Chemical Kinetics Rates of chemical reactions Mechanisms of



![• Rate = -1 D[HI] = D[H 2] = D[I 2] 2 Dt • Rate = -1 D[HI] = D[H 2] = D[I 2] 2 Dt](https://slidetodoc.com/presentation_image_h2/ce29874ad7caf91abf24f3479426f574/image-8.jpg)




![• Look at rate comparing [A] to [B] • If [B] is changed(exp. • Look at rate comparing [A] to [B] • If [B] is changed(exp.](https://slidetodoc.com/presentation_image_h2/ce29874ad7caf91abf24f3479426f574/image-15.jpg)


![• Solving for rate of reaction if [A]=0. 050 M and [B]=0. 100 • Solving for rate of reaction if [A]=0. 050 M and [B]=0. 100](https://slidetodoc.com/presentation_image_h2/ce29874ad7caf91abf24f3479426f574/image-19.jpg)



















- Slides: 45

Chemical Kinetics • Rates of chemical reactions • Mechanisms of chemical reactions

• Chemical reaction • Particles involved can be ions, atoms and/or molecules

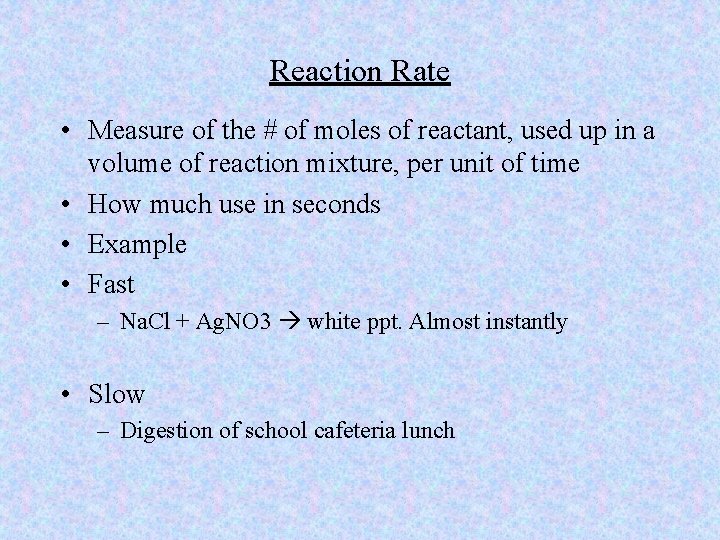
Reaction Rate • Measure of the # of moles of reactant, used up in a volume of reaction mixture, per unit of time • How much use in seconds • Example • Fast – Na. Cl + Ag. NO 3 white ppt. Almost instantly • Slow – Digestion of school cafeteria lunch

• Avg. rate = change in # moles of product/reactant Dtime • = D (moles Product/reactant) Dt

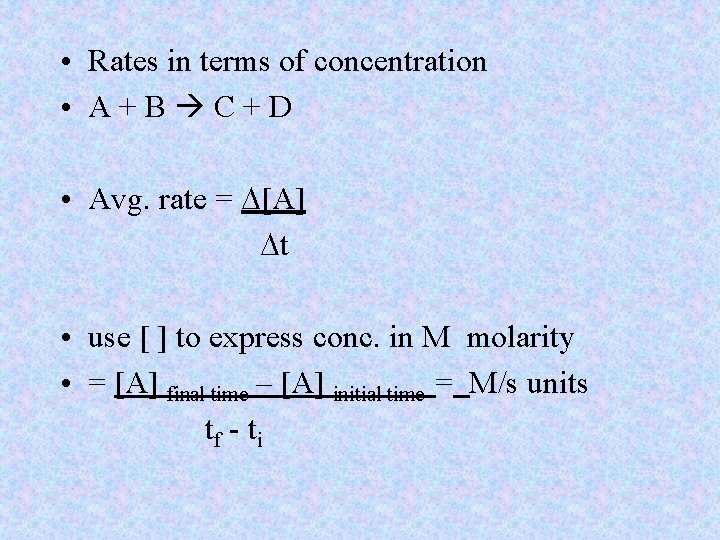
• Rates in terms of concentration • A+B C+D • Avg. rate = D[A] Dt • use [ ] to express conc. in M molarity • = [A] final time – [A] initial time = M/s units tf - ti

• Plot data on graph • Graph curve used to determine instantaneous rate at any point • Instantaneous rate is the slope of the straight line tangent that touches the curve at the point of interest

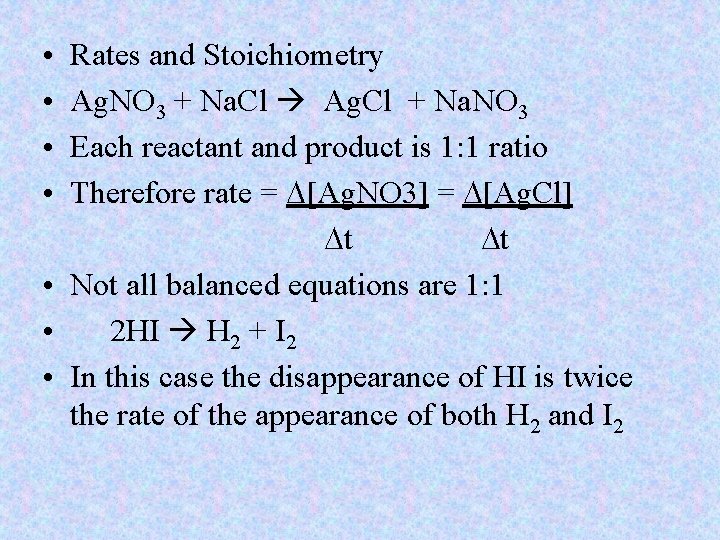
• • Rates and Stoichiometry Ag. NO 3 + Na. Cl Ag. Cl + Na. NO 3 Each reactant and product is 1: 1 ratio Therefore rate = D[Ag. NO 3] = D[Ag. Cl] Dt Dt • Not all balanced equations are 1: 1 • 2 HI H 2 + I 2 • In this case the disappearance of HI is twice the rate of the appearance of both H 2 and I 2
![Rate 1 DHI DH 2 DI 2 2 Dt • Rate = -1 D[HI] = D[H 2] = D[I 2] 2 Dt](https://slidetodoc.com/presentation_image_h2/ce29874ad7caf91abf24f3479426f574/image-8.jpg)
• Rate = -1 D[HI] = D[H 2] = D[I 2] 2 Dt Dt Dt • General equation is • a. A + b. B c. C + d. D • Rate= 1 D[A] = 1 D[B] = 1 D[C] = 1 D[D] a Dt b Dt c Dt d Dt

• Do following: • 2 N 2 O 5 4 NO 2 + O 2 • Rate of decomposition of N 2 O 5 is 4. 2 x 10 -7 M/s, what is the rate of appearance of NO 2 and O 2 • 1 D[NO 2] = 1 D[N 2 O 5] = 4(4. 2 x 10 -7 M/s) = 4 Dt 2 • 8. 4 x 10 -7 M/s • D[O 2] = 1 D[N 2 O 5] = 1(4. 2 x 10 -7 M/s) = Dt 2 • 2. 1 x 10 -7 M/s

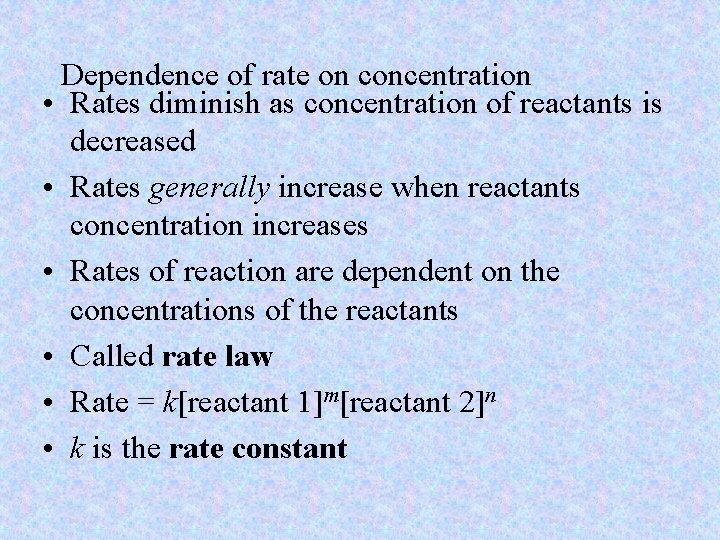
Dependence of rate on concentration • Rates diminish as concentration of reactants is decreased • Rates generally increase when reactants concentration increases • Rates of reaction are dependent on the concentrations of the reactants • Called rate law • Rate = k[reactant 1]m[reactant 2]n • k is the rate constant

• Given a rate law for a reaction and its rate for a set of reactant concentrations, the value of k at a specific temp. and pressure can be calculated • Magnitude of k provides valuable info about reactions • Exponents m and n are called reaction orders • Sum of exponents is the overall reaction order

• Reaction order exponents are determined experimentally and do not necessarily correspond to the coefficients of a balanced equation • Most rate laws have reaction orders of 0, 1 or 2 • Some rate laws have fractional orders or even negative • Example: H 2 + I 2 2 HI • Rate = k[H 2][I 2] • Since altering conc. of either reactant has same effect on rate, they are both first order and have exponent values of 1 • Overall reaction order is 1+1=2 ; second order overall

• Rate law for a reaction must be determined experimentally • Observe the effect of changing initial concentration of reactants on initial rate of a reaction • Example: if changing the conc. of a reactant has no effect on the reaction rate as long as some of the reactant is present, it has a zero order • If reaction is first order in a reactant, changes in conc. of that reactant will produce proportional changes in the rate • Rate law second order, doubling conc. will increase rate by a factor of 22=4 • Important to note; rate of reaction depends on conc. , rate constant does not

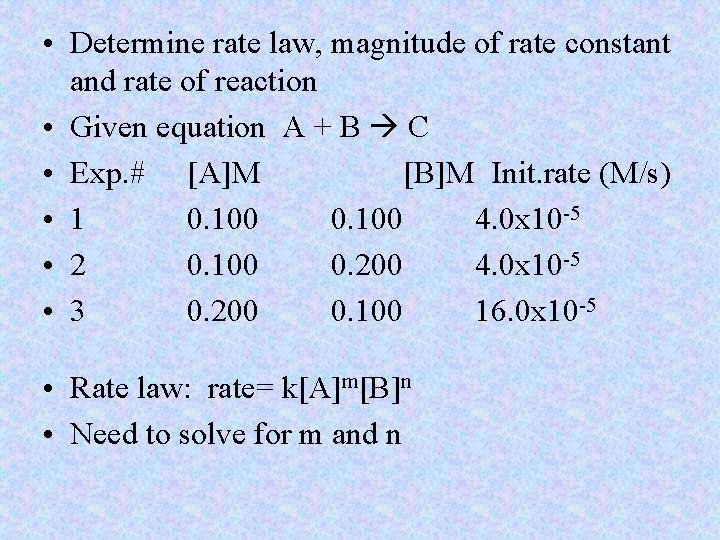
• Determine rate law, magnitude of rate constant and rate of reaction • Given equation A + B C • Exp. # [A]M [B]M Init. rate (M/s) • 1 0. 100 4. 0 x 10 -5 • 2 0. 100 0. 200 4. 0 x 10 -5 • 3 0. 200 0. 100 16. 0 x 10 -5 • Rate law: rate= k[A]m[B]n • Need to solve for m and n
![Look at rate comparing A to B If B is changedexp • Look at rate comparing [A] to [B] • If [B] is changed(exp.](https://slidetodoc.com/presentation_image_h2/ce29874ad7caf91abf24f3479426f574/image-15.jpg)
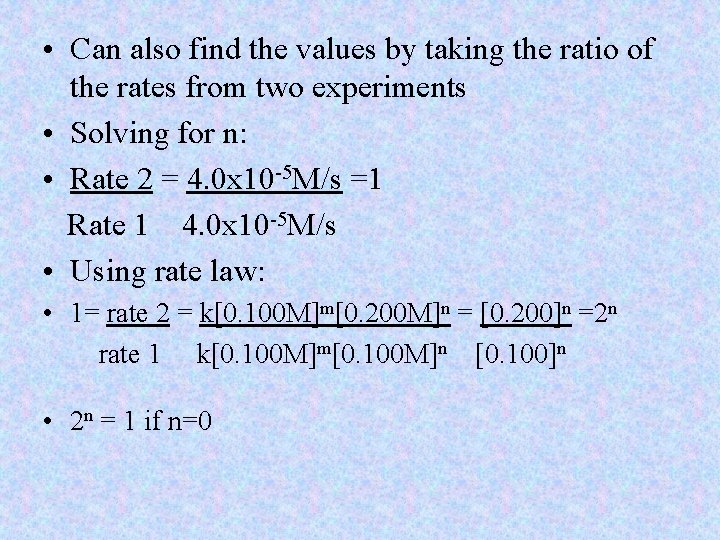
• Look at rate comparing [A] to [B] • If [B] is changed(exp. 2 doubled) it had no effect([A] held constant) (remember only change one variable at a time) • Therefore rate law is zero order in B • If [A] is doubled: rate increases fourfold • Rate is proportional to [A]2 • Rate law of A is 2 nd order • Rate = k[A]2[B]0

• Can also find the values by taking the ratio of the rates from two experiments • Solving for n: • Rate 2 = 4. 0 x 10 -5 M/s =1 Rate 1 4. 0 x 10 -5 M/s • Using rate law: • 1= rate 2 = k[0. 100 M]m[0. 200 M]n = [0. 200]n =2 n rate 1 k[0. 100 M]m[0. 100 M]n [0. 100]n • 2 n = 1 if n=0

• Solving for m: • Rate 3 = 16. 0 x 10 -5 M/s = 4 Rate 1 4. 0 x 10 -5 M/s • Using rate law • 4 = rate 3 = k[0. 200 M]m[0. 100 M]n = [0. 200]m = 2 m rate 1 k[0. 100 M]m[0. 100 M]n [0. 100]m • 2 m = 4 so therefore m = 2

• Solving for magnitude of k • k = rate = 4. 0 x 10 -5 M/s = 4. 0 x 10 -3 M-1 s-1 [A]2 (0. 100 M)2
![Solving for rate of reaction if A0 050 M and B0 100 • Solving for rate of reaction if [A]=0. 050 M and [B]=0. 100](https://slidetodoc.com/presentation_image_h2/ce29874ad7caf91abf24f3479426f574/image-19.jpg)
• Solving for rate of reaction if [A]=0. 050 M and [B]=0. 100 M • Rate = k[A]2 = (4. 0 x 10 -3 M-1 s-1)(. 050 M)2 = • 1. 0 x 10 -5 M/s • Since B is not part of the rate law, it is immaterial to the rate, provided there is at least some B to react with A

Reaction rates • • • Why necessary to understand? Body uses chem. Reactions to stay healthy Medicines Industry Curtail rust Speed up production of products

5 Main Factors affecting rate • 1. Nature of reactants • In reactions bonds are rearranged • Bond types affect rate due to electronegativities • Slight e- arrangement, fast reaction at room temp. • Ex. Ionic double displacement • Covalent bonds • Large # of rearrangements • Slow reactions at room temp. • Ex. CH 4 +2 O 2 2 H 2 O + CO 2

• 2. Concentration • An increase conc. of one or more reactants will usually but not always increase the reaction rate if the mixture is homogenous

3. Temperature • Increase temp. increase rate of most reactions • An increase in temp. of 100 C will approximately double reaction rate • Due to collision theory – More KE to change to PE – More and faster collisions – More chance for an Effective collision – Laser disc #9 Cpt. 30

4. Catalysts • Substance which speeds up a reaction without being permanently altered • Lower the amount of energy (activation energy) required for a reaction to occur – Recoverable – Ex. Enzymes, catalytic converter • Inhibitors – Chemicals that slow down reactions

5. Phase • Reactants in same or more than one phase • Homogenous – Same phase – Fast reaction rate • Heterogeneous – Reactants in different phases • Slower reaction rate – Due to reactants only being able to react at the interface site (where meet) • Pressure in gases – Higher pressure forces closer-faster – Low pressure further apart-slower

Reaction Mechanism • Series of steps by which reacting particles rearrange themselves to form the products of a chemical reaction • Hard to do • Intermediate products often short lived

Effective Collision • Based on collision theory • Particles must collide for chem. reaction to occur • Ex. –A+B C • • • As A approaches B they begin to interact e- shift positions Old bonds are broken New bonds are formed Collision must be EFFECTIVE

• The reactants must approach each other at Proper angle ( CORRECT GEOMETRY) and with Sufficient energy • Activation energy • Minimum amount of energy required for a reaction to occur • Electron rearrangement needs energy • Laser disc #9 Cpt. 20

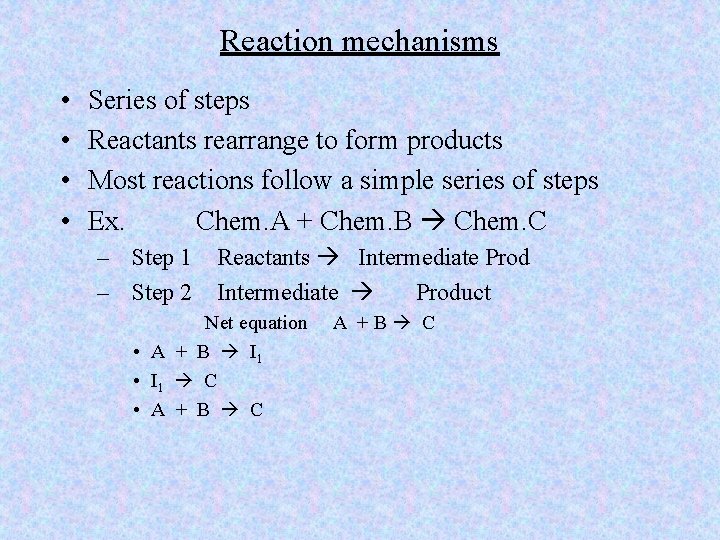
Reaction mechanisms • • Series of steps Reactants rearrange to form products Most reactions follow a simple series of steps Ex. Chem. A + Chem. B Chem. C – Step 1 – Step 2 Reactants Intermediate Prod Intermediate Product Net equation • A + B I 1 • I 1 C • A + B C A +B C

• • Step 1 A + B I 1 Step 2 I 1 + A I 2 Step 3 I 2 C Net Eq 2 A + B C

• • • A collides with B to form intermediate product I 1 collides with another A particle to form I 2 rearranges into the final product C When final equation is written it is in the net form Done this way due to difficulty in actually knowing what the intermediate is • Intermediate products are highly unstable and short lived

• • • Increase conc. usually increases rate Not always true Remember there are steps Steps reactions occur at different rates Slowest step is known as RATE DETERMINING STEP • To speed up reaction must increase conc. Of reactants in rate determining step • Demo

• Example 2 A + B + C D STEP 1 A + B I 1 STEP 2 I 1 + A I 2 rate determining step (slowest) STEP 3 I 2 + C I 3 STEP 4 I 3 D Net Eq. 2 A + B + C D

• A + B have collided with an Effective collision • The compound initially formed that has eundergoing rearrangement is called the Activated Complex or Transition State Complex – It is an intermediate – Unstable and short lived – 2 processes it can undergo • Break down back into A + B • Form product(s) • Shown using a Potential Energy Diagram using Activation energy




Thermodynamics • Study of changes in energy in chemical reactions and the influence of temperature on those changes

• Every system or form of matter has stored energy • Energy in bonds • P. E. due to phase, pressure and volume • K. E. from random motion of particles • Total of all these forms of energy referred to as heat content • Internal energy is symbolized as DU

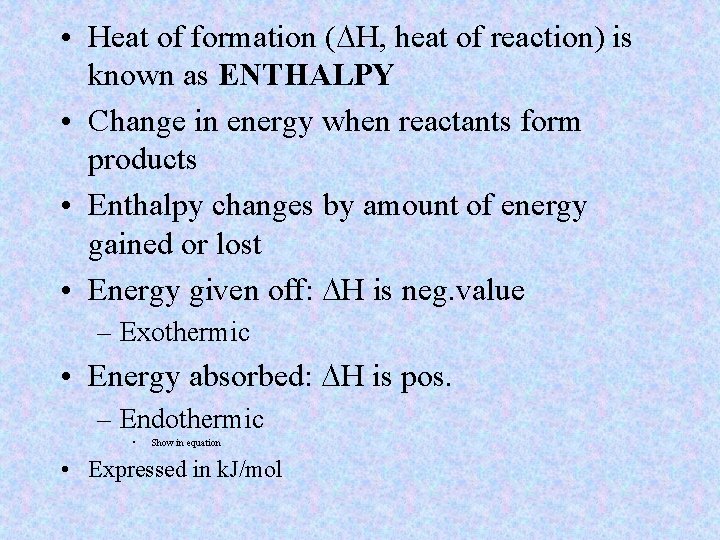
• Heat of formation (DH, heat of reaction) is known as ENTHALPY • Change in energy when reactants form products • Enthalpy changes by amount of energy gained or lost • Energy given off: DH is neg. value – Exothermic • Energy absorbed: DH is pos. – Endothermic • Show in equation • Expressed in k. J/mol

• Heat of formation depends on temp. and press. at which reaction occurs and phase • Standard Heat of Formation DHfo is at 250 C and 1 Atm. (101. 325 k. Pa)pressure • Expressed as 298 K and 101. 3 k. Pa plus phase • Ex. Water liquid DHf 0 = 286 k. J/mol H 2(g) + 1/2 O 2(g) D H 2 O(l) + 286 k. J Since energy is released(part of product) DH is neg. value ΔH= -286 k. J Elements in naturally found form have DHf 0 of 0.

• Hess’s Law of Constant Heat Summation – When a reaction can be expressed as the algebraic sum of two or more other reactions, then the heat of the reaction is the algebraic sum of the heats of these other reaction • If a reaction is carried out in a series of steps, DH for the reaction will be equal to the sum of the enthalpy changes for the individual steps • Therefore we can calculate DH for a reaction by “adding” up known DH of reactions • Used to obtain energy changes that are difficult to measure or predetermine

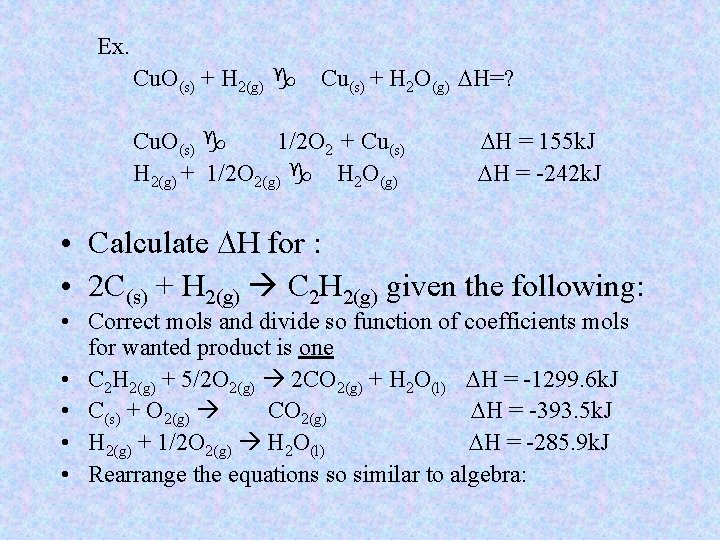
Ex. Cu. O(s) + H 2(g) g Cu(s) + H 2 O(g) DH=? Cu. O(s) g 1/2 O 2 + Cu(s) H 2(g) + 1/2 O 2(g) g H 2 O(g) DH = 155 k. J DH = -242 k. J • Calculate DH for : • 2 C(s) + H 2(g) C 2 H 2(g) given the following: • Correct mols and divide so function of coefficients mols for wanted product is one • C 2 H 2(g) + 5/2 O 2(g) 2 CO 2(g) + H 2 O(l) DH = -1299. 6 k. J • C(s) + O 2(g) CO 2(g) DH = -393. 5 k. J • H 2(g) + 1/2 O 2(g) H 2 O(l) DH = -285. 9 k. J • Rearrange the equations so similar to algebra:

Entropy • Measure of disorder, randomness or lack of organization of a system • Large entropy- great state of disorder – Solid is organized pattern with low entropy and as melts the particles become random and have higher disorder of larger entropy; increases as change to gas • Represented by DS DS = Sf – Si DS value pos. spontaneous change can occur

Gibbs Free Energy Equation • Combines enthalpy and entropy changes to determine spontaneity • Neg. value spontaneous • Pos. value non spontaneous DG = DH - TDS
 Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Ratios rates and unit rates
Ratios rates and unit rates A rate is a ratio that compares
A rate is a ratio that compares Ratios guided notes
Ratios guided notes Ratios rates and unit rates
Ratios rates and unit rates Section 1 chemical changes
Section 1 chemical changes Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Molecularity of reaction
Molecularity of reaction Chemical kinetics experiment
Chemical kinetics experiment Chemistry grade 11 unit 4 chemical kinetics
Chemistry grade 11 unit 4 chemical kinetics Define steady state approximation
Define steady state approximation Applications of chemical kinetics
Applications of chemical kinetics Order of kinetics
Order of kinetics Chemical kinetics definition
Chemical kinetics definition Balance redox half reactions
Balance redox half reactions Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Section 2 chemical reactions answer key
Section 2 chemical reactions answer key Chemical reactions classification
Chemical reactions classification Unit 5 chemical equations and reactions
Unit 5 chemical equations and reactions The calculation of quantities in chemical reactions
The calculation of quantities in chemical reactions Synthesis reaction predicting products
Synthesis reaction predicting products Solvent in chemical reactions
Solvent in chemical reactions Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes How to identify types of chemical reactions
How to identify types of chemical reactions Chemical reactions summary
Chemical reactions summary Toxic reactions chemical equations
Toxic reactions chemical equations Chemical equations and reactions chapter 8
Chemical equations and reactions chapter 8 What is this
What is this 5 general types of chemical reactions
5 general types of chemical reactions Equilibrium occurs when
Equilibrium occurs when A balanced chemical reaction obeys the law of
A balanced chemical reaction obeys the law of Four types of chemical reactions
Four types of chemical reactions Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry What are active metals
What are active metals 4 types of chemical reactions
4 types of chemical reactions Describing chemical reactions
Describing chemical reactions Noncompetitive inhibitor
Noncompetitive inhibitor Stoichiometry mole island diagram
Stoichiometry mole island diagram Chapter 11 chemical reactions answer key
Chapter 11 chemical reactions answer key Solvent in chemical reactions
Solvent in chemical reactions Four types of chemical reactions
Four types of chemical reactions Solvent in chemical reactions
Solvent in chemical reactions Understanding chemical reactions worksheet answer key
Understanding chemical reactions worksheet answer key Examples of chemical reactions in everyday life
Examples of chemical reactions in everyday life