CHEMISTRY The Central Science 9 th Edition Chapter










- Slides: 38

CHEMISTRY The Central Science 9 th Edition Chapter 5 Thermochemistry David P. White Prentice Hall © 2003 Chapter 5

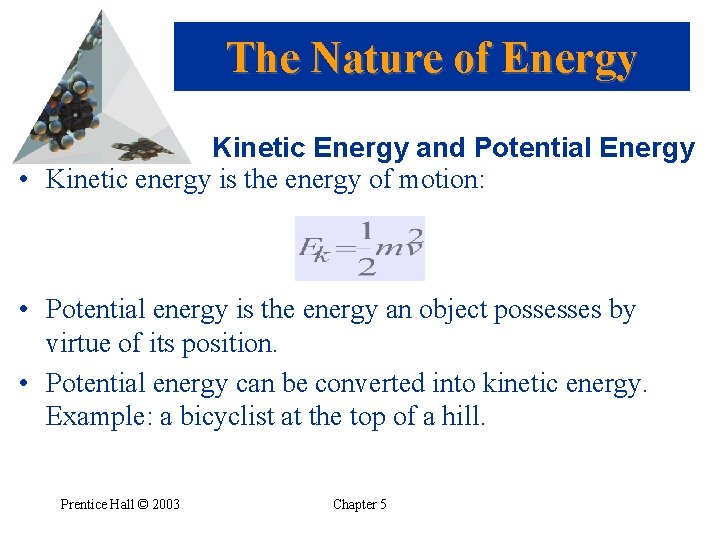
The Nature of Energy Kinetic Energy and Potential Energy • Kinetic energy is the energy of motion: • Potential energy is the energy an object possesses by virtue of its position. • Potential energy can be converted into kinetic energy. Example: a bicyclist at the top of a hill. Prentice Hall © 2003 Chapter 5

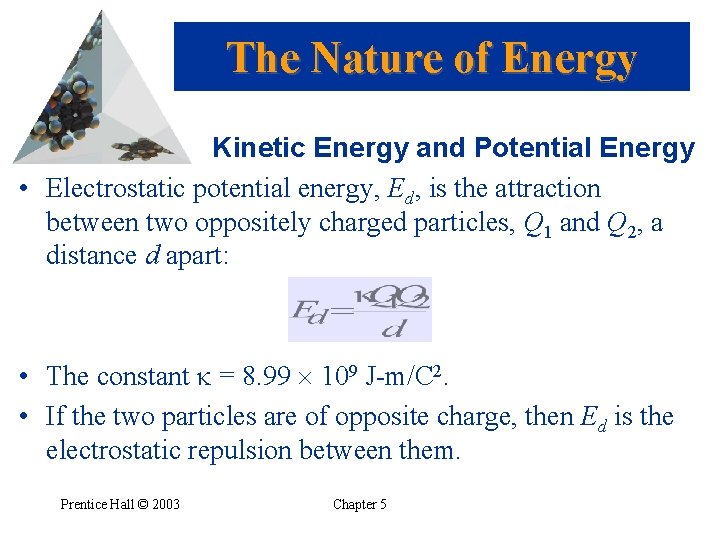
The Nature of Energy Kinetic Energy and Potential Energy • Electrostatic potential energy, Ed, is the attraction between two oppositely charged particles, Q 1 and Q 2, a distance d apart: • The constant = 8. 99 109 J-m/C 2. • If the two particles are of opposite charge, then Ed is the electrostatic repulsion between them. Prentice Hall © 2003 Chapter 5

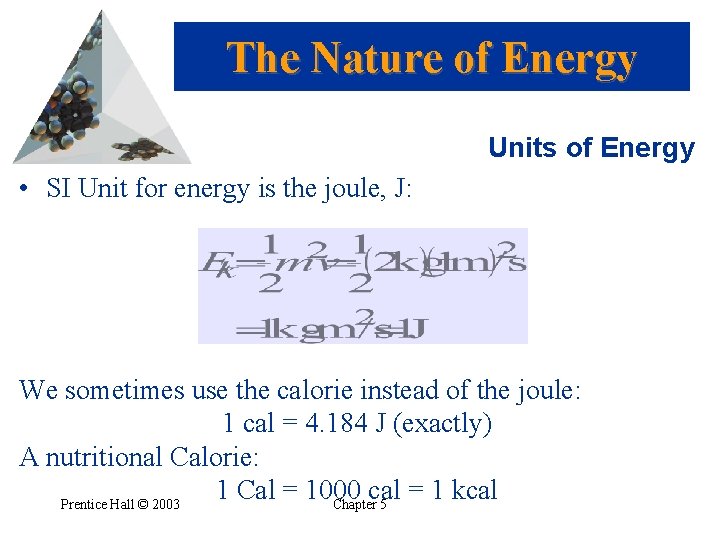
The Nature of Energy Units of Energy • SI Unit for energy is the joule, J: We sometimes use the calorie instead of the joule: 1 cal = 4. 184 J (exactly) A nutritional Calorie: 1 Cal = 1000 cal = 1 kcal Prentice Hall © 2003 Chapter 5

The Nature of Energy Systems and Surroundings • System: part of the universe we are interested in. • Surroundings: the rest of the universe. Prentice Hall © 2003 Chapter 5

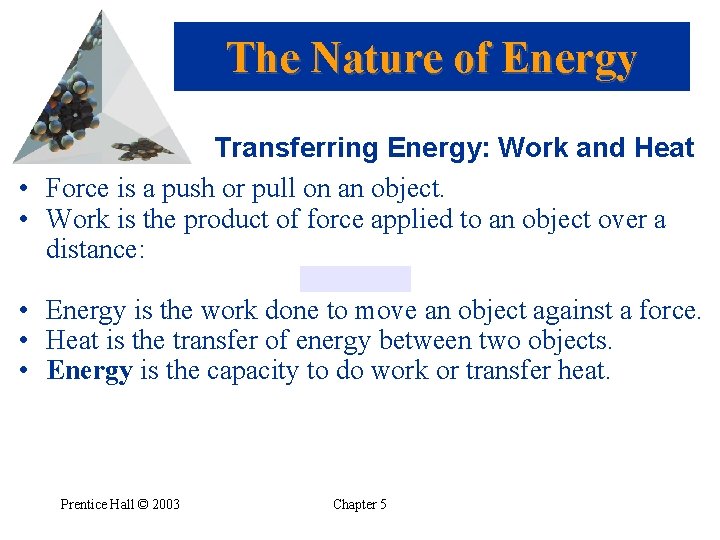
The Nature of Energy Transferring Energy: Work and Heat • Force is a push or pull on an object. • Work is the product of force applied to an object over a distance: • Energy is the work done to move an object against a force. • Heat is the transfer of energy between two objects. • Energy is the capacity to do work or transfer heat. Prentice Hall © 2003 Chapter 5

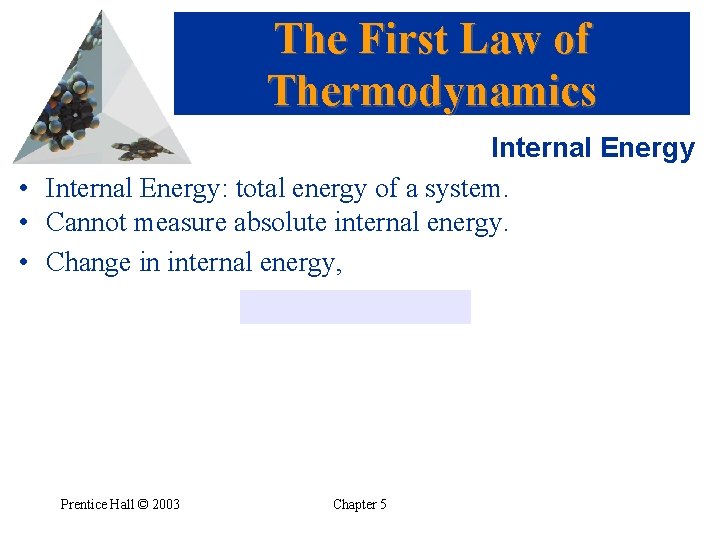
The First Law of Thermodynamics Internal Energy • Internal Energy: total energy of a system. • Cannot measure absolute internal energy. • Change in internal energy, Prentice Hall © 2003 Chapter 5

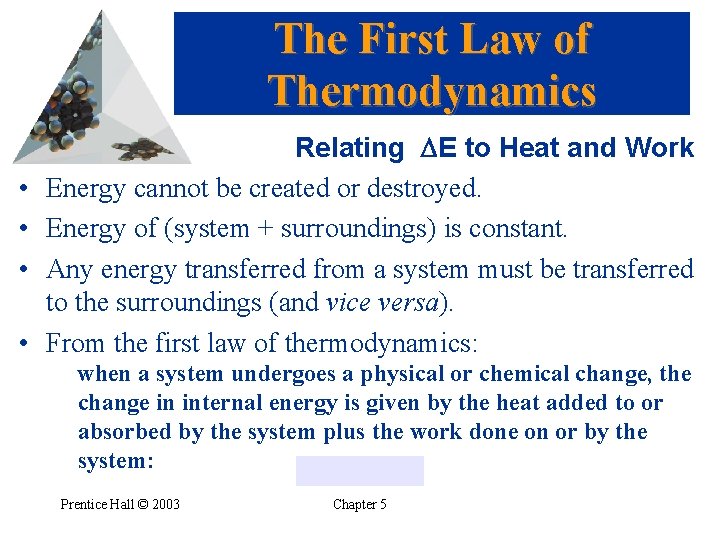
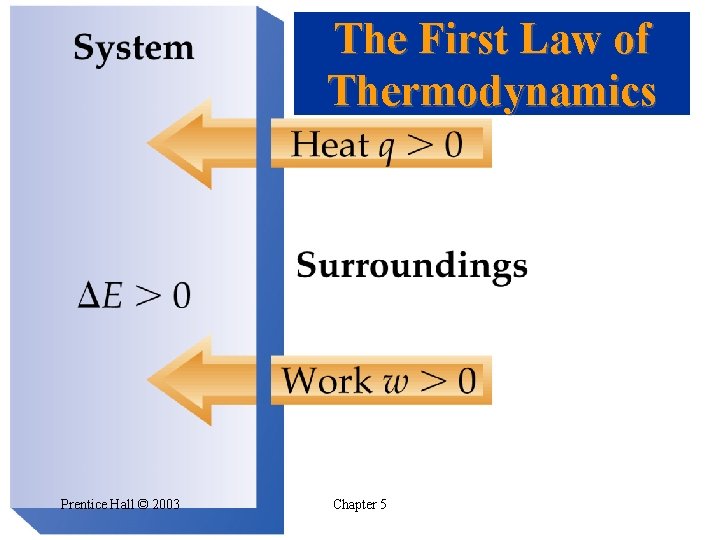
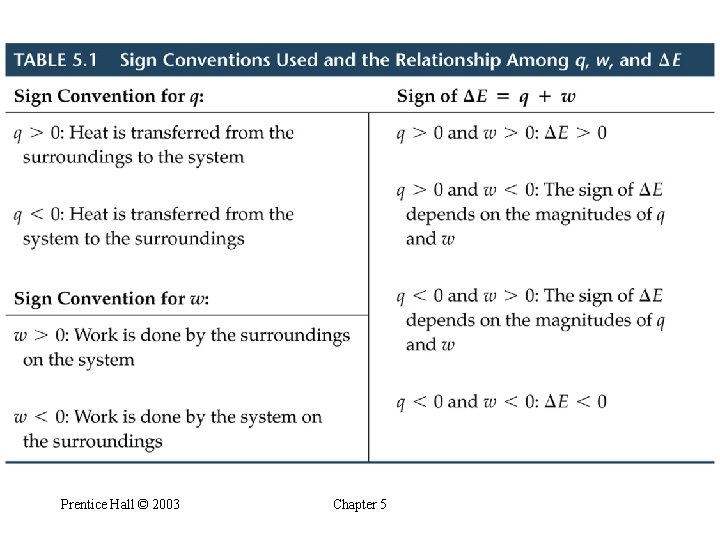
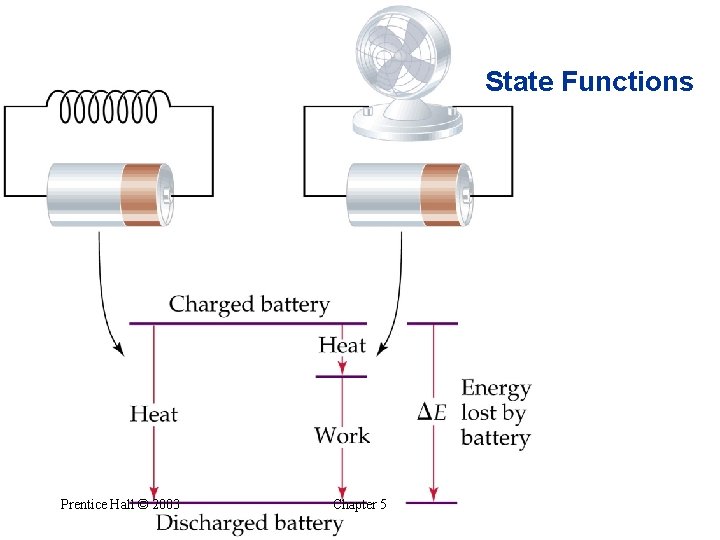
The First Law of Thermodynamics • • Relating DE to Heat and Work Energy cannot be created or destroyed. Energy of (system + surroundings) is constant. Any energy transferred from a system must be transferred to the surroundings (and vice versa). From the first law of thermodynamics: when a system undergoes a physical or chemical change, the change in internal energy is given by the heat added to or absorbed by the system plus the work done on or by the system: Prentice Hall © 2003 Chapter 5

The First Law of Thermodynamics Prentice Hall © 2003 Chapter 5

Prentice Hall © 2003 Chapter 5

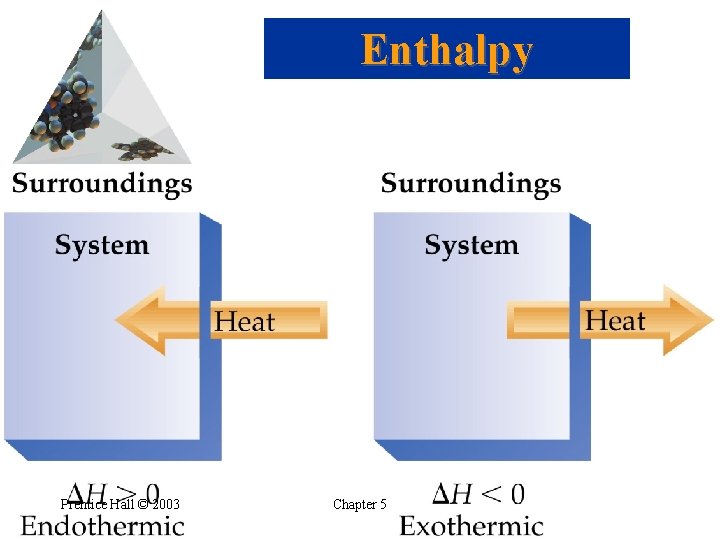
The First Law of Thermodynamics • • Exothermic and Endothermic Processes Endothermic: absorbs heat from the surroundings. Exothermic: transfers heat to the surroundings. An endothermic reaction feels cold. An exothermic reaction feels hot. Prentice Hall © 2003 Chapter 5

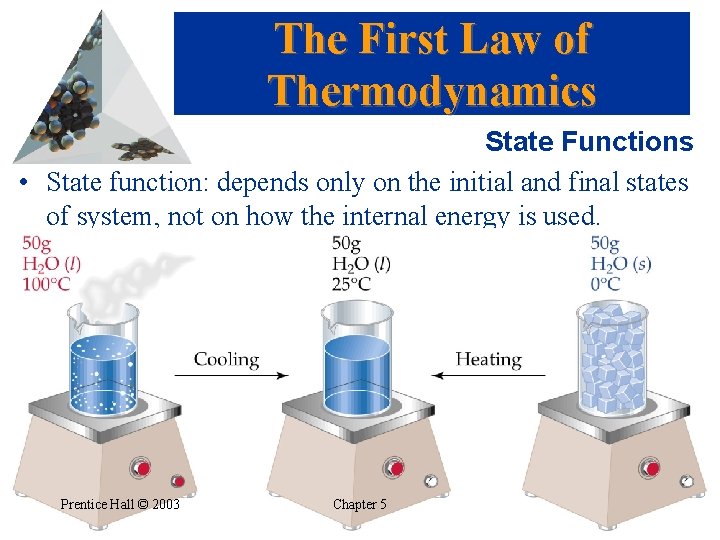
The First Law of Thermodynamics State Functions • State function: depends only on the initial and final states of system, not on how the internal energy is used. Prentice Hall © 2003 Chapter 5

State Functions Prentice Hall © 2003 Chapter 5

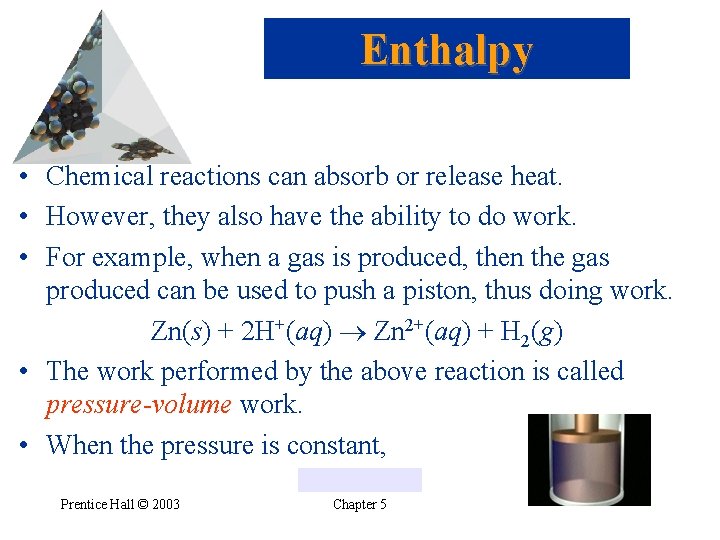
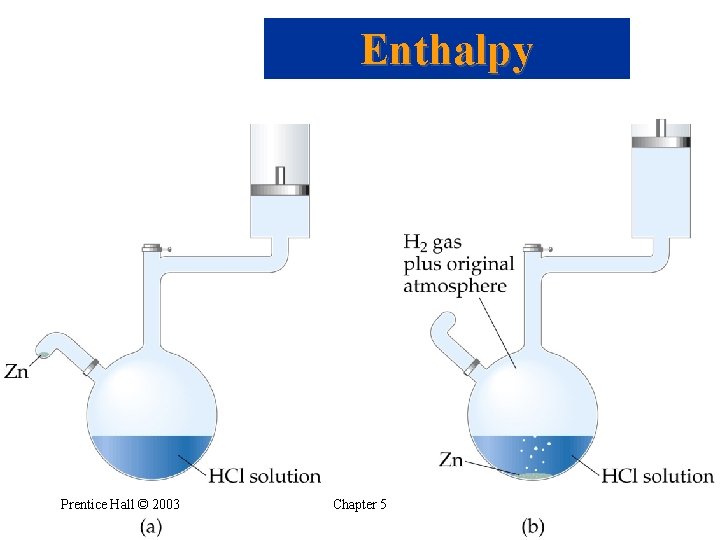
Enthalpy • Chemical reactions can absorb or release heat. • However, they also have the ability to do work. • For example, when a gas is produced, then the gas produced can be used to push a piston, thus doing work. Zn(s) + 2 H+(aq) Zn 2+(aq) + H 2(g) • The work performed by the above reaction is called pressure-volume work. • When the pressure is constant, Prentice Hall © 2003 Chapter 5

Enthalpy Prentice Hall © 2003 Chapter 5

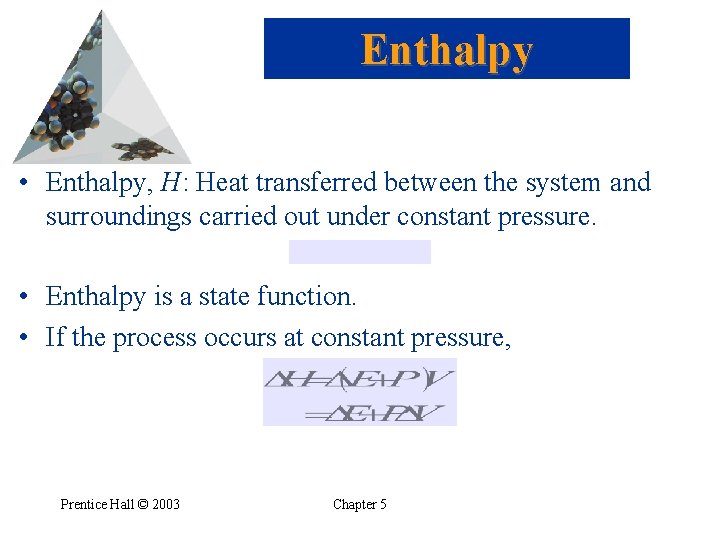
Enthalpy • Enthalpy, H: Heat transferred between the system and surroundings carried out under constant pressure. • Enthalpy is a state function. • If the process occurs at constant pressure, Prentice Hall © 2003 Chapter 5

Enthalpy • Since we know that • We can write • When H, is positive, the system gains heat from the surroundings. • When H, is negative, the surroundings gain heat from the system. Prentice Hall © 2003 Chapter 5

Enthalpy Prentice Hall © 2003 Chapter 5

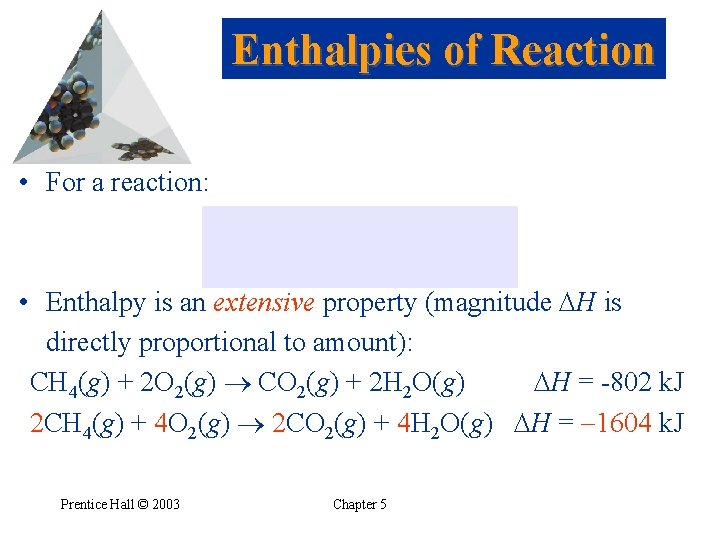
Enthalpies of Reaction • For a reaction: • Enthalpy is an extensive property (magnitude H is directly proportional to amount): CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(g) H = -802 k. J 2 CH 4(g) + 4 O 2(g) 2 CO 2(g) + 4 H 2 O(g) H = -1604 k. J Prentice Hall © 2003 Chapter 5

Enthalpies of Reaction • When we reverse a reaction, we change the sign of H: CO 2(g) + 2 H 2 O(g) CH 4(g) + 2 O 2(g) H = +802 k. J • Change in enthalpy depends on state: 2 H 2 O(g) 2 H 2 O(l) H = -88 k. J Prentice Hall © 2003 Chapter 5

Calorimetry • • • Heat Capacity and Specific Heat Calorimetry = measurement of heat flow. Calorimeter = apparatus that measures heat flow. Heat capacity = the amount of energy required to raise the temperature of an object (by one degree). Molar heat capacity = heat capacity of 1 mol of a substance. Specific heat = specific heat capacity = heat capacity of 1 g of a substance. Prentice Hall © 2003 Chapter 5

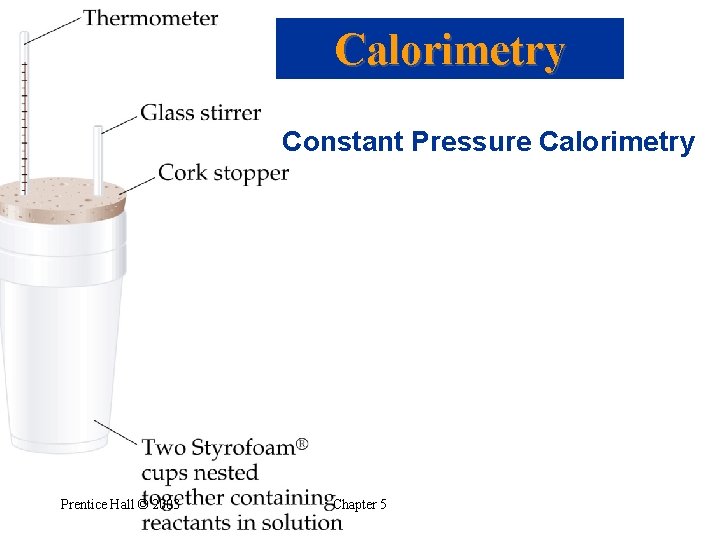
Calorimetry Constant Pressure Calorimetry • Atmospheric pressure is constant! Prentice Hall © 2003 Chapter 5

Calorimetry Constant Pressure Calorimetry Prentice Hall © 2003 Chapter 5

Calorimetry Bomb Calorimetry (Constant Volume Calorimetry) • Reaction carried out under constant volume. • Use a bomb calorimeter. • Usually study combustion. Prentice Hall © 2003 Chapter 5

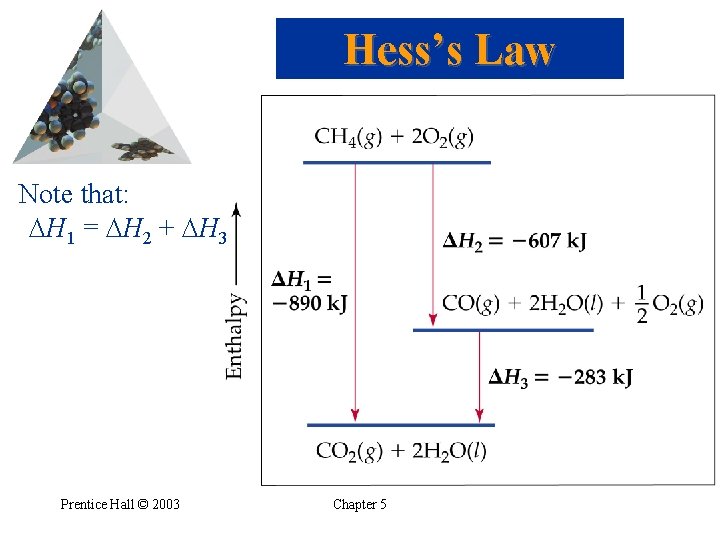
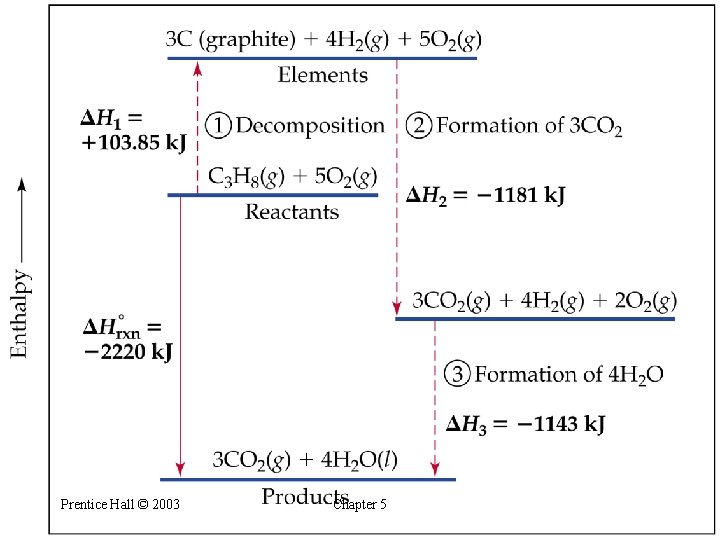
Hess’s Law • Hess’s law: if a reaction is carried out in a number of steps, H for the overall reaction is the sum of H for each individual step. • For example: CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(g) H = -802 k. J 2 H 2 O(g) 2 H 2 O(l) H = -88 k. J CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(l) H = -890 k. J Prentice Hall © 2003 Chapter 5

Hess’s Law Note that: H 1 = H 2 + H 3 Prentice Hall © 2003 Chapter 5

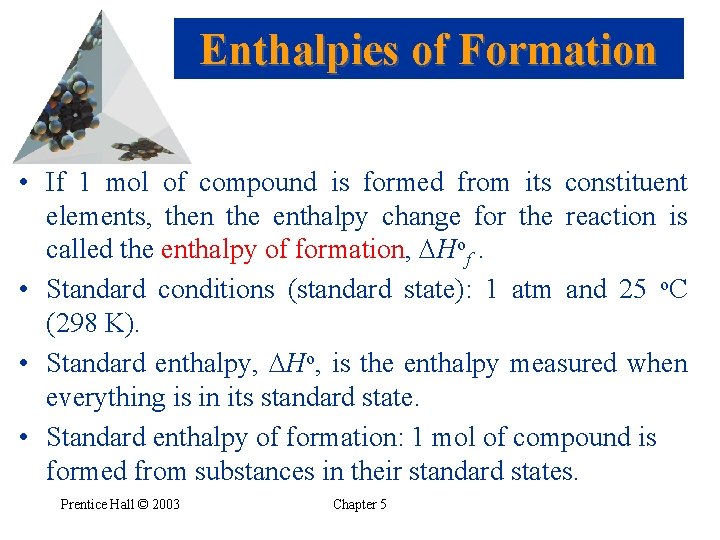
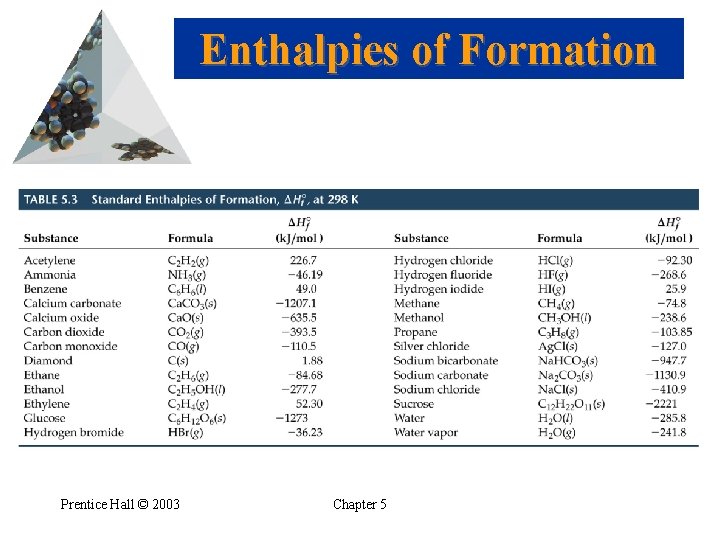
Enthalpies of Formation • If 1 mol of compound is formed from its constituent elements, then the enthalpy change for the reaction is called the enthalpy of formation, Hof. • Standard conditions (standard state): 1 atm and 25 o. C (298 K). • Standard enthalpy, Ho, is the enthalpy measured when everything is in its standard state. • Standard enthalpy of formation: 1 mol of compound is formed from substances in their standard states. Prentice Hall © 2003 Chapter 5

Enthalpies of Formation • If there is more than one state for a substance under standard conditions, the more stable one is used. • Standard enthalpy of formation of the most stable form of an element is zero. Prentice Hall © 2003 Chapter 5

Enthalpies of Formation Prentice Hall © 2003 Chapter 5

Enthalpies of Formation Using Enthalpies of Formation of Calculate Enthalpies of Reaction • We use Hess’ Law to calculate enthalpies of a reaction from enthalpies of formation. Prentice Hall © 2003 Chapter 5

Prentice Hall © 2003 Chapter 5

Enthalpies of Formation Using Enthalpies of Formation of Calculate Enthalpies of Reaction • For a reaction Prentice Hall © 2003 Chapter 5

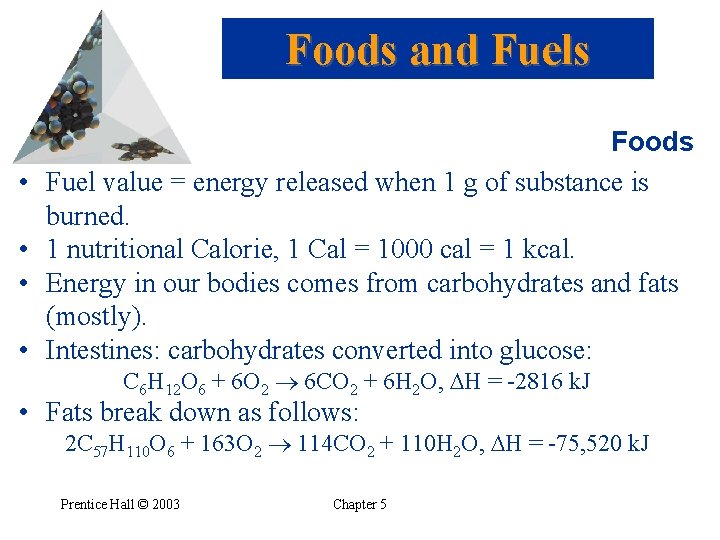
Foods and Fuels • • Foods Fuel value = energy released when 1 g of substance is burned. 1 nutritional Calorie, 1 Cal = 1000 cal = 1 kcal. Energy in our bodies comes from carbohydrates and fats (mostly). Intestines: carbohydrates converted into glucose: C 6 H 12 O 6 + 6 O 2 6 CO 2 + 6 H 2 O, H = -2816 k. J • Fats break down as follows: 2 C 57 H 110 O 6 + 163 O 2 114 CO 2 + 110 H 2 O, H = -75, 520 k. J Prentice Hall © 2003 Chapter 5

Foods and Fuels Foods • Fats: contain more energy; are not water soluble, so are good for energy storage. Prentice Hall © 2003 Chapter 5

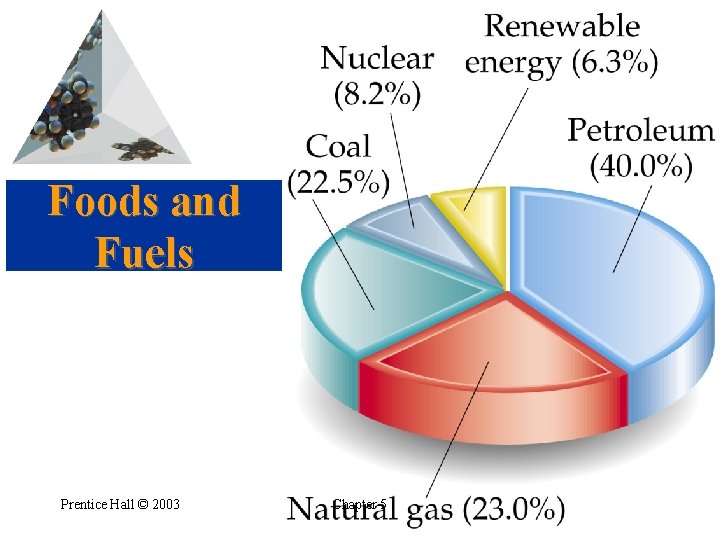
Foods and Fuels • • Fuels In 2000 the United States consumed 1. 03 1017 k. J of fuel. Most from petroleum and natural gas. Remainder from coal, nuclear, and hydroelectric. Fossil fuels are not renewable. Prentice Hall © 2003 Chapter 5

Foods and Fuels Prentice Hall © 2003 Chapter 5

Foods and Fuels • Fuel value = energy released when 1 g of substance is burned. • Hydrogen has great potential as a fuel with a fuel value of 142 k. J/g. Prentice Hall © 2003 Chapter 5

End of Chapter 5: Thermochemistry Prentice Hall © 2003 Chapter 5