Clostridium difficile disease Management Flagyl to Fecal Transplants























- Slides: 50

Clostridium difficile disease Management: Flagyl to Fecal Transplants – A Step Wise Approach to C. difficile colitis September 22, 2017 CSGNA Pamela Kibsey MD, FRCPC Medical Director, Medical Microbiology and Infection Prevention and Control, IH

Objectives 1. Refresh disease epidemiology and definitions 2. Diagnostic methods and clinical interpretation of laboratory results 3. Treatment options and updates

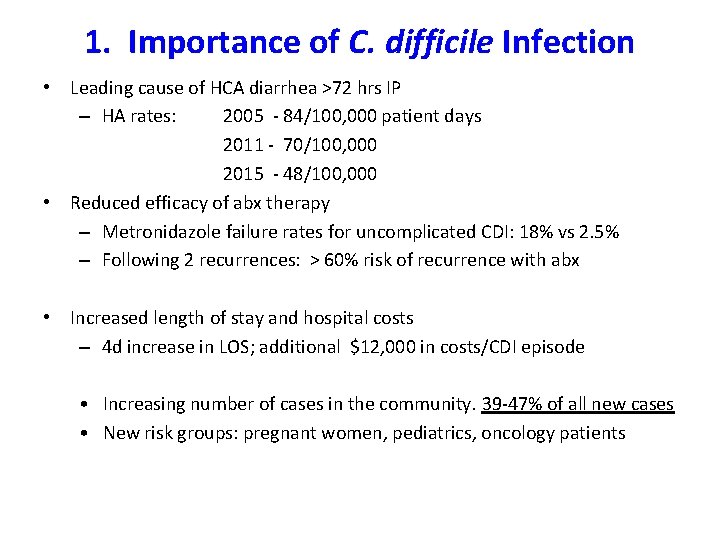
1. Importance of C. difficile Infection • Leading cause of HCA diarrhea >72 hrs IP – HA rates: 2005 - 84/100, 000 patient days 2011 - 70/100, 000 2015 - 48/100, 000 • Reduced efficacy of abx therapy – Metronidazole failure rates for uncomplicated CDI: 18% vs 2. 5% – Following 2 recurrences: > 60% risk of recurrence with abx • Increased length of stay and hospital costs – 4 d increase in LOS; additional $12, 000 in costs/CDI episode • Increasing number of cases in the community. 39 -47% of all new cases • New risk groups: pregnant women, pediatrics, oncology patients

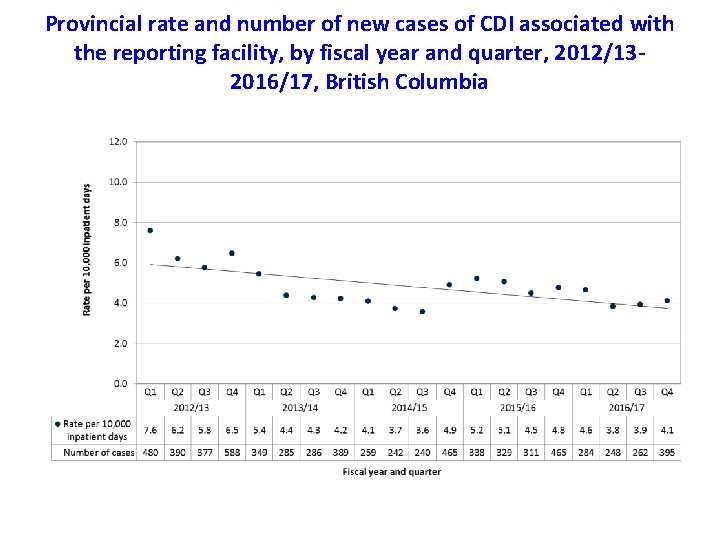
Provincial rate and number of new cases of CDI associated with the reporting facility, by fiscal year and quarter, 2012/132016/17, British Columbia

Definition of a Case Sustained watery diarrhea 3/24 hrs or 6+/36 hrs +/- elevated WBC, fever, dehydration Abdominal pain, distention, ileus Sometimes bloody Not induced by laxatives, enema, bowel prep in the previous 48 hrs or when co-existing viruses present (norovirus or rotavirus) • Risk factors: previous antibiotics 8 wks, chemotherapy, abdominal surgery, malnutrition, deconditioning, age>65, co-morbidities, 027 ribotype • • •

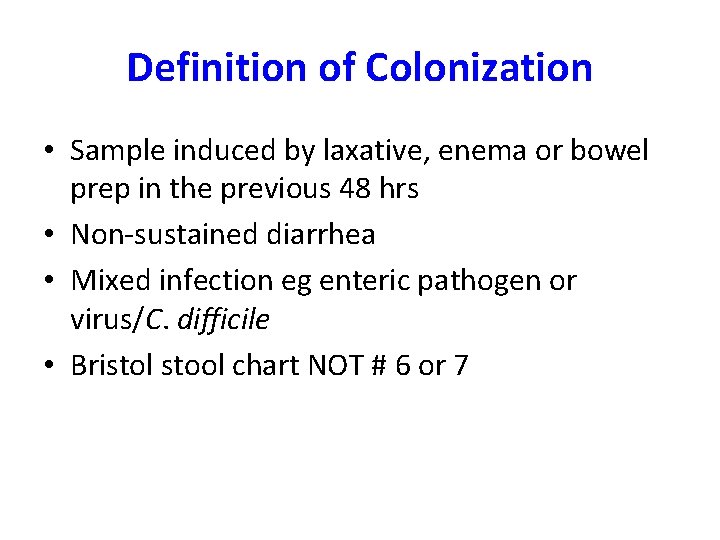
Definition of Colonization • Sample induced by laxative, enema or bowel prep in the previous 48 hrs • Non-sustained diarrhea • Mixed infection eg enteric pathogen or virus/C. difficile • Bristol stool chart NOT # 6 or 7

Environmental control • Vegetative forms susceptible to most detergents • Spores R to dessication and disinfectants • High concentration H₂O₂ or 10 % bleach • Many spores removed with neutral detergent and microfibre cloth • New agents: Xenon or blue light, H₂O₂ vapor, UV light • HH: ABHR or soap/water/friction when soiled

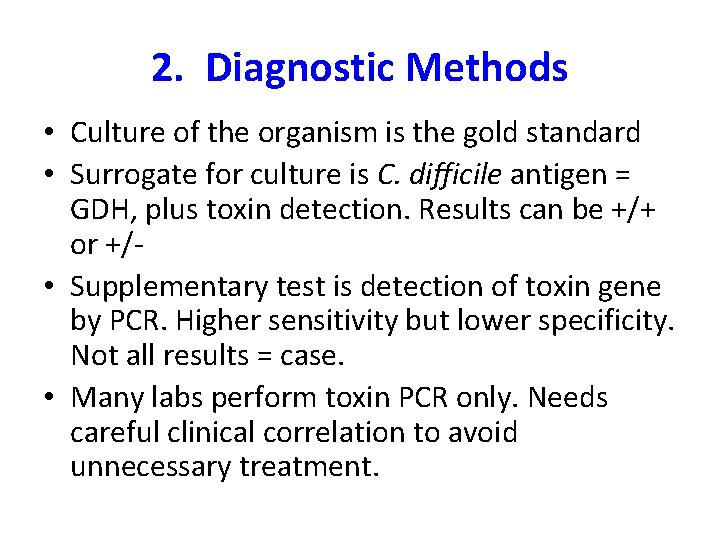
2. Diagnostic Methods • Culture of the organism is the gold standard • Surrogate for culture is C. difficile antigen = GDH, plus toxin detection. Results can be +/+ or +/ • Supplementary test is detection of toxin gene by PCR. Higher sensitivity but lower specificity. Not all results = case. • Many labs perform toxin PCR only. Needs careful clinical correlation to avoid unnecessary treatment.

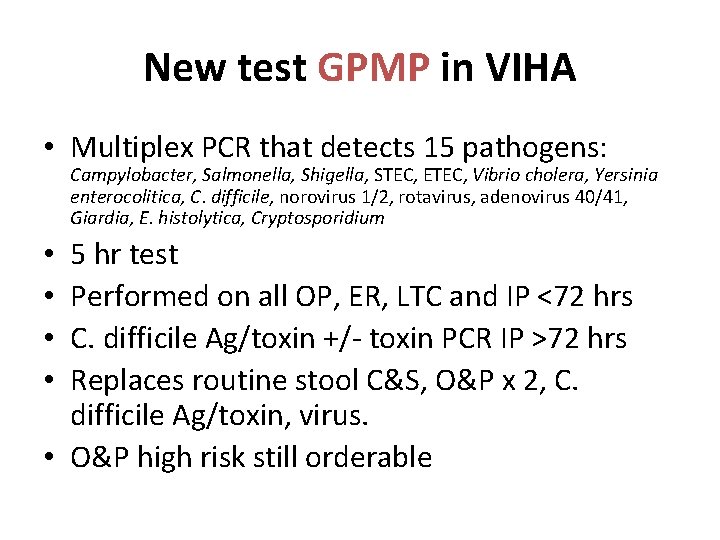
New test GPMP in VIHA • Multiplex PCR that detects 15 pathogens: Campylobacter, Salmonella, Shigella, STEC, ETEC, Vibrio cholera, Yersinia enterocolitica, C. difficile, norovirus 1/2, rotavirus, adenovirus 40/41, Giardia, E. histolytica, Cryptosporidium 5 hr test Performed on all OP, ER, LTC and IP <72 hrs C. difficile Ag/toxin +/- toxin PCR IP >72 hrs Replaces routine stool C&S, O&P x 2, C. difficile Ag/toxin, virus. • O&P high risk still orderable • •

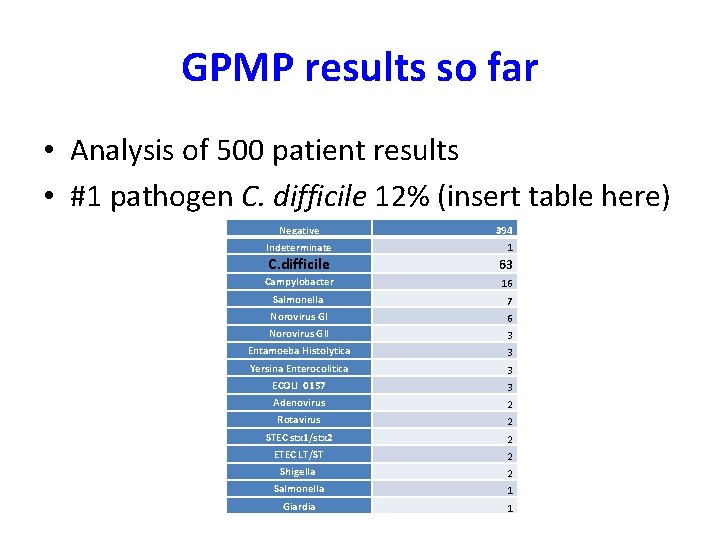
GPMP results so far • Analysis of 500 patient results • #1 pathogen C. difficile 12% (insert table here) Negative Indeterminate 394 1 C. difficile 63 Campylobacter 16 Salmonella 7 Norovirus GI 6 Norovirus GII 3 Entamoeba Histolytica 3 Yersina Enterocolitica 3 ECOLI 0157 3 Adenovirus 2 Rotavirus 2 STEC stx 1/stx 2 2 ETEC LT/ST 2 Shigella 2 Salmonella 1 Giardia 1

3. Treatment options and updates • Efficacy of current treatments for CDI – Primary and 1 st recurrent episode – Recurrent CDI treatment/prevention • Current evidence for use of FMT • Review of cases

Severity Criteria Treatment Comment Mild-tomoderate disease Diarrhea plus any additional signs or symptoms not meeting severe or complicated criteria Metronidazole 500 mg orally three times a day for 10 days. If unable to take metronidazole, vancomycin 125 mg orally four times a day for 10 days If no improvement in 5– 7 days, consider change to vancomycin at standard dose (vancomycin 125 mg four times a day for 10 days) Severe disease Serum albumin <3 g/dl plus ONE of the following: WBC ≥ 15, 000 cells/mm 3, Abdominal tenderness Vancomycin 125 mg orally four times a day for 10 days Severe and Any of the following complicated attributable to CDI: disease Admission to intensive care unit for CDI Hypotension with or without required use of vasopressors Fever ≥ 38. 5 °C Ileus or significant abdominal distention Mental status changes WBC ≥ 35, 000 cells/mm 3 or <2, 000 cells/mm 3 Serum lactate levels >2. 2 mmol/l End organ failure (mechanical ventilation, renal failure, etc. ) Vancomycin 500 mg orally four times a day and metronidazole 500 mg IV every 8 h, and vancomycin per rectum (vancomycin 500 mg in 500 ml saline as enema) four times a day Surgical consultation suggested Recurrent CDI Repeat metronidazole or vancomycin pulse regimen Consider FMT after 3 recurrences Recurrent CDI within 8 weeks of completion of therapy CDI, Clostridium difficile infection; FMT, fecal microbiota transplant; IV, intravenous; WBC, white blood cell.

Case 1 CA Mild CDI • 48 y. F. Recently completed Rx clindamycin for abscessed tooth • 2 wk later presents with abdominal cramping, watery diarrhea 4 -6/day for 3 days • Lab positive C. difficile toxin PCR • What treatment would you pick first line?

? ? Rx • • Metronidazole 500 mg po bid for 7 days Metronidazole 500 mg po tid for 10 days Metronidazole 500 mg po tid for 14 days Vancomycin 125 mg po qid for 10 days

? ? Rx • • Metronidazole 500 mg po bid for 7 days Metronidazole 500 mg po tid for 10 days Metronidazole 500 mg po tid for 14 days Vancomycin 125 mg po qid for 10 days

Case 2 Mild CDI, compromised pt • 67 y. F – fever, nausea, copious diarrhea and abdominal pain following ingesting of raspberries • Stool O & P: Cyclospora • Started on TMP/SMX 160/800 mg 2 – improved on day 4 of treatment • Recurrent diarrhea 7 d following Rx. • Next steps? ?

Repeat stool investigations • • O & P: negative C & S: negative Enteric viruses: negative C. difficile toxin- pending

Patient’s current state and management plan 67 F, now has 5 watery bowel movements/day Admitted for monitoring (immunocompromised) • Normal temperature, WBC, lactate • Maintained baseline creatinine • Empiric treatment for suspected C. difficile infection?

• Wait for laboratory confirmation for mild CDI • Patient’s stool: C. difficile Ag/toxin positive • Which antibiotic? – Metronidazole 500 mg po tid – Vancomycin 125 mg po qid – Fidaxomicin 200 mg po bid – Combination therapy? ?

Vancomycin 125 mg po qid for 10 days • Multinational, RCT. S Johnson. CID Aug 2014 • Clinical success: MTZ (72. 7%)was inferior to VM (81. 8%) (p = 0. 02) • Clinical success: 4% (mild); 8. 3% (mod); 12. 2% (severe cases) more in VM than MTZ

Back to Mild Case of CDI • Patient unable to take any oral medications due to intractable nausea and vomiting • Is IV metronidazole the only option? • Is it equivalent to oral treatment?

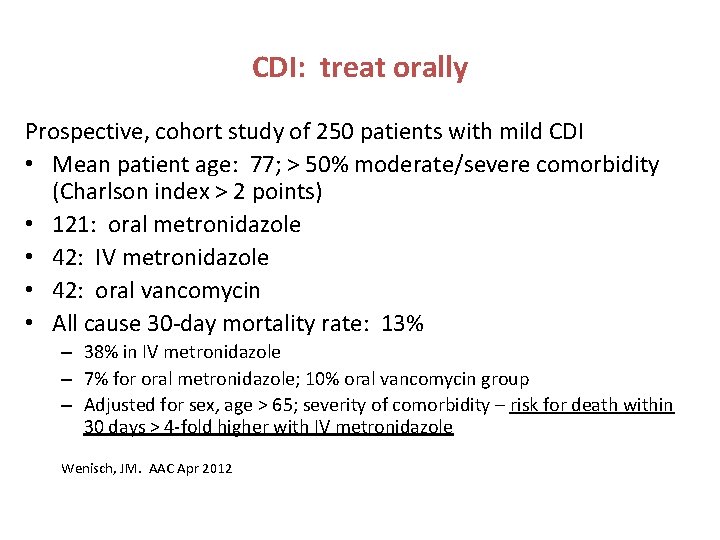
CDI: treat orally Prospective, cohort study of 250 patients with mild CDI • Mean patient age: 77; > 50% moderate/severe comorbidity (Charlson index > 2 points) • 121: oral metronidazole • 42: IV metronidazole • 42: oral vancomycin • All cause 30 -day mortality rate: 13% – 38% in IV metronidazole – 7% for oral metronidazole; 10% oral vancomycin group – Adjusted for sex, age > 65; severity of comorbidity – risk for death within 30 days > 4 -fold higher with IV metronidazole Wenisch, JM. AAC Apr 2012

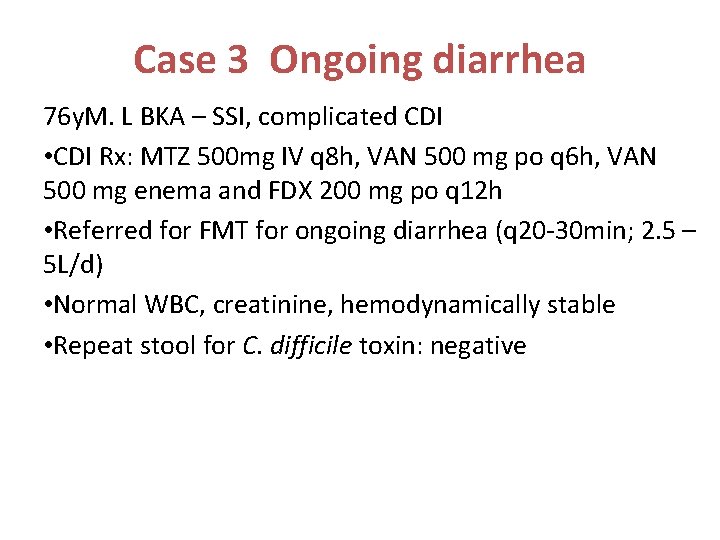
Case 3 Ongoing diarrhea 76 y. M. L BKA – SSI, complicated CDI • CDI Rx: MTZ 500 mg IV q 8 h, VAN 500 mg po q 6 h, VAN 500 mg enema and FDX 200 mg po q 12 h • Referred for FMT for ongoing diarrhea (q 20 -30 min; 2. 5 – 5 L/d) • Normal WBC, creatinine, hemodynamically stable • Repeat stool for C. difficile toxin: negative

Ongoing diarrhea • When should you consider switching therapy or making an alternate diagnosis?

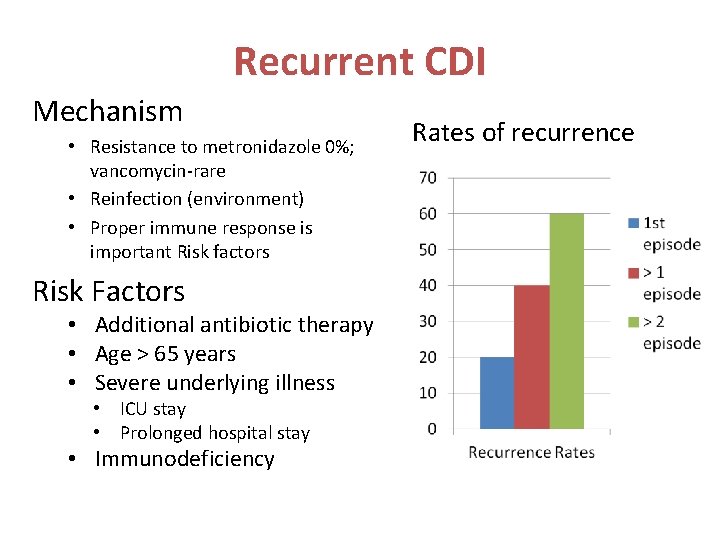
Recurrent CDI Mechanism • Resistance to metronidazole 0%; vancomycin-rare • Reinfection (environment) • Proper immune response is important Risk factors Risk Factors • Additional antibiotic therapy • Age > 65 years • Severe underlying illness • ICU stay • Prolonged hospital stay • Immunodeficiency Rates of recurrence

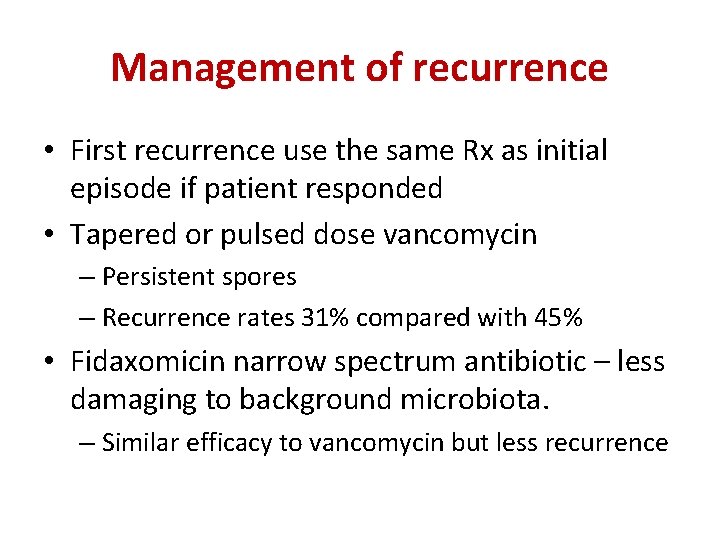
Management of recurrence • First recurrence use the same Rx as initial episode if patient responded • Tapered or pulsed dose vancomycin – Persistent spores – Recurrence rates 31% compared with 45% • Fidaxomicin narrow spectrum antibiotic – less damaging to background microbiota. – Similar efficacy to vancomycin but less recurrence IPS Liverpool 29 th September 2015

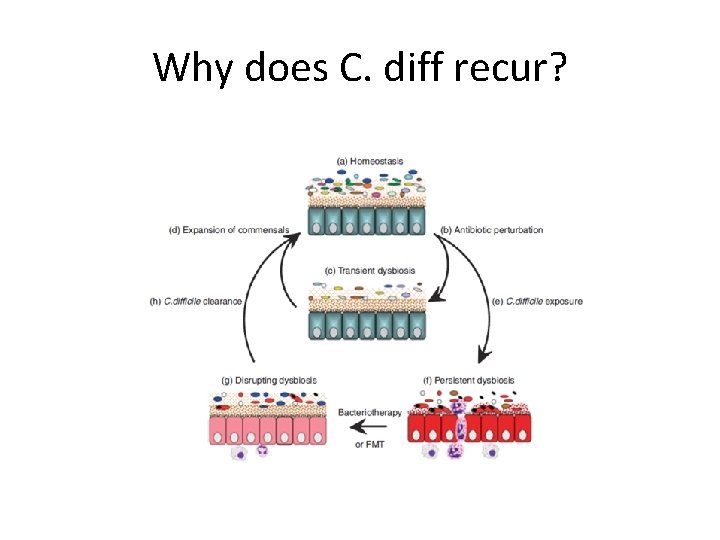
Why does C. diff recur? IPS Liverpool 29 th September 2015

From me. . . to poo IPS Liverpool 29 th September 2015

Overview of FMT • • • Case history Recurrent CDI Why FMT? History of FMT Our journey The future? IPS Liverpool 29 th September 2015

Case history FMT • Mr MW 76 year old man • Admitted to RJH October 2016 – – – Severe CAP- managed in RICU Cardiac arrest Coronary angioplasty Developed ARF Haemodialysis • Developed C difficile diarrhea – – Metronidazole 14 days Vancomycin + metronidazole 28 days Fidaxomicin 10 days IPS Liverpool 29 th September 2015

Referral to MM Sent: Friday, December 23, 2016, 10: 41 AM To: Dr Christine Lee Subject: Stool transplant Hi, I am trying to get hold of the gastroenterologist who does stool transplants. Is that you? We have a patient I would like to discuss. Thanks IPS Liverpool 29 th September 2015

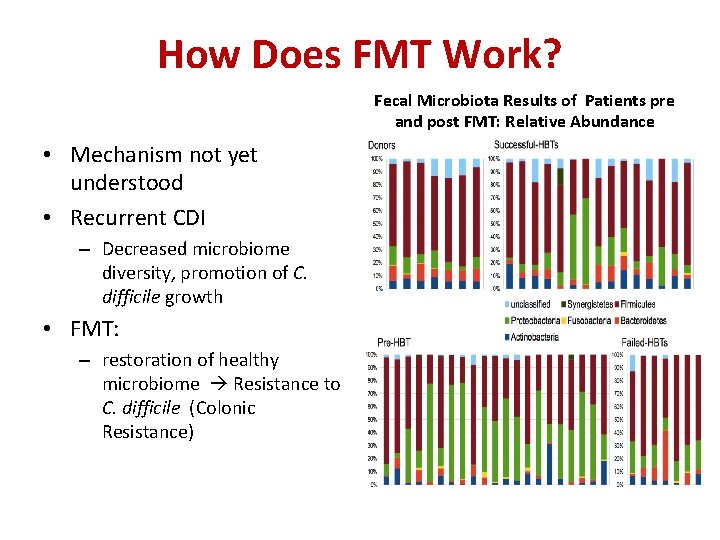
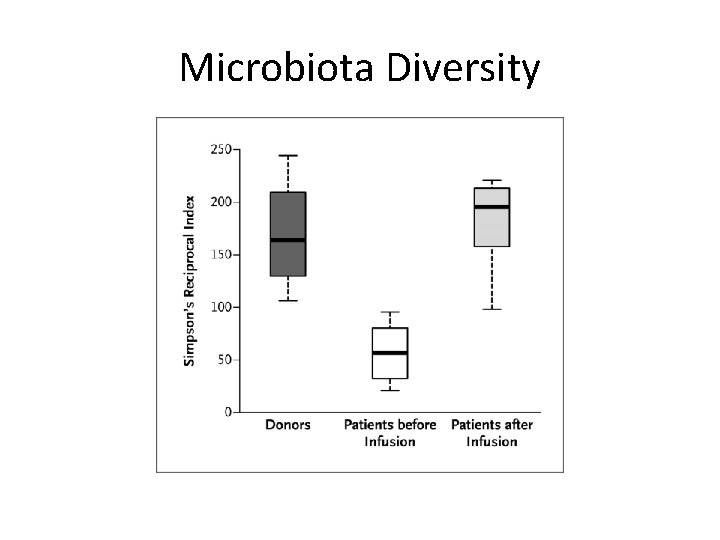
How Does FMT Work? Fecal Microbiota Results of Patients pre and post FMT: Relative Abundance • Mechanism not yet understood • Recurrent CDI – Decreased microbiome diversity, promotion of C. difficile growth • FMT: – restoration of healthy microbiome Resistance to C. difficile (Colonic Resistance) Acitinobacteria (blue); Bacteroidetes (yellow); Firmicutes (white); Fusobacteria (red);

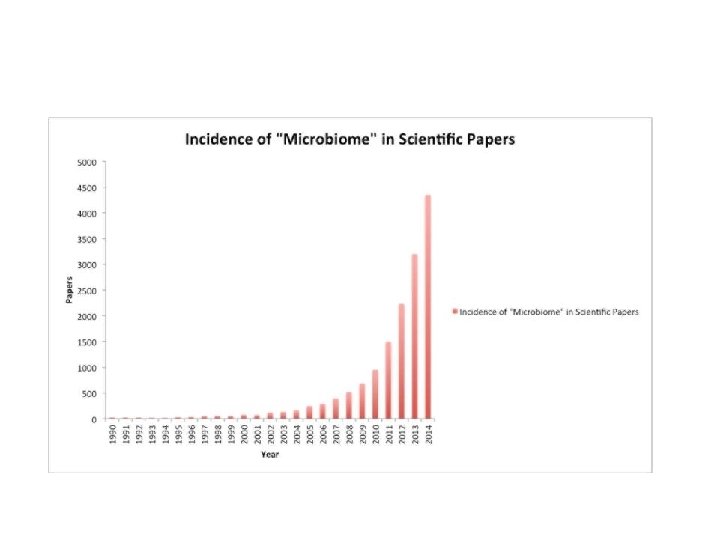
Ant A t n ibio o i ib tics s c ti Emma Allen-Vercoe, Univ Guelph , Canada IPS Liverpool 29 th September 2015 FMT

IPS Liverpool 29 th September 2015

FMT Donor Screening • No standardized exclusion criteria • Exclusion criteria: • Positive for any of the following: HIV, HCV, HBs. Ag, HTLV 1/II, syphilis, Salmonella, Shigella, E. coli O 157 H 7, Yersinia and Campylobacter, VRE, MRSA, ESBL, CRO • Detection of ova, protozoa, C. difficile toxin, norovirus, adenovirus, rotavirus • History of risk factors for acquisition of blood-borne pathogens; prion or any neurological disease as determined by the donor questionnaire, • History of gastrointestinal comorbidites, e. g. , inflammatory bowel disease, irritable bowel syndrome, chronic constipation or diarrhea • Antibiotic use or any systemic immunosuppressive agents in the 3 months prior to stool donation • Receipt of any type of live vaccine within 3 months prior to stool donation • Ingestion of nut or shell fish 3 days preceding donation • History of GI cancer • Family history of colon cancer • History of any type of active cancer or autoimmune disease • History of depression, anxiety or panic disorder • Body mass index > 29

IPS Liverpool 29 th September 2015

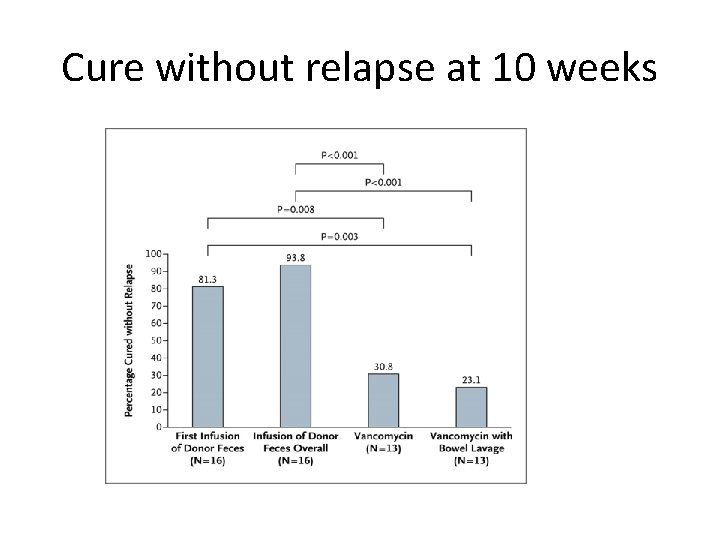
Efficacy and safety of FMT 9 Randomized Controlled Trials. Duodenal Infusion of Donor Feces for Recurrent C. difficile van Nood, et. al. N Eng J Med. 2013 – 3 treatment groups (NJ infusion of FMT: oral vancomycin; bowel lavage and oral vancomycin – Study halted following interim analysis as FMT superior to other treatments ( P <0. 001 ) and almost all of the other pts had recurrences. • FMT 13/16 (81% , 1 st infusion); 2/3 resolved with 2 nd infusion: overall efficacy 94% • Vancomycin 4/13 (31%) • Bowel lavage and oral vancomycin 3/13 (23%) • Similar AE’s between 3 groups; mild diarrhea and abd cramps in FMT group

Cure without relapse at 10 weeks IPS Liverpool 29 th September 2015

Microbiota Diversity IPS Liverpool 29 th September 2015

Acceptable to patients? • Clinicians often state no patient would ever agree to this procedure • No patients who have been considered for procedure has refused it. Zipursky J. et al. Can J Gastroenterol Hepatol 2014 28(6): 319 -24 IPS Liverpool 29 th September 2015

Efficacy and safety of FMT: 6 Randomized Controlled Trials Van Nood 20 Cammarota 21 Youngster 22 Lee 23 Kelly 24 Hota 25 16 20 20 178 22 16 enema colonoscopy enema 1 (91) 1 (41. 7)6 fresh No patients 1 Route of administration ND 2 No. of FMTs/response rate (%) 1(81) FMT type fresh Follow-up (weeks) 1 no tube 2(93. 7) colonoscopy 1 (65) 2 (90) fresh 10 10(NG 3) 10 (colonoscopy 4) 2 via NG/NG (80) 2 via colonos & NG (100)5 1 - NG (60) 1 - colonosc (80) frozen 24 of patients treated with FMT 2 ND = nasoduodenal 52 nd FMT via NG only 6 FMT for acute episode of r. CDI 1(55) 2 (84) fresh & frozen 8 3 NG 13 8 = nasogastric 4 colonos = colonoscopy 16

Outcomes of FMT at IH • • • 30 patients enrolled to date 20/25 cured with 2 FMT. f/u 60 days 3/5 cured with 3 FMT. Remainder are being followed. Efficacy is 80% with 2 and 92% with 3 FMT.

Outcome of Patients Non-Responsive to FMT • Pts refractory to CDI • Multiple FMTs – no response • Response to oral vancomycin post FMT relapse – 4/94 in SJHH observational study – 6/232 in RCT • 4/6 unresponsive to VAN pre-FMT • 6/6 post FMT, symptom-free on VAN 125 mg 1 24 – 36 m f/up – Ruben, Bakken. Anaerobe 2013 – Brandt. Am J Gastroenterol 2012 – Lee, et. al. Eur J Microbiol Infect Dis 2014

Safety of FMT • Most current data is retrospective • Minor – Abdominal symptoms immediately post FMT are common • Serious – Related to mode of administration – Transmission of infection • Potential – Transmission of infective agent – Induction of chronic disease by altering the microbiome IPS Liverpool 29 th September 2015

Multidisciplinary approach • Interested in the concept • Lack of effective treatment for recurrent disease • Discussed with local microbiologists • Discussed with gastroenterology and ID/MAP colleagues • RCT was trigger to look at developing a service IPS Liverpool 29 th September 2015

Who, where and how? • Must have had 2 relapses – tapered vancomycin • MAP any hospital, ward, at home • 50 ml enema given by physician, nurse or patient. 15 minute procedure. No bowel preparation, no loperimide, d/c vancomycin 24 -48 hrs before. Refractory cases 6 hr. • Referral to OPAT or Dr Christine Lee MM IH IPS Liverpool 29 th September 2015

Easier? • • Material provided as frozen or lyophilized 6 months shelf life in a -20⁰C freezer No more donor finding or screening Allows patients to be treated quickly IPS Liverpool 29 th September 2015

Safer? • More extensively screened • Better quality control • Traceability – Tracker codes – Deep frozen reference samples • Established adverse event reporting system • Reduced hazard of processing locally IPS Liverpool 29 th September 2015

Take Home Messages for CDI • Metronidazole: no longer routinely recommended for IP • Empiric therapy for ill patients only • Mean time to response: 3 – 5 days • Treat for 10 days for primary or 1 st recurrence • Assess for risk of recurrences • Do not perform test of cure assays • Avoid antibiotic treatment in C. difficile colonized pts • Consider FMT after 2 nd relapse and in all severe cases

Thank you!
 Embryo transplant gcse
Embryo transplant gcse Mixed amebicides
Mixed amebicides Metronidazol en walgreens
Metronidazol en walgreens Clostridium botulinum disease name
Clostridium botulinum disease name Dingbat difficile
Dingbat difficile Forti et fideli nihil difficile
Forti et fideli nihil difficile Puzzle difficile
Puzzle difficile Burp intubazione
Burp intubazione Mot difficile à faire deviner
Mot difficile à faire deviner Un difficile dopoguerra
Un difficile dopoguerra Oral-fecal
Oral-fecal Is used for
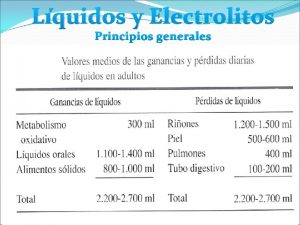
Is used for Liquidos corporales
Liquidos corporales Fecal loop
Fecal loop Gasto fecal
Gasto fecal Clasificacion de waterlow desnutricion
Clasificacion de waterlow desnutricion Malabsorption stool pictures
Malabsorption stool pictures Fecal
Fecal Fecal matter
Fecal matter Fecal matter
Fecal matter Fecal coliform
Fecal coliform Impacted colon pictures
Impacted colon pictures Hemosure ifob test instructions
Hemosure ifob test instructions Communicable disease and non communicable disease
Communicable disease and non communicable disease Livor mortis vs rigor mortis
Livor mortis vs rigor mortis Clostridium botulinum reino
Clostridium botulinum reino Morfologi clostridium perfringens
Morfologi clostridium perfringens Gentamicina que bacterias ataca
Gentamicina que bacterias ataca Litmus milk broth
Litmus milk broth Tetanus
Tetanus Leucorrea profusa
Leucorrea profusa Measles vs chicken pox
Measles vs chicken pox Clostridium tetani
Clostridium tetani Clostridium tetani
Clostridium tetani Granulos de cianoficina
Granulos de cianoficina Mr test
Mr test Membrane
Membrane Clostridium tetani
Clostridium tetani Clostridium tetani morphology
Clostridium tetani morphology Clostridium perfringens sintomas
Clostridium perfringens sintomas Clostridium sporogenes
Clostridium sporogenes Clostridium welchii
Clostridium welchii Clostridium perfringens ppt
Clostridium perfringens ppt Clostridium spores
Clostridium spores Hirschsprung disease nursing management
Hirschsprung disease nursing management Hirschsprung disease nursing management
Hirschsprung disease nursing management Kate lorig chronic disease self-management
Kate lorig chronic disease self-management Disease management association of america
Disease management association of america Who developed the chronic care model
Who developed the chronic care model Stage 3 liver disease
Stage 3 liver disease Disease management roi
Disease management roi