Chemical Engineering Department Sodium Polyacrylate as Sacrificial Adsorption









- Slides: 27

Chemical Engineering Department Sodium Polyacrylate as Sacrificial Adsorption Agent for Anionic Surfactants* Hadi Shamsi. Jazeyi, George J. Hirasaki ______________________________ * (Patent Pending) Presented at Rice Consortium for Processes in Porous Media 16 th Annual Meeting Rice University, Houston TX. April 23, 2012

Agenda • Introduction • Problem Definition • Materials and Methods ü Potentiometric titration (surfactant), Colorimetric Measurement (polyacrylate) • Results and Discussions ü Polyacrylate vs. different chemicals for Reducing Adsorption of Surfactant ü Polyacrylate and Sodium Carbonate on Different Outcrop Minerals ü Sodium Polyacrylate for Reducing Adsorption of different Surfactants ü Effect of Molecular Weight of Sodium Polyacrylate on its Sacrificial Property • Conclusions • Future Work 2

3 Introduction

4 Why is Surfactant Adsorption important? • Enhanced Oil Recovery (EOR) • Surfactant Adsorption & EOR ü Economy of Surfactant Injection ü Chromatographic Separation ü Wettability Alteration

5 Problem Definition

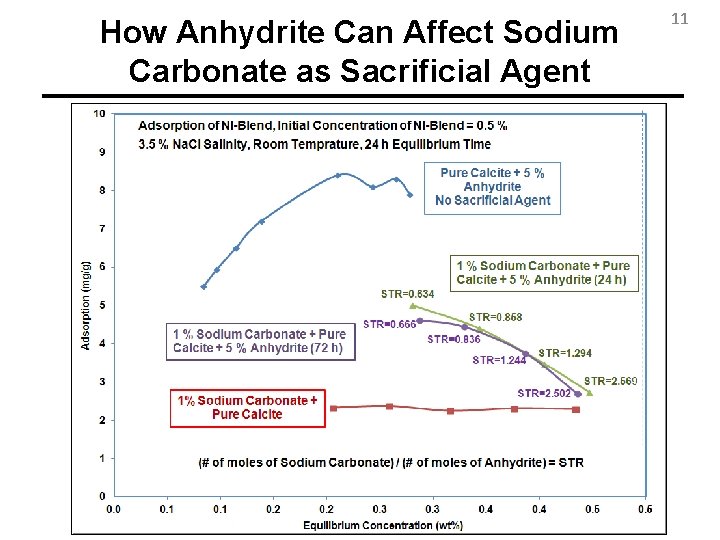
Anhydrite reacts with Sodium Carbonate 6 • Sodium Carbonate is traditionally used as Sacrificial Agent • Carbonates sometimes contain Anhydrite • Sodium Carbonate is not an effective Sacrificial Agent when Anhydrite is present Na 2 CO 3 + Ca. SO 4 Na 2 SO 4 + Ca. CO 3 ↓ • Sodium Polyacrylate as Sacrificial Agent?

7 Materials and Methods

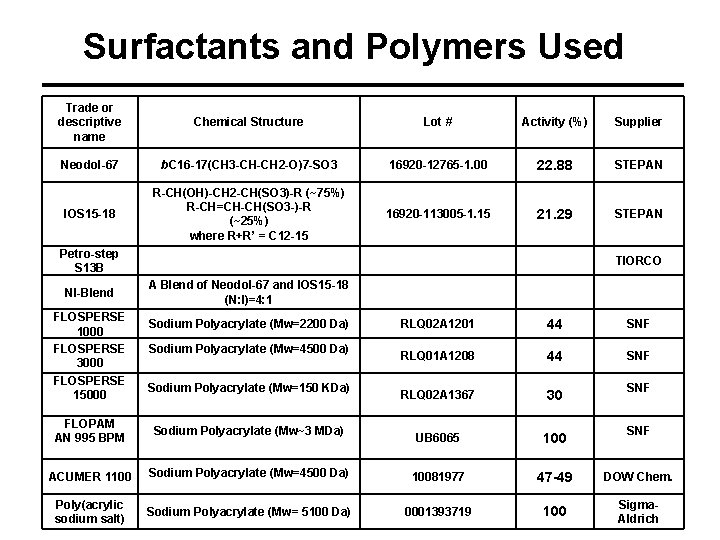
Surfactants and Polymers Used Trade or descriptive name Chemical Structure Lot # Activity (%) Supplier Neodol-67 b. C 16 -17(CH 3 -CH-CH 2 -O)7 -SO 3 16920 -12765 -1. 00 22. 88 STEPAN IOS 15 -18 R-CH(OH)-CH 2 -CH(SO 3)-R (~75%) R-CH=CH-CH(SO 3 -)-R (~25%) where R+R’ = C 12 -15 16920 -113005 -1. 15 21. 29 STEPAN Petro-step S 13 B NI-Blend FLOSPERSE 1000 FLOSPERSE 3000 FLOSPERSE 15000 TIORCO A Blend of Neodol-67 and IOS 15 -18 (N: I)=4: 1 Sodium Polyacrylate (Mw=2200 Da) Sodium Polyacrylate (Mw=4500 Da) Sodium Polyacrylate (Mw=150 KDa) RLQ 02 A 1201 44 SNF RLQ 01 A 1208 44 SNF RLQ 02 A 1367 30 UB 6065 100 SNF FLOPAM AN 995 BPM Sodium Polyacrylate (Mw~3 MDa) ACUMER 1100 Sodium Polyacrylate (Mw=4500 Da) 10081977 47 -49 DOW Chem. Poly(acrylic sodium salt) Sodium Polyacrylate (Mw= 5100 Da) 0001393719 100 Sigma. Aldrich SNF

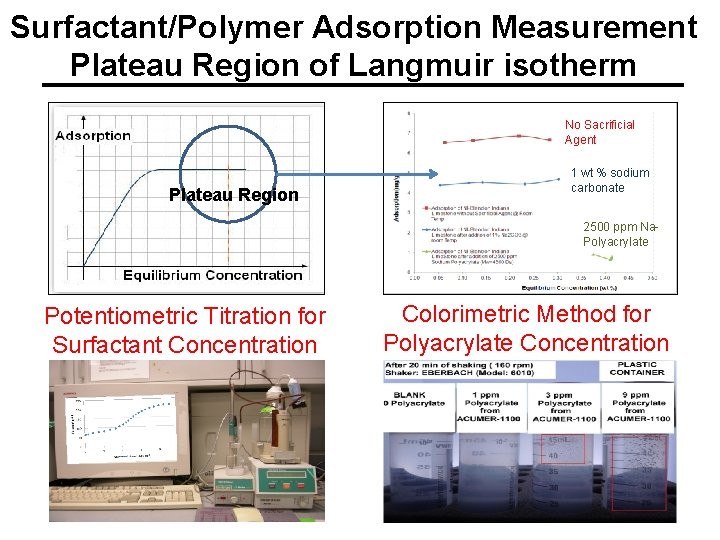
Surfactant/Polymer Adsorption Measurement Plateau Region of Langmuir isotherm No Sacrificial Agent Plateau Region 1 wt % sodium carbonate 2500 ppm Na. Polyacrylate Potentiometric Titration for Surfactant Concentration Colorimetric Method for Polyacrylate Concentration

10 Results And Discussions

How Anhydrite Can Affect Sodium Carbonate as Sacrificial Agent 11

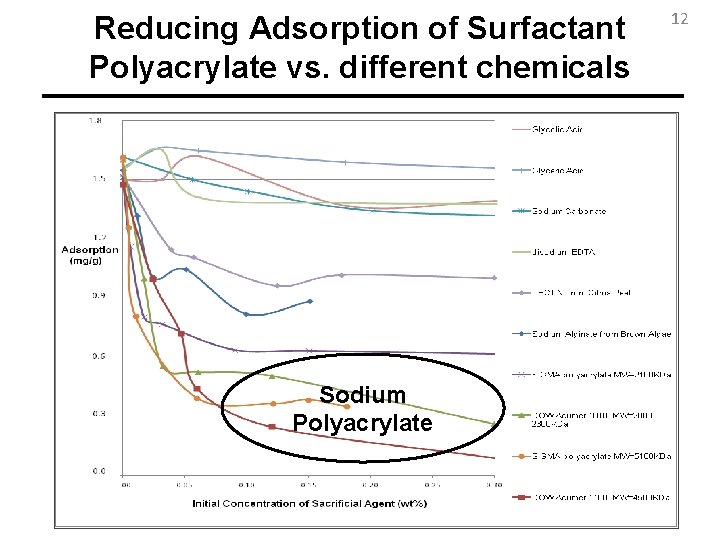
Reducing Adsorption of Surfactant Polyacrylate vs. different chemicals Sodium Polyacrylate 12

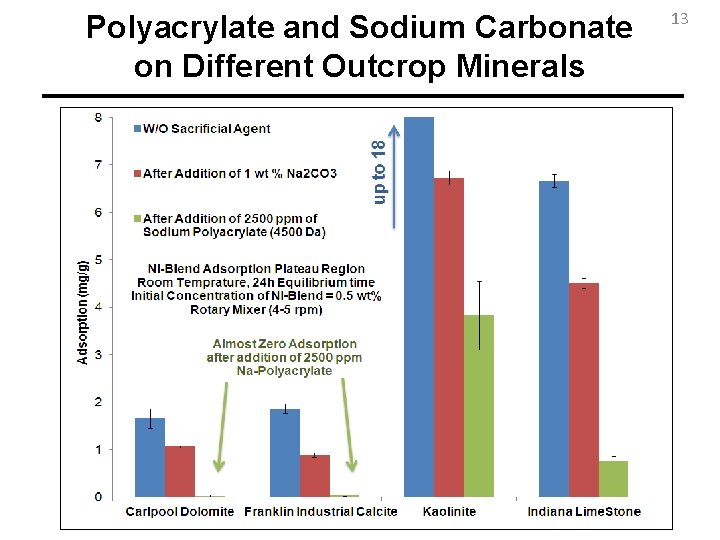
Polyacrylate and Sodium Carbonate on Different Outcrop Minerals 13

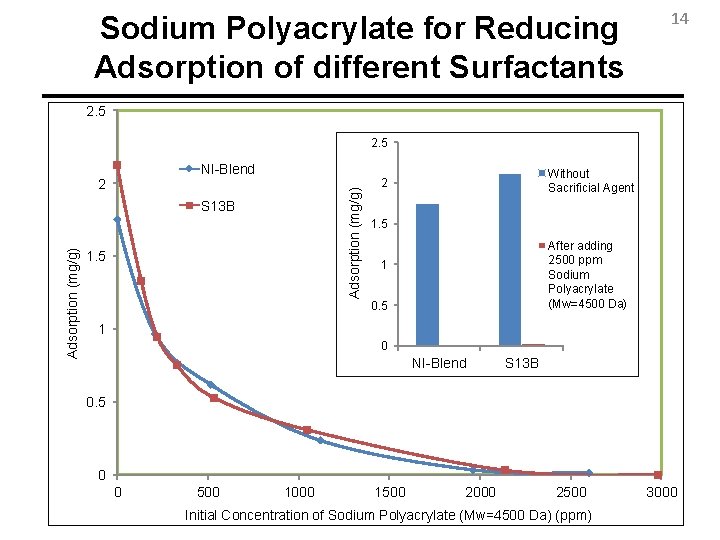
Sodium Polyacrylate for Reducing Adsorption of different Surfactants 14 2. 5 2 Adsorption (mg/g) NI-Blend Adsorption (mg/g) S 13 B 1. 5 1 Without Sacrificial Without Agent Sacrificial Agent 2 2 1. 5 After adding 2500 Afterppm adding Sodium 2500 ppm Polyacrylate Sodium (Mw=4500 Da) Polyacrylate (Mw=4500 Da) 1 1 0. 5 0 0 NI-Blend S 13 B 0. 5 0 0 500 1000 1500 2000 2500 Initial Concentration of Sodium Polyacrylate (Mw=4500 Da) (ppm) 3000

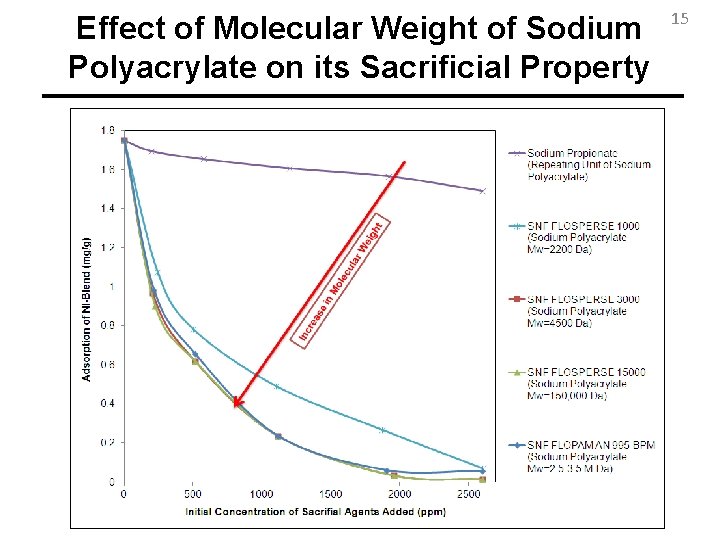
Effect of Molecular Weight of Sodium Polyacrylate on its Sacrificial Property 15

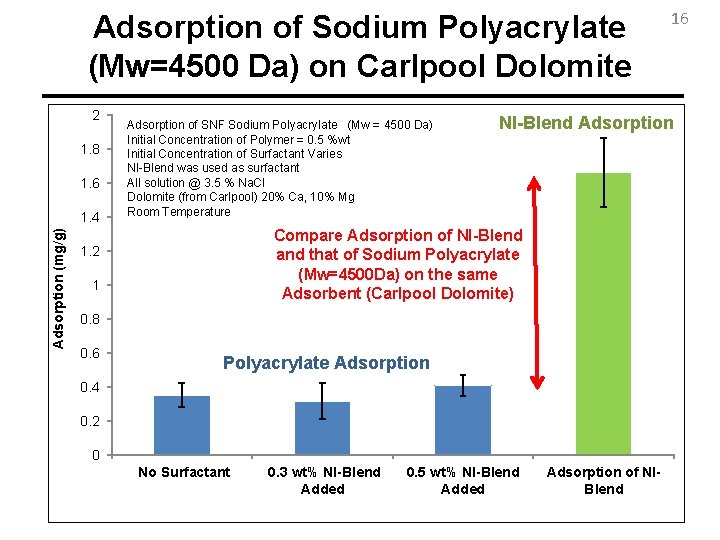
Adsorption of Sodium Polyacrylate (Mw=4500 Da) on Carlpool Dolomite 2 1. 8 1. 6 Adsorption (mg/g) 1. 4 Adsorption of SNF Sodium Polyacrylate (Mw = 4500 Da) Initial Concentration of Polymer = 0. 5 %wt Initial Concentration of Surfactant Varies NI-Blend was used as surfactant All solution @ 3. 5 % Na. Cl Dolomite (from Carlpool) 20% Ca, 10% Mg Room Temperature NI-Blend Adsorption Compare Adsorption of NI-Blend and that of Sodium Polyacrylate (Mw=4500 Da) on the same Adsorbent (Carlpool Dolomite) 1. 2 1 0. 8 0. 6 Polyacrylate Adsorption 0. 4 0. 2 0 No Surfactant 0. 3 wt% NI-Blend Added 16 0. 5 wt% NI-Blend Added Adsorption of NIBlend

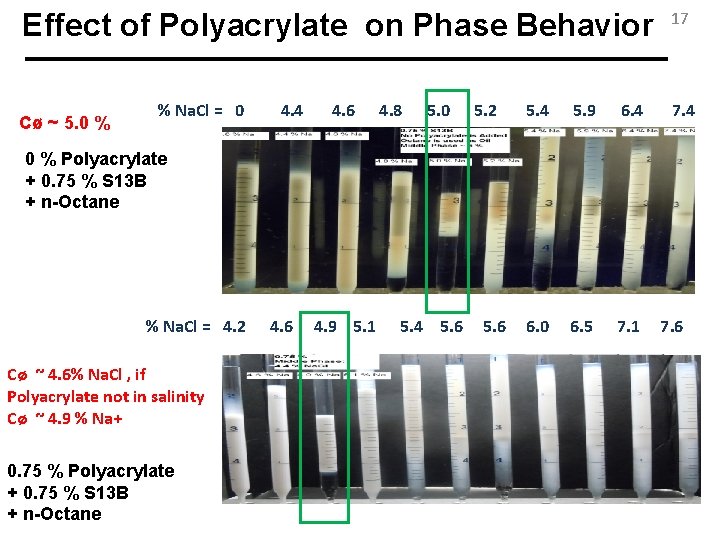
Effect of Polyacrylate on Phase Behavior Cø ~ 5. 0 % % Na. Cl = 0 4. 4 4. 6 4. 8 5. 0 5. 2 5. 4 5. 9 6. 4 6. 0 6. 5 7. 1 17 7. 4 0 % Polyacrylate + 0. 75 % S 13 B + n-Octane % Na. Cl = 4. 2 Cø ~ 4. 6% Na. Cl , if Polyacrylate not in salinity Cø ~ 4. 9 % Na+ 0. 75 % Polyacrylate + 0. 75 % S 13 B + n-Octane 4. 6 4. 9 5. 1 5. 4 5. 6 7. 6

18 Conclusions

Conclusions 19 • Sodium Polyacrylate shows the highest reduction in adsorption of anionic surfactants among many other candidates. • Regardless of type of anionic surfactant, mineral, or the commercial provider of the polyacrylate, it reduces the adsorption of surfactant much better than sodium carbonate. • Higher Molecular Weights of Polyacrylate lead to higher reductions in adsorption of surfactant until Mw=4500 Da after which Molecular Weight Does not Play Role.

Conclusions (…Continued) 20 • The fact that Mw not higher than 4500 Da is needed for Sacrificial Agent wipes out any worries about possible limitations of this application due to precipitation or degradation of high molecular weight polymers in the presence of hardness. • Adsorption of Sodium Polyacrylate (MW=4500 Da) on Carlpool Dolomite is at least ¼ of adsorption of NI-Blend. • Adsorption of Polyacrylate does not change significantly with the presence of surfactant. • Polyacrylate does not seem to change phase behavior if the ionic strength effect is considered.

Future Work 21 • Effect of Sodium Polyacrylate on Adsorption of other Surfactants, Especially Betaines. • Effect of Salinity and Hardness on Effectiveness of Sodium Polyacrylate (Mw=4500 Da). • Dynamic Adsorption Tests. • High-Temperature Long-Term Stability of Sodium Polyacrylate (Mw=4500 Da).

22 Thank You!

23 Back-Up Slides

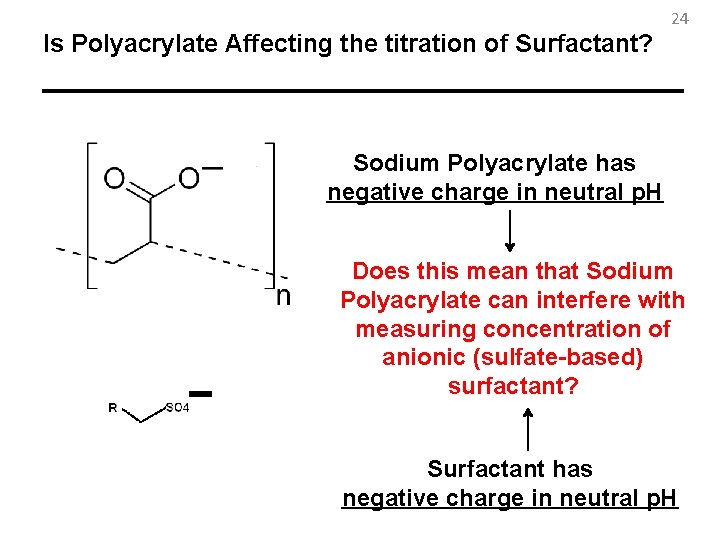
24 Is Polyacrylate Affecting the titration of Surfactant? Sodium Polyacrylate has negative charge in neutral p. H Does this mean that Sodium Polyacrylate can interfere with measuring concentration of anionic (sulfate-based) surfactant? Surfactant has negative charge in neutral p. H

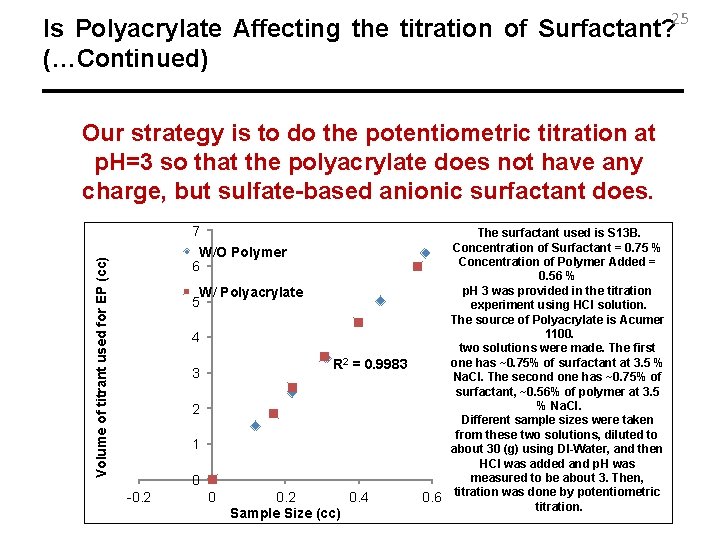
25 Is Polyacrylate Affecting the titration of Surfactant? (…Continued) Our strategy is to do the potentiometric titration at p. H=3 so that the polyacrylate does not have any charge, but sulfate-based anionic surfactant does. 7 Volume of titrant used for EP (cc) W/O Polymer 6 W/ Polyacrylate 5 4 R 2 = 0. 9983 3 2 1 0 -0. 2 0. 4 Sample Size (cc) The surfactant used is S 13 B. Concentration of Surfactant = 0. 75 % Concentration of Polymer Added = 0. 56 % p. H 3 was provided in the titration experiment using HCl solution. The source of Polyacrylate is Acumer 1100. two solutions were made. The first one has ~0. 75% of surfactant at 3. 5 % Na. Cl. The second one has ~0. 75% of surfactant, ~0. 56% of polymer at 3. 5 % Na. Cl. Different sample sizes were taken from these two solutions, diluted to about 30 (g) using DI-Water, and then HCl was added and p. H was measured to be about 3. Then, 0. 6 titration was done by potentiometric titration.

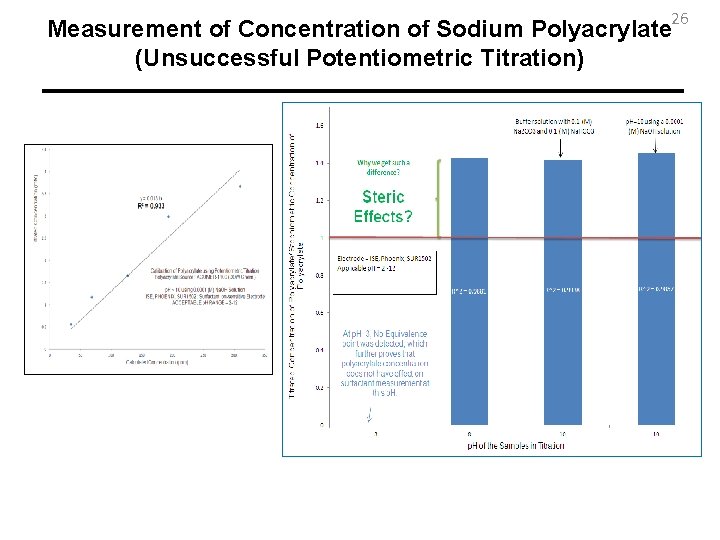
26 Measurement of Concentration of Sodium Polyacrylate (Unsuccessful Potentiometric Titration)

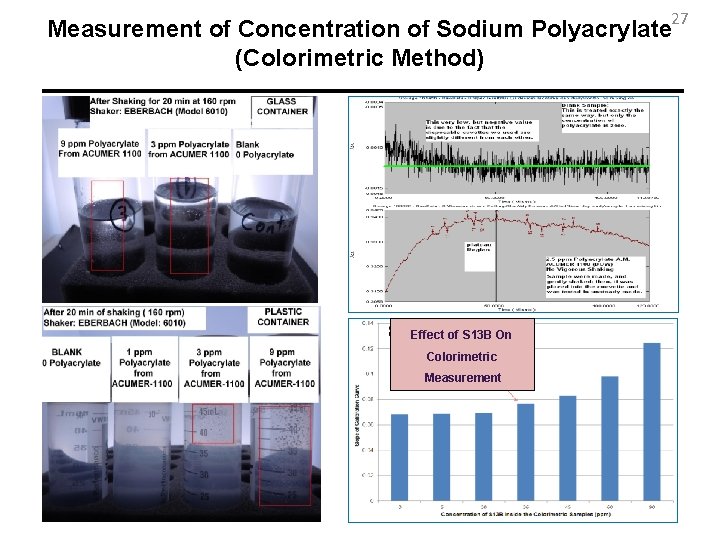
27 Measurement of Concentration of Sodium Polyacrylate (Colorimetric Method) Effect of S 13 B On Colorimetric Measurement
 Is sodium polyacrylate safe for plants
Is sodium polyacrylate safe for plants Thermal polyaspartate
Thermal polyaspartate Why does sodium polyacrylate absorb water
Why does sodium polyacrylate absorb water Sacrificial anode method
Sacrificial anode method An archetype is a ________.
An archetype is a ________. Hcl+nahco3
Hcl+nahco3 Sodium thiosulfate and sodium hypochlorite reaction
Sodium thiosulfate and sodium hypochlorite reaction Balance the following chemical equations
Balance the following chemical equations Sodium hypochlorite and sodium hydroxide
Sodium hypochlorite and sodium hydroxide What is the iupac name of the base naoh?
What is the iupac name of the base naoh? Sodium oxidanide
Sodium oxidanide Sodium chloride chemical equation
Sodium chloride chemical equation Li reaction with oxygen
Li reaction with oxygen Adsorption chromatography types
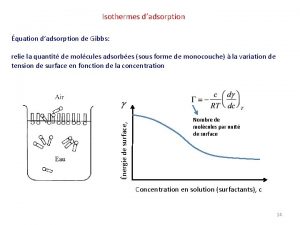
Adsorption chromatography types Isotherme de gibbs
Isotherme de gibbs Physisorption and chemisorption graph
Physisorption and chemisorption graph Principles of chromatography
Principles of chromatography Adsorption blood bank
Adsorption blood bank Adsorption
Adsorption What is adsorption in physical pharmacy
What is adsorption in physical pharmacy Fajans titration
Fajans titration Chromatography mobile phase and stationary phase
Chromatography mobile phase and stationary phase Eosin adsorption indicator
Eosin adsorption indicator Freundlich adsorption isotherm formula
Freundlich adsorption isotherm formula Les types d'adsorption
Les types d'adsorption Organic precipitating agents examples
Organic precipitating agents examples What is dry gum method
What is dry gum method What is adsorption
What is adsorption