Chemistry This Powerpoint is hosted on www worldofteaching


















- Slides: 35

Chemistry This Powerpoint is hosted on www. worldofteaching. com Please 100’s more free More visit freefor powerpoints atpowerpoints www. worldofteaching. com

Matter What is Matter? Where is Matter made? Matter = any material substance with Mass, Density & Volume

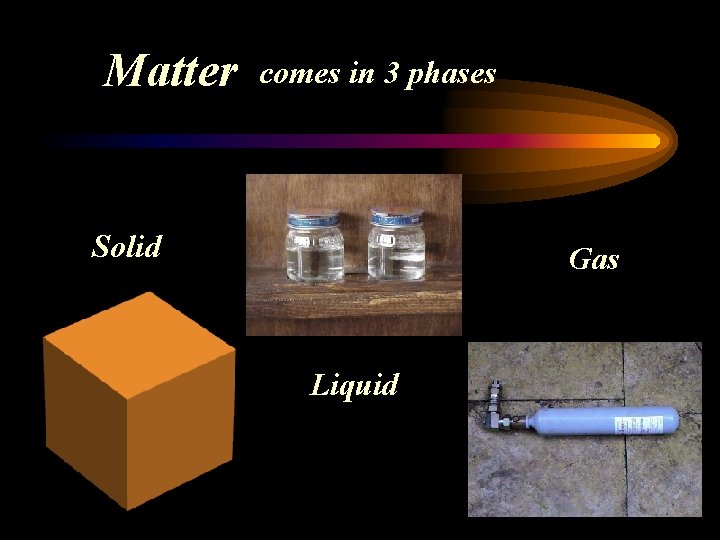
Matter comes in 3 phases Solid Gas Liquid

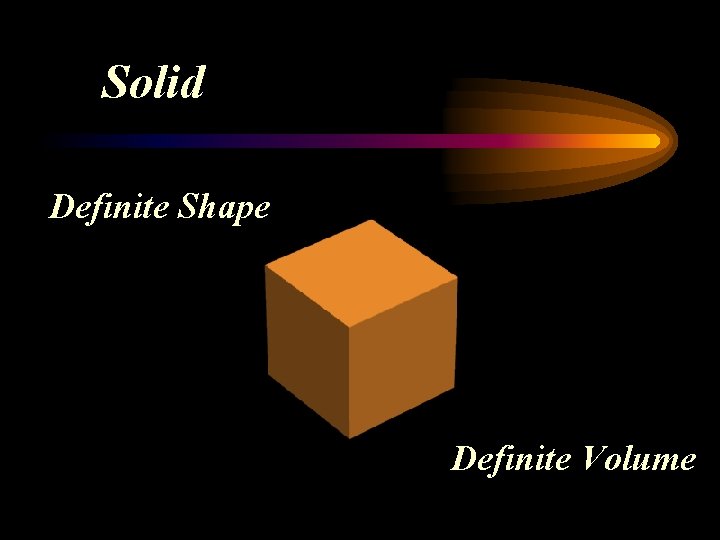
Solid Definite Shape Definite Volume

Liquid Indefinite Shape – takes the shape of the container Definite Volume

Gas Indefinite Shape – takes the shape of the container Indefinite Volume – can expand be compressed

Elements one of the 100+ pure substances that make up everything in the universe

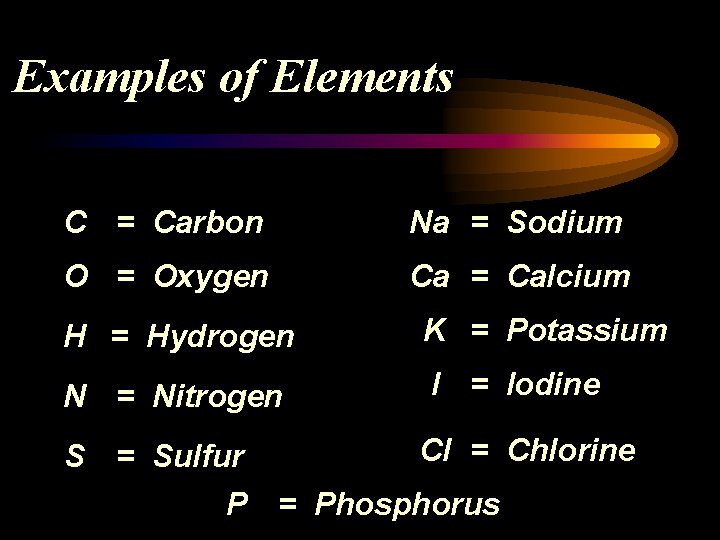
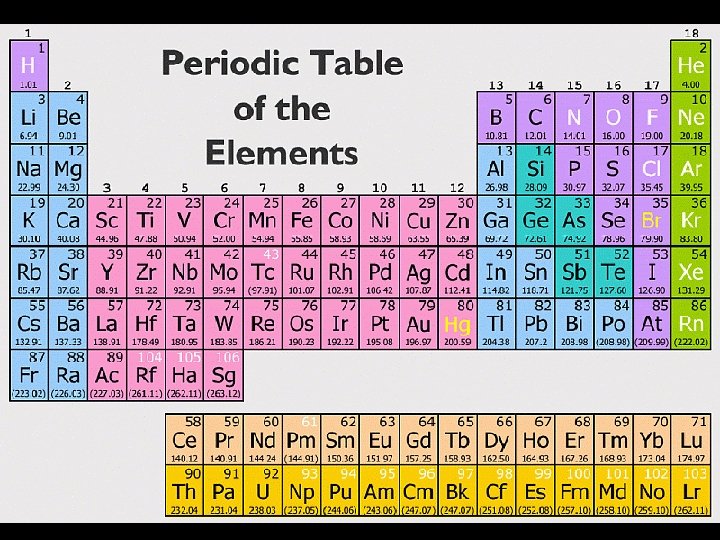
Examples of Elements C = Carbon Na = Sodium O = Oxygen Ca = Calcium H = Hydrogen K = Potassium N = Nitrogen I = Iodine Cl = Chlorine S = Sulfur P = Phosphorus

C. Elements 1. All matter is composed of elements 2. Substances that cannot be broken into simple substances by chemical means Most Common Elements in Earth’s Crust 46. 6% Oxygen (O) 27. 7% Silicon (Si) 8. 1% Aluminum (Al) 5. 0% Iron (Fe) 3. 6% Calcium (Ca) 2. 8% Sodium (Na) 2. 6% Potassium (K) 2. 1% Magnesium (Mg)

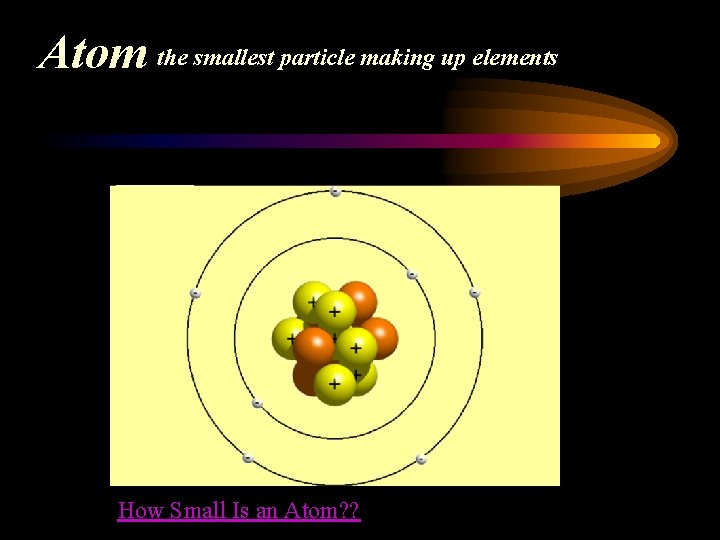
Atom the smallest particle making up elements How Small Is an Atom? ?

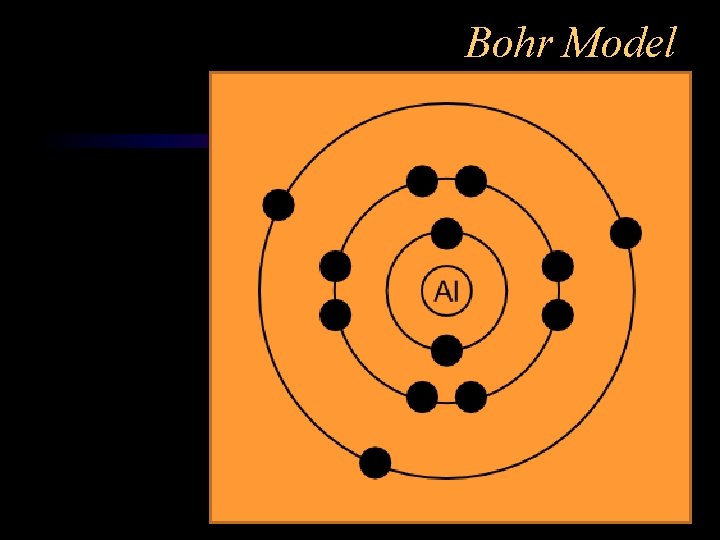
Bohr Model

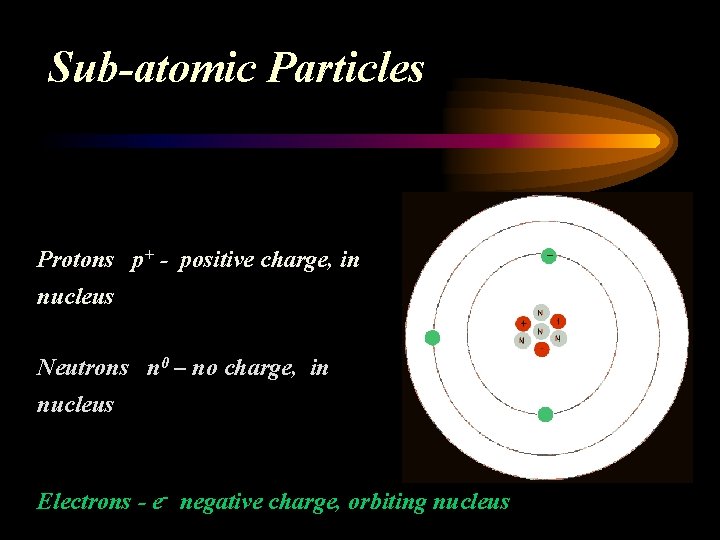
Sub-atomic Particles Protons p+ - positive charge, in nucleus Neutrons n 0 – no charge, in nucleus Electrons - e- negative charge, orbiting nucleus

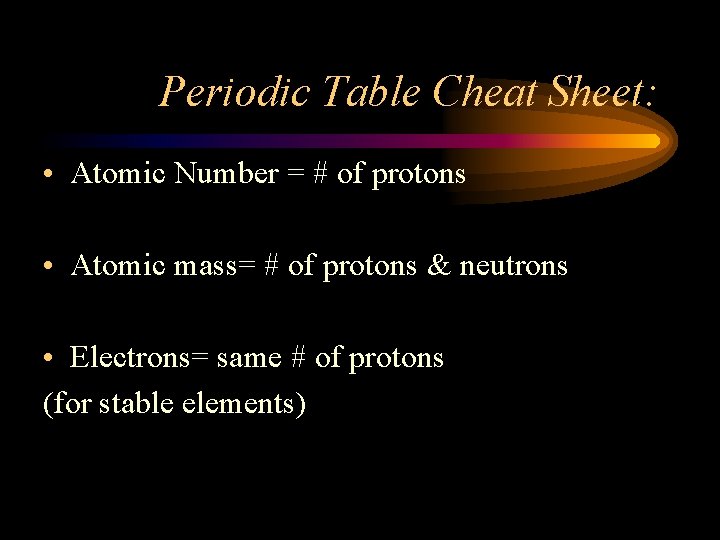
Periodic Table Cheat Sheet: • Atomic Number = # of protons • Atomic mass= # of protons & neutrons • Electrons= same # of protons (for stable elements)

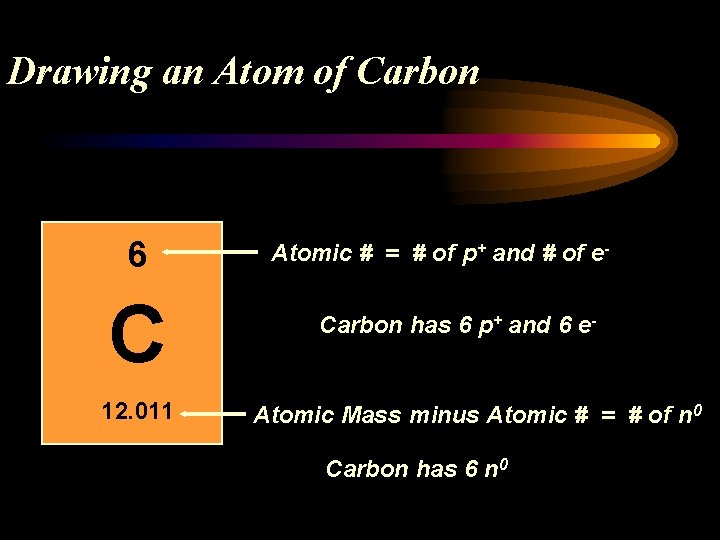
Drawing an Atom of Carbon 6 C 12. 011 Atomic # = # of p+ and # of e. Carbon has 6 p+ and 6 e- Atomic Mass minus Atomic # = # of n 0 Carbon has 6 n 0

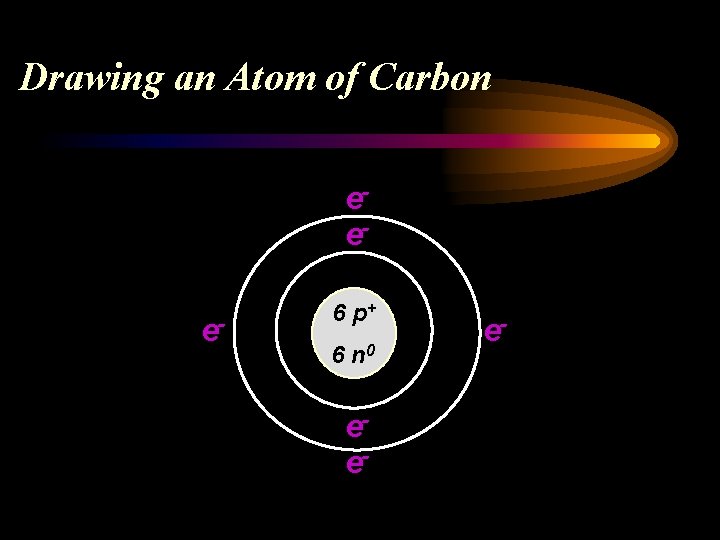
Drawing an Atom of Carbon eee- 6 p+ 6 n 0 ee- e-

E. Compounds - A substance made of atoms combined in a fixed proportion. Ex. H – O = water Ex. H 2 O + Na = Saltwater

Reading Chemical Compounds How many different elements? H 2 O How many total atoms? Na. Cl H 2 SO 4 Be(OH)2

Compounds - 2 or more elements chemically combined to form a new substance with new properties Properties – The way a chemical substance looks and behaves

Compounds – are made of 2 or more different atoms combined to form Molecules O H+O H 2 O = H H Chemical formula lists the number of different atoms in a single molecule Structural formula shows the arrangement of the atoms in a single molecule

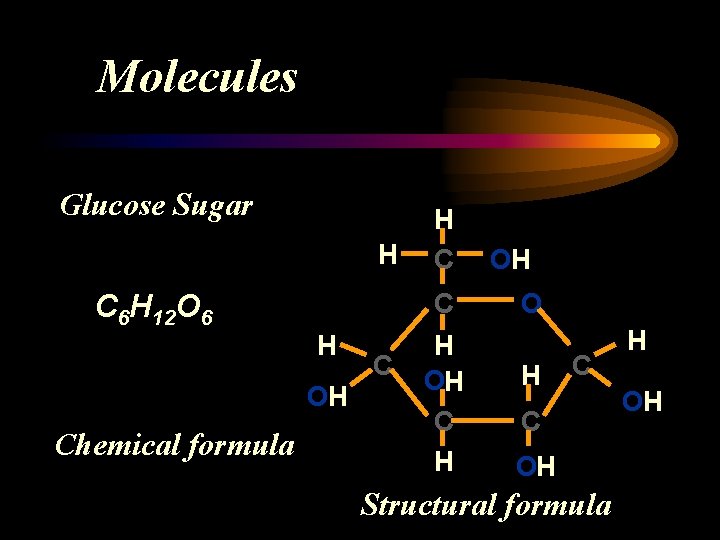
Molecules Glucose Sugar H C C 6 H 12 O 6 H OH Chemical formula H C C H OH O H C C OH Structural formula H OH

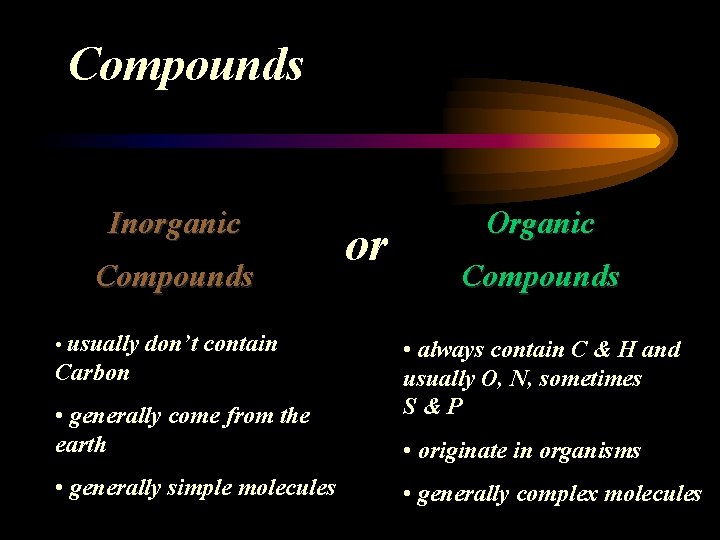
Compounds Inorganic Compounds • usually don’t contain Carbon • generally come from the earth • generally simple molecules or Organic Compounds • always contain C & H and usually O, N, sometimes S&P • originate in organisms • generally complex molecules

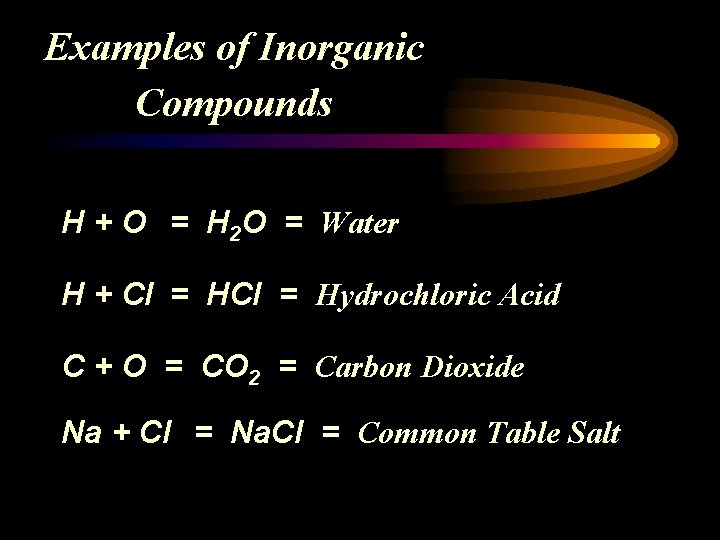
Examples of Inorganic Compounds H + O = H 2 O = Water H + Cl = Hydrochloric Acid C + O = CO 2 = Carbon Dioxide Na + Cl = Na. Cl = Common Table Salt

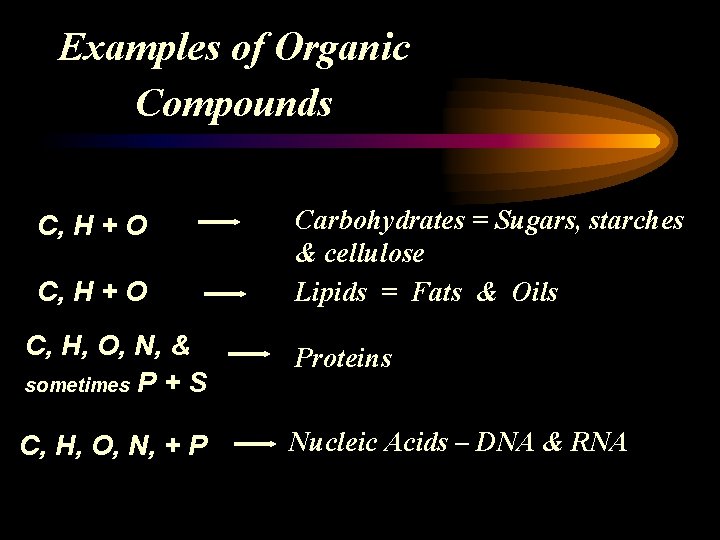
Examples of Organic Compounds C, H + O Carbohydrates = Sugars, starches & cellulose Lipids = Fats & Oils C, H, O, N, & sometimes P + S Proteins C, H, O, N, + P Nucleic Acids – DNA & RNA


PT Interactive

D. Isotopes 1. Definition: Atoms of the same number of protons, different number of neutrons Ex. Carbon-12 & Carbon-14 2. Useful because they decay at a specific rate. -tells us the age of a rock or fossil

1. Atom attraction a. Atoms in their normal state have an equal # of protons & electrons. b. Said to be “stable” 2. Ion a. An atom that gains or loses one or more electrons becoming either more positively or negatively charged

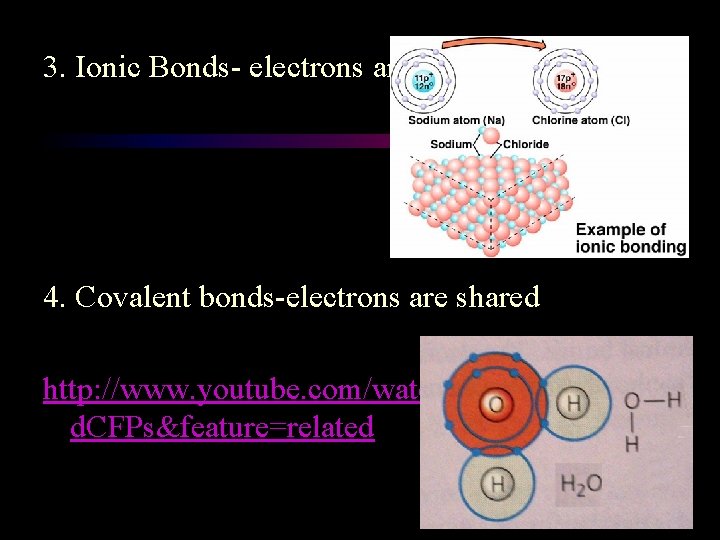
3. Ionic Bonds- electrons are transferred 4. Covalent bonds-electrons are shared http: //www. youtube. com/watch? v=yjge 1 W d. CFPs&feature=related

1. When an atom gains or loses an electron it is now called an ____. Ion

2. How many different elements are in the following compound? (CH 3)2 CO Answer- 3

3. How many total atoms are in the following compound? (CH 3)2 CO Answer- 10

4. The atomic number tells how many ______ are in the element. protons

5. What type of chemically bonding involves the sharing of electrons? Covalent

What is your stone?

Chemistry Diga, diga, that’s all folks!
 Worldofteaching.com
Worldofteaching.com Worldofteaching
Worldofteaching Hosted lync
Hosted lync Lync hosted
Lync hosted Hosted lync voice
Hosted lync voice New-omeconfiguration
New-omeconfiguration Hosted bi
Hosted bi Hosted telefonie betekenis
Hosted telefonie betekenis Hosted uc
Hosted uc Branchcache hosted cache server
Branchcache hosted cache server Self hosted wikipedia
Self hosted wikipedia Color 06142010
Color 06142010 Hosted collaboration solution
Hosted collaboration solution Voip presentation
Voip presentation Crmpro 360
Crmpro 360 Hosted data warehouse
Hosted data warehouse Ib organic chemistry functional groups
Ib organic chemistry functional groups Organic vs inorganic chemistry
Organic vs inorganic chemistry Soluzioni performer heritage 1
Soluzioni performer heritage 1 Template
Template Advantages of hydroelectricity
Advantages of hydroelectricity Greenhouse effect powerpoint
Greenhouse effect powerpoint Fil d'ariane ppt
Fil d'ariane ppt Pravila dobre prezentacije
Pravila dobre prezentacije Science fair powerpoint presentation examples
Science fair powerpoint presentation examples Trinity
Trinity Drag and drop ppt
Drag and drop ppt Civil air patrol powerpoint template
Civil air patrol powerpoint template Divine principle powerpoint
Divine principle powerpoint Advantages of power point
Advantages of power point Vehicle search procedures powerpoint
Vehicle search procedures powerpoint Feuerwehrleinen arten
Feuerwehrleinen arten Pop music facts
Pop music facts Free driver diagram template word
Free driver diagram template word Enron case study corporate governance
Enron case study corporate governance Principles of economics powerpoint lecture slides
Principles of economics powerpoint lecture slides