Discuss the optical and electrical properties of colloids
Discuss the optical and electrical properties of colloids Lyophilic colloids and lyophobic colloids difference
Lyophilic colloids and lyophobic colloids difference Lyophilic colloids and lyophobic colloids difference
Lyophilic colloids and lyophobic colloids difference Caldwell county child protective services
Caldwell county child protective services Smaw welding ppe
Smaw welding ppe Dfw security protective force reviews
Dfw security protective force reviews Protective capacities
Protective capacities Ultimate protection security services
Ultimate protection security services West virginia adult protective services
West virginia adult protective services Aces protective factors
Aces protective factors Define topical agents
Define topical agents Protective custom choice ul
Protective custom choice ul Ppe list
Ppe list Child protective investigations pasco county
Child protective investigations pasco county Integrated protective services
Integrated protective services Protective factors definition
Protective factors definition Adult protective services delaware
Adult protective services delaware Gabi gabi plant
Gabi gabi plant Crs 19-3-304
Crs 19-3-304 Microgard protective clothing
Microgard protective clothing Warships covered with protective iron plates
Warships covered with protective iron plates