Chemical Reaction Engineering Chapter 3 Part 4 Reaction





- Slides: 23

Chemical Reaction Engineering Chapter 3, Part 4: Reaction Stoichiometry Measures Other Than Conversion


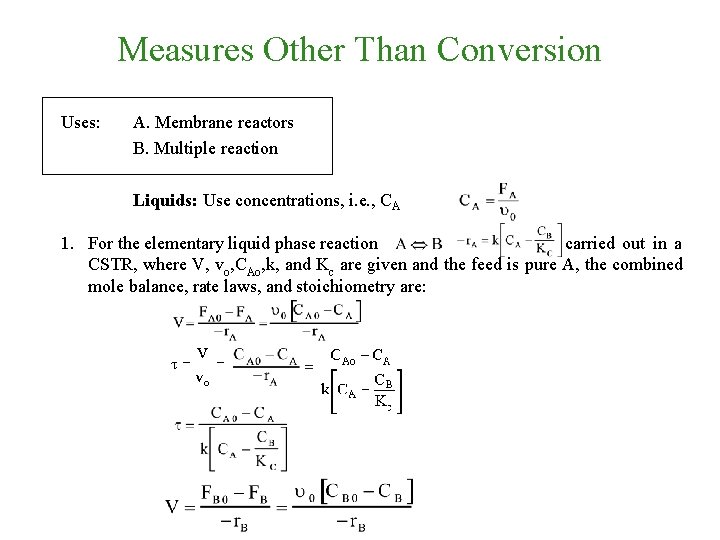
Measures Other Than Conversion Uses: A. Membrane reactors B. Multiple reaction Liquids: Use concentrations, i. e. , CA

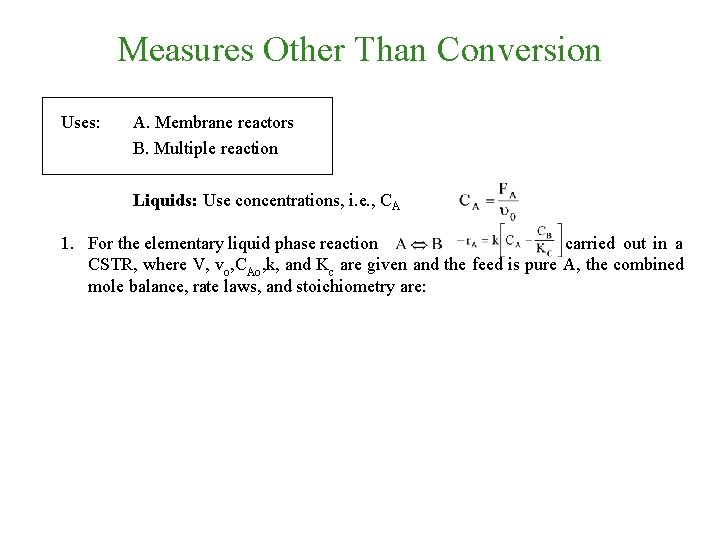
Measures Other Than Conversion Uses: A. Membrane reactors B. Multiple reaction Liquids: Use concentrations, i. e. , CA 1. For the elementary liquid phase reaction carried out in a CSTR, where V, vo, CAo, k, and Kc are given and the feed is pure A, the combined mole balance, rate laws, and stoichiometry are:

Measures Other Than Conversion Uses: A. Membrane reactors B. Multiple reaction Liquids: Use concentrations, i. e. , CA 1. For the elementary liquid phase reaction carried out in a CSTR, where V, vo, CAo, k, and Kc are given and the feed is pure A, the combined mole balance, rate laws, and stoichiometry are:

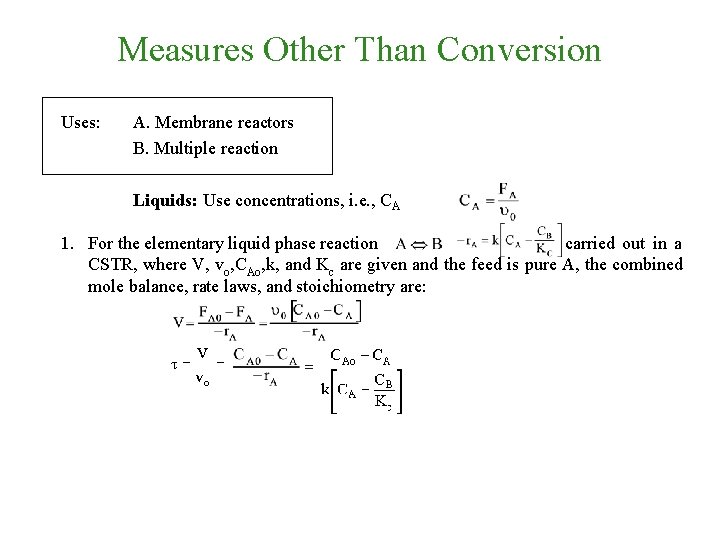
Measures Other Than Conversion Uses: A. Membrane reactors B. Multiple reaction Liquids: Use concentrations, i. e. , CA 1. For the elementary liquid phase reaction carried out in a CSTR, where V, vo, CAo, k, and Kc are given and the feed is pure A, the combined mole balance, rate laws, and stoichiometry are:

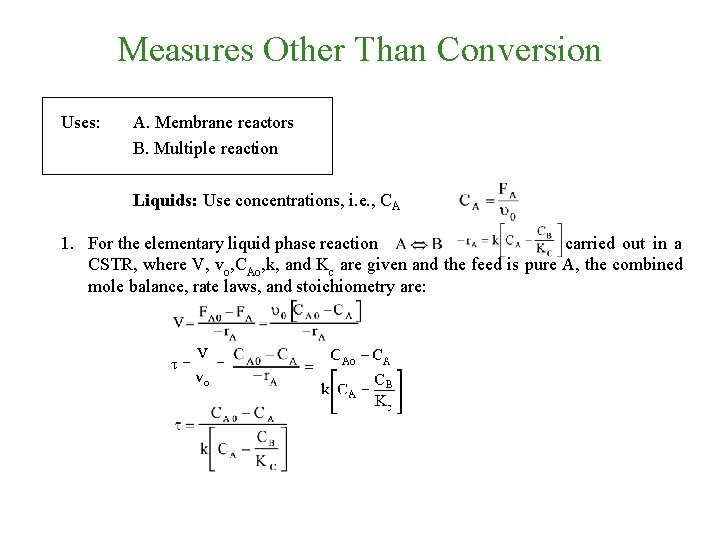
Measures Other Than Conversion Uses: A. Membrane reactors B. Multiple reaction Liquids: Use concentrations, i. e. , CA 1. For the elementary liquid phase reaction carried out in a CSTR, where V, vo, CAo, k, and Kc are given and the feed is pure A, the combined mole balance, rate laws, and stoichiometry are:

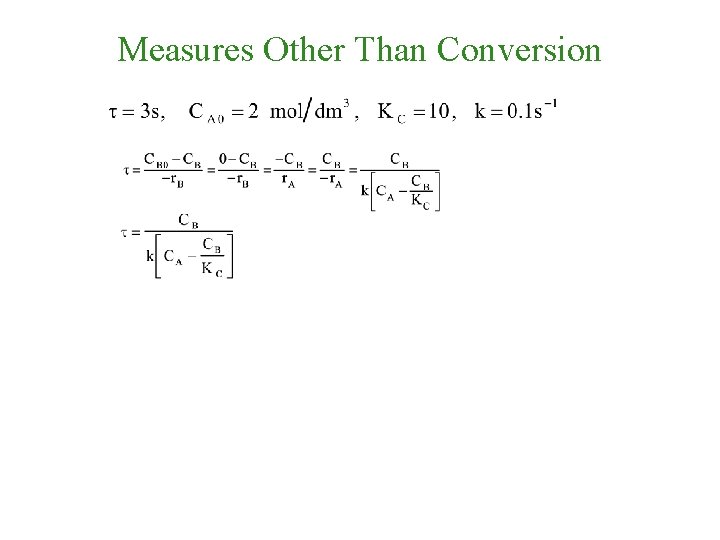
Measures Other Than Conversion

Measures Other Than Conversion

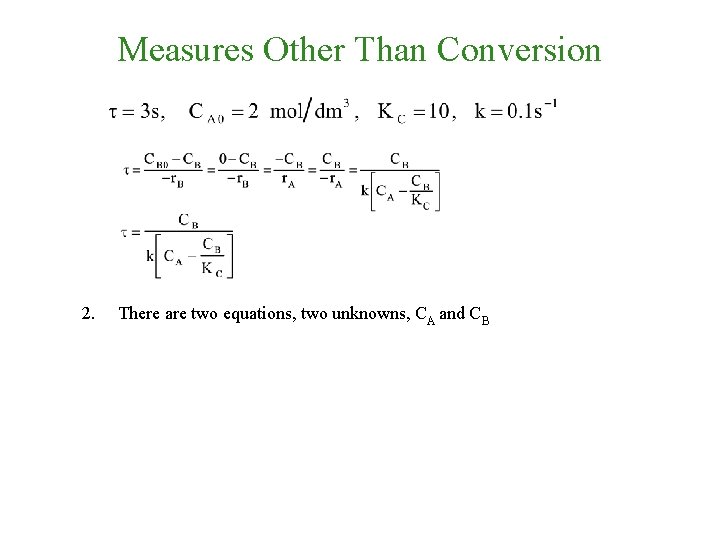
Measures Other Than Conversion 2. There are two equations, two unknowns, CA and CB

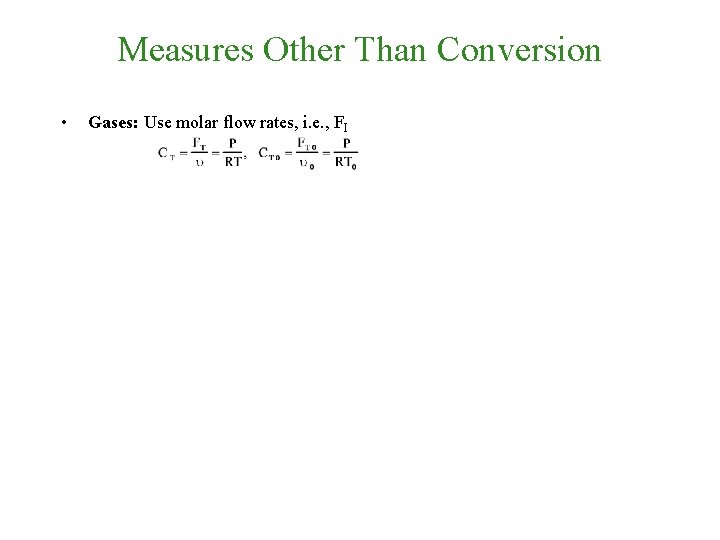
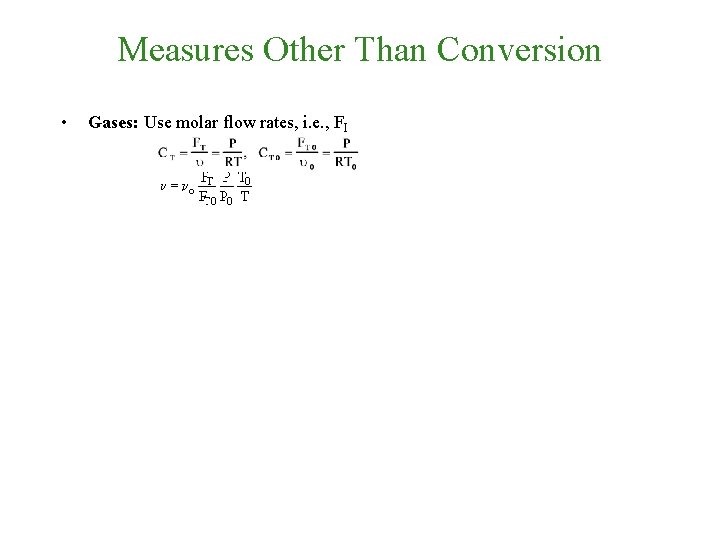
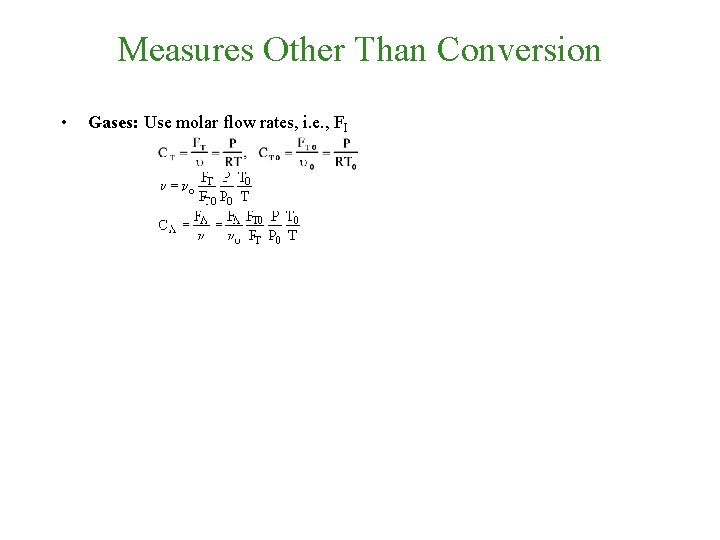
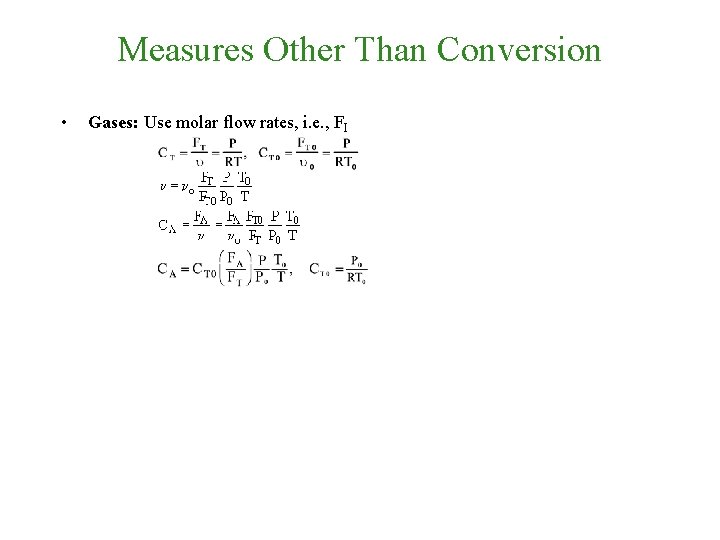
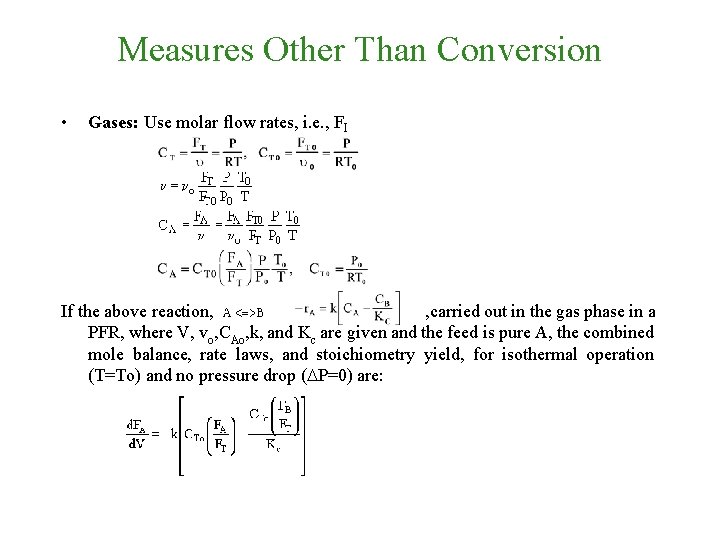
Measures Other Than Conversion • Gases: Use molar flow rates, i. e. , FI

Measures Other Than Conversion • Gases: Use molar flow rates, i. e. , FI

Measures Other Than Conversion • Gases: Use molar flow rates, i. e. , FI

Measures Other Than Conversion • Gases: Use molar flow rates, i. e. , FI

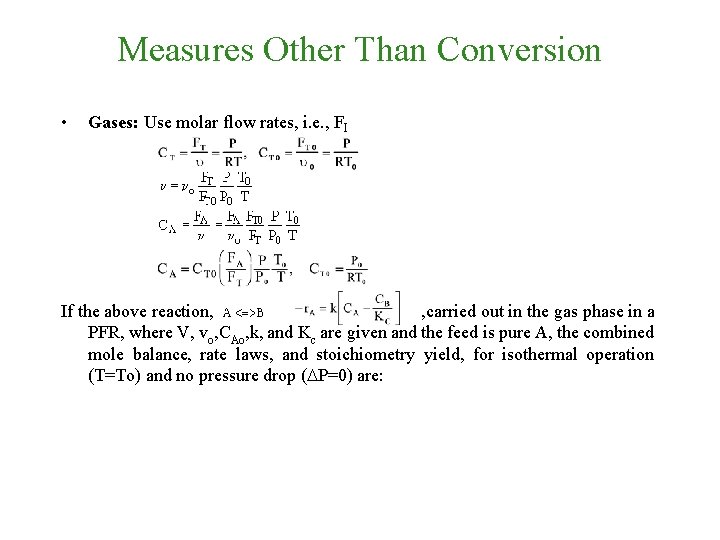
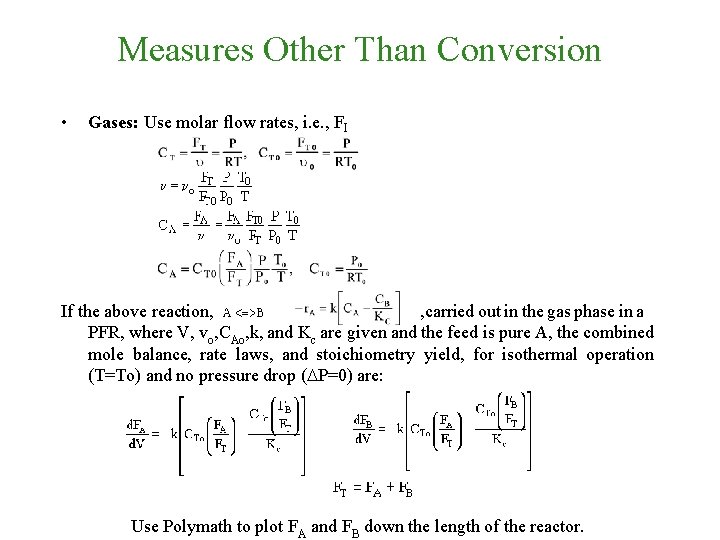
Measures Other Than Conversion • Gases: Use molar flow rates, i. e. , FI If the above reaction, , carried out in the gas phase in a PFR, where V, vo, CAo, k, and Kc are given and the feed is pure A, the combined mole balance, rate laws, and stoichiometry yield, for isothermal operation (T=To) and no pressure drop (DP=0) are:

Measures Other Than Conversion • Gases: Use molar flow rates, i. e. , FI If the above reaction, , carried out in the gas phase in a PFR, where V, vo, CAo, k, and Kc are given and the feed is pure A, the combined mole balance, rate laws, and stoichiometry yield, for isothermal operation (T=To) and no pressure drop (DP=0) are:

Measures Other Than Conversion • Gases: Use molar flow rates, i. e. , FI If the above reaction, , carried out in the gas phase in a PFR, where V, vo, CAo, k, and Kc are given and the feed is pure A, the combined mole balance, rate laws, and stoichiometry yield, for isothermal operation (T=To) and no pressure drop (DP=0) are: Use Polymath to plot FA and FB down the length of the reactor.

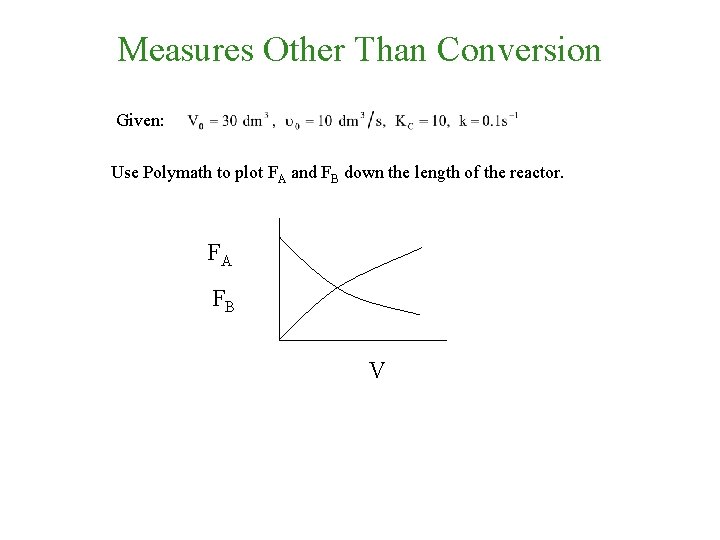
Measures Other Than Conversion Given: Use Polymath to plot FA and FB down the length of the reactor.

Measures Other Than Conversion Given: Use Polymath to plot FA and FB down the length of the reactor. FA FB V

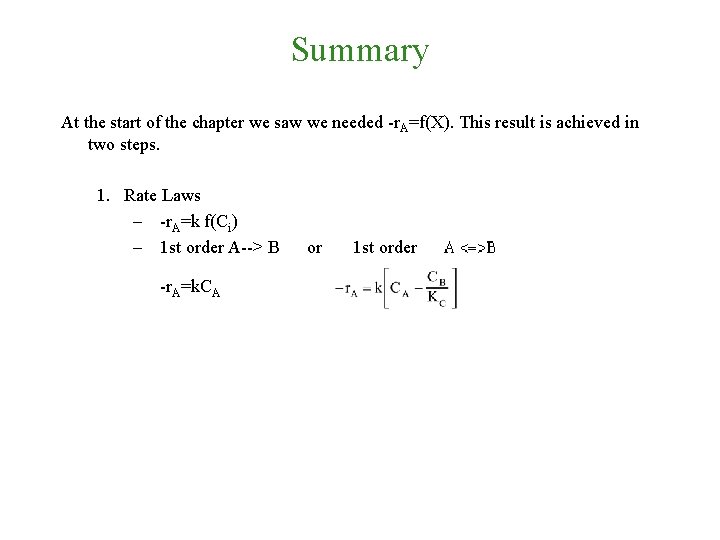
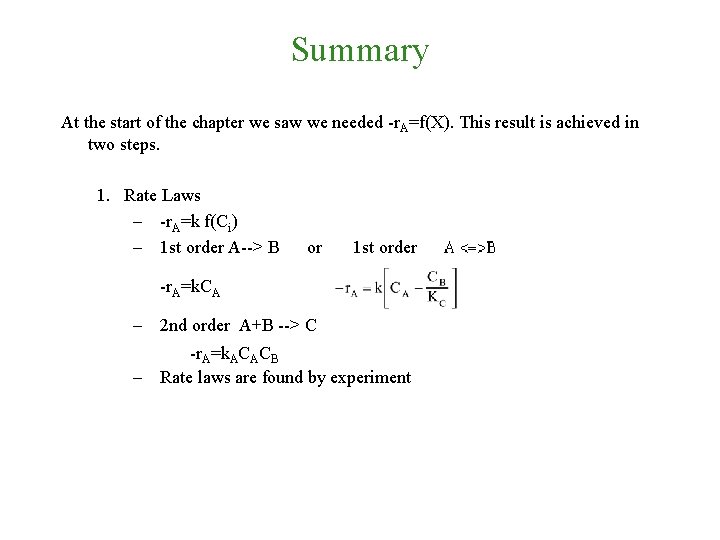
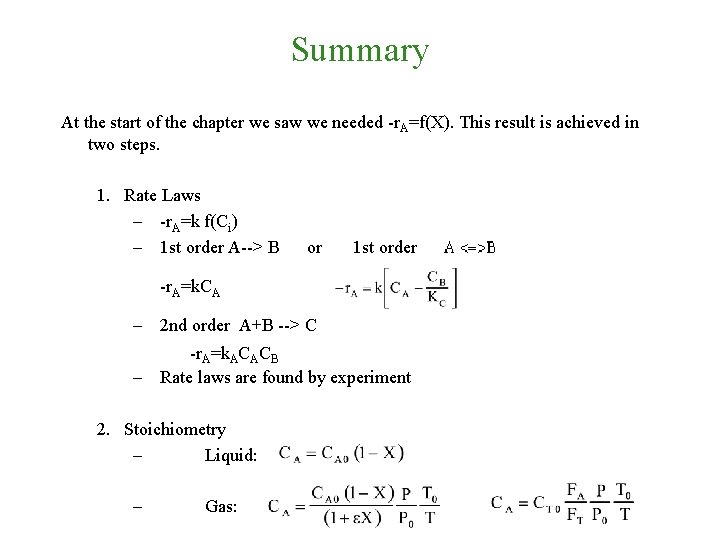
Summary At the start of the chapter we saw we needed -r. A=f(X). This result is achieved in two steps.

Summary At the start of the chapter we saw we needed -r. A=f(X). This result is achieved in two steps. 1. Rate Laws – -r. A=k f(Ci) – 1 st order A--> B -r. A=k. CA or 1 st order

Summary At the start of the chapter we saw we needed -r. A=f(X). This result is achieved in two steps. 1. Rate Laws – -r. A=k f(Ci) – 1 st order A--> B or 1 st order -r. A=k. CA – 2 nd order A+B --> C -r. A=k. ACACB – Rate laws are found by experiment

Summary At the start of the chapter we saw we needed -r. A=f(X). This result is achieved in two steps. 1. Rate Laws – -r. A=k f(Ci) – 1 st order A--> B or 1 st order -r. A=k. CA – 2 nd order A+B --> C -r. A=k. ACACB – Rate laws are found by experiment 2. Stoichiometry – Liquid: – Gas: