Chapter 2 History From ancient Greeks to early





































- Slides: 41

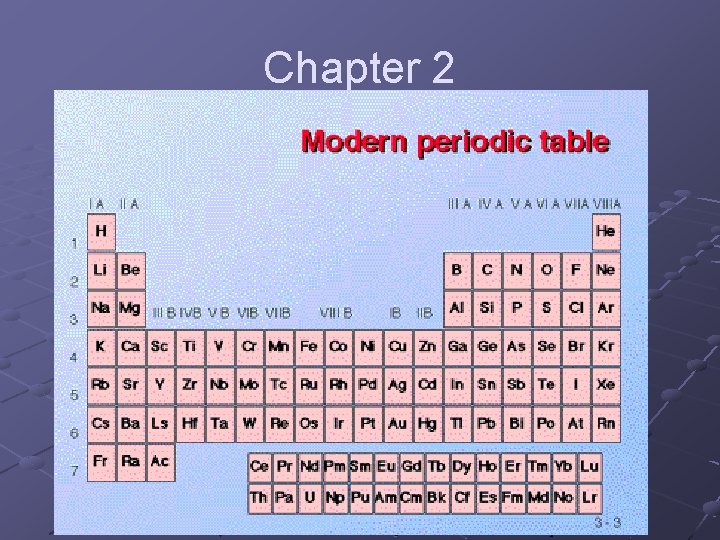
Chapter 2

History: From ancient Greeks to early 1900’s Democritus vs. Aristotle Joseph Proust (1799) – Law of Definite Proportions (see #2 below)

John Dalton (1808) – Modern Atomic Theory: 1. Elements composed of small, spherical, indestructible particles, called atoms. Atoms of the same element are identical. Atoms of different elements are different. 2. Compounds are composed of 2 or more atoms from different elements combined together in a fixed ratio of small whole numbers.

3. A chemical reaction involves only the rearrangement of atoms; it does not result in the creation or destruction of atoms. Law of Multiple Proportions: If 2 elements form more than one compound, the masses of one element that combine with a fixed mass of the other element are in ratios of small whole #’s.

Law of Conservation of Mass: Matter cannot be created nor destroyed, thus in a chemical reaction, no atoms are created or destroyed; only rearranged (#3 above) Crookes’ Tube: (1877) – Cathode Rays and the Cathode Ray tube Remove gas - not quite completely - light disappears - but if a fluorescent screen placed in tube it glows. This glow was called cathode rays, because they seemed to flow from the cathode electrode to the anode electrode. What caused this glow ?

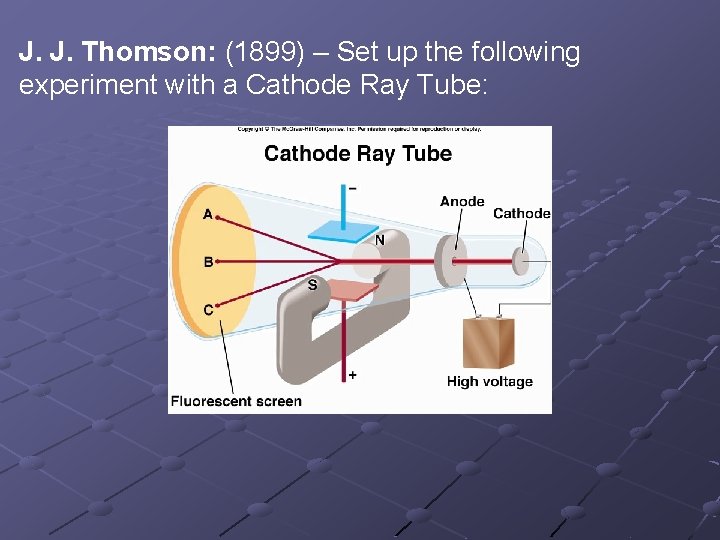
J. J. Thomson: (1899) – Set up the following experiment with a Cathode Ray Tube:

What this showed was that this glow was a stream of negative charge, because it bent toward the positive plate. The cathode ray was also bent by a magnetic field. This showed that the stream of negative charge was composed of material particles. He could actually measure the ratio of mass to charge; m / e. He performed this experiment, using different gases and different metals for the anode and cathode. In every experiment, the cathode rays were always the same with the same m / e ratio.

He concluded that these negative particles must be coming from either the cathode metal or the gas in the tube, but since they were always the same, they must be a common part of all substances and since they were negatively charged, he said they must be the basic particle of negative charge, which Ben Franklin had described over a century earlier and named the electron.

Thomson went one step further and concluded that since all substances, according to Dalton, contained only neutral atoms, these electrons must be coming from inside the atoms. This was a contradiction to Dalton, who said the atom was indivisible, and the smallest possible particle. Dalton’s Theory was modified as a result.

Thomson proposed his own model for the atom, which he named the “Plum-Pudding” Model:

Subsequently, An American Physicist was able to determine the exact charge on this electron: 1. 6022 x 10 -19 coulombs (C). he was also able to determine the mass of the electron: 9. 10 x 10 -28 g. Obviously the electron is a very small particle.

In 1893, Henri Becquerel accidentally discovered a new phenomenon. Some substances spontaneously gave off certain particles and radiation. This process was named radioactivity. Three types of radioactive emissions had been discovered by 1900: 1. Alpha particles + charged particles of high energy (a) 2. beta particles - charged particles of very high energy (b) 3. gamma rays High energy electromagnetic radiation (g)

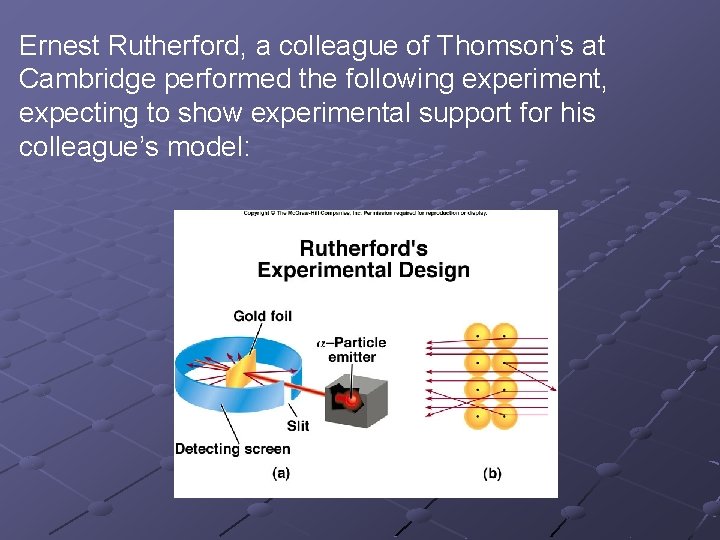
Ernest Rutherford, a colleague of Thomson’s at Cambridge performed the following experiment, expecting to show experimental support for his colleague’s model:

Rutherford expected some very slight scattering of a particles due to their attraction to the electrons and due to the pudding like inside of the atom. Instead over 99% of a particles were unscattered, while a few were scattered at high angles. Some even bounced straight back. His only conclusion was that Thomson’s Model was incorrect.

Rutherford proposed what is called the Nuclear Model of the atom. He said that almost all the mass of the atom was concentrated in a tiny space in the center of the atom, which also contained all of the positive charge. Electrons were scattered around, as in the Thomson Model. But, quite remarkably, Rutherford concluded that 99% of all the space in atoms was a perfect vacuum, totally empty.

If you took all atoms of earth and removed all the empty space you would have a 0. 8 mile diameter sphere. Proton discovered at about same time – Found to be equal in charge to the electron but about 1800 times more mass. Prediction of neutron around 1910. Discovered in 1932 – Found to have no charge but a mass about equal to the proton.

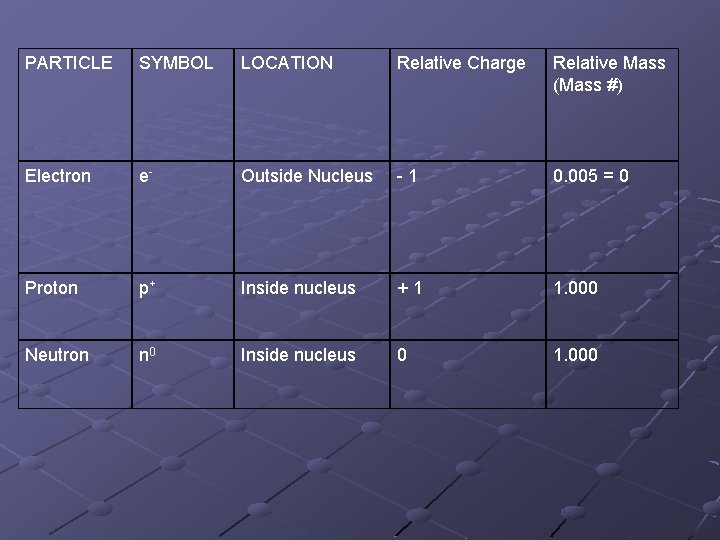
PARTICLE SYMBOL LOCATION Relative Charge Relative Mass (Mass #) Electron e- Outside Nucleus -1 0. 005 = 0 Proton p+ Inside nucleus +1 1. 000 Neutron n 0 Inside nucleus 0 1. 000

Two new terms based on these discoveries: Atomic # (Z) - # of protons inside a nucleus. Also equal to # electrons outside the nucleus of a neutral atom. Atomic # is unique for each element. Mass # = # of protons + # of neutrons in any atom.

Also, with these new discoveries, another flaw in Dalton’s Atomic Theory is brought out. He said that all atoms of the same element are identical. It turns out that every element, except F, has more than one type of atom occurring in nature. They have the same # of protons (they have to, in order to be the same element), but they have different #’s of neutrons.

Isotopes – 2 or more atoms of the same element with different Mass #’s (different # of neutrons) The common way to designate different isotopes; where Z represents the Atomic #, A the Mass # and X the symbol of the element.

The Periodic Table: There were many attempts to organize elements into some kind of logical pattern, but none made sense until Dimitri Mendeleev, a Russian chemist presented his model in 1869.

Mendeleev organized the elements by atomic weight, left to right, but also vertically by similar properties. There were a few exceptions, which he attributed to inaccurate atomic weights. In actuality, the mistake he made, was that the arrangement should not be by atomic weight, but rather by Atomic Number.

The Periodic Table gets its name from the fact that properties of elements repeat themselves (vertical columns), just like a pendulum swings back and forth and each swing is called a period, which is each row in the Table. Each column is called a group or family.

The elements are arranged in other ways, also, in the Periodic Table. They are divided into metals, nonmetals, metalloids and Noble gases. Metals – For now we will define metals in terms of physical properties: 1. Conduct heat and electricity 2. Are malleable – Solid state can be banged into thin sheets without shattering

3. Are ductile – Solid state can be formed into thin wires 4. Solid state can be polished 5. Have a wide range of melting and boiling points Metals are the largest group on the Periodic Table. Every element left of the bold stepped line beginning between B and C are elements. This includes the 2 rows at the bottom of the Table.

Non-metals – Basically the opposite of metals. They don’t conduct heat or electricity, are not ductile or malleable, can not be polished and generally have low to medium melting and boiling points. Nonmetals are found between the bold stepped line and the last column. Metalloids – They are the bridge between metals and non-metals. They have some properties of both. They are the elements that touch the bold stepped line.

Noble Gases – The elements in the last column (beginning with He). All are gases at room temperature. They have the lowest melting and boiling points of all the elements. They either do not chemically react at all or react very little. We will discuss this later.

Some elements only exist naturally as 2 atoms bonded together in a molecule, such as H 2. These are called diatomic elements. There are 7 common ones: H 2, N 2, O 2, F 2, Cl 2, Br 2 and I 2.

Atoms form ions by gaining (anions) or losing (cations) electrons. Nothing happens to the nucleus in these type of reactions.

Molecular Formula is the actual formula of a compound. Empirical Formula is the formula with the smallest possible ratio of whole numbers. Many times the molecular and empirical formulas for a substance are the same. Some examples are H 2 O, Na. Cl, H 2 SO 4 however there are many cases in which the molecular formula is not the same as the empirical formula. Some examples are (the empirical formula will be in parentheses): H 2 O 2 (HO), Na 2 S 2 O 4 (Na. SO 2) and C 6 H 12 O 6 (CH 2 O).

We can usually predict the molecular formula of binary (containing 2 elements) ionic compounds or compounds containing any 2 ions. The subscript of the cation is the same number as the charge on the anion and the subscript of the anion is the same as the charge on the cation. Examples: potassium bromide, zinc iodide, ammonium nitrate

Chemical Nomenclature – The naming of compounds. To become a chemist, it is necessary to be able to speak and understand the language of chemistry. The Naming of compounds, at one time, was somewhat haphazard. We call those names today, common names. Some are still used, but by and large, we now have systematic methods for naming compounds. We will begin to learn how to do this with the simplest cases, those of binary salts. A binary salt is a compound between a metal and a non-metal. In other words, binary means there are 2 and only 2 elements in the compound.

To name a binary compound involving only Representative elements, the metal is named first (with no changes) followed by the non-metal (with any ending, such as “gen”, “ine”, “ur”, “orous”, “ic” or “ium”, replaced by “ide”. For example: Na. Cl = sodium chloride Al 2 O 3 = aluminum oxide Note that there is no need to tell the # of each type of atom with these names.

When the 2 elements are both non-metals, it frequently becomes necessary to use prefixes in front of both names to indicate the number of each of these elements in the compound. The prefixes are listed in Table 2 -4 on page 56. If there is only one atom of the first element, the prefix mono is usually omitted. “ide” is still used for the ending. If the compound is an oxide, frequently the last “a” of the prefix in front of oxide is frequently omitted. For example N 2 O 4 is named dinitrogen tetroxide

When a transition metal is involved, the naming becomes a little more complicated. Usually, transition metals have 2 common valences (Cu can be +1 or +2, Fe can be +2 or +3). It thus becomes necessary to distinguish between the 2 possibilities. The modern method is to use Roman Numerals in parentheses after the metal name to indicate its charge. For example: Fe. O is iron(II)oxide. (We know that Fe is +2 here because O is almost always – 2) Fe 2 O 3 is iron(III)oxide

There is an older method that is still used by some chemists and books, which attaches the suffix “ic” or “ous” to the metal name, depending on its charge. For the higher of the 2 possible charges, “ic” is used and for the lower of the 2 charges “ous” is used. Also, the Latin name for the metal is frequently used because it sounds better, thus Fe. O is ferrous oxide and Fe 2 O 3 is ferric oxide.

For now, we can define an acid as a substance that yields H+ ions when dissolved in water. All acids contain at least one H atom. Naming acids: Binary Acids: HX Name as "hydro" followed by X's name with "ine" or "ium" ending replaced with ic acid. Example: HCl is hydrochloric acid.

Oxygen containing acids (oxoacids): 1. If there are 2 possibilities, with only # O atoms different, such as HNO 3 and HNO 2 or H 2 SO 4 and H 2 SO 3: Use only the anion name: Replace "ate" ending with "ic acid" or sometimes "uric acid" and "ite" ending with "ous acid" or sometimes "urous acid". The above acids are nitric acid and nitrous acid and sulfuric acid and sulfurous acid.

2. If there are 4 possibilities, add the prefix "hypo" to the one with less O atoms than the "ous acid" and add the prefix "per" to the one with more O atoms than the "ic acid". For example: HCl. O 4 = perchloric acid HCl. O 3 = chloric acid HCl. O 2 = chlorous acid HCl. O = hypochlorous acid

A base can be defined as a substance that produces OH-1 ions when dissolved in water. Most are ionic compounds containing OH-1 and are named as any ionic compound.

Hydrate – Sometimes, if a crystalline solid forms in water, as the solid crystal forms, molecules of water get trapped inside the crystal. This process is not random but rather occurs the same way every time the situation arises. The water molecules are only weakly attracted to the rest of the substance and basically retain their own identity. To indicate this, a hydrate is written with the # of water molecules shown separately from the rest of the formula, separated by a . For example Cu. SO 4 5 H 2 O is copper sulfate pentahydrate.
 What are the values of ancient greek culture
What are the values of ancient greek culture The difference between geocentric and heliocentric
The difference between geocentric and heliocentric Ancient rome and early christianity
Ancient rome and early christianity Chapter 6 ancient rome and early christianity
Chapter 6 ancient rome and early christianity Who were the persians
Who were the persians Which statement is accurate?
Which statement is accurate? Balkan peninsula ancient greece map
Balkan peninsula ancient greece map Byzantine floral arrangements
Byzantine floral arrangements Romans and greeks
Romans and greeks Byzantine flower arrangements
Byzantine flower arrangements Above all else i must be saved
Above all else i must be saved Where does the word theatre come from
Where does the word theatre come from Timeo danaos et dona ferentes
Timeo danaos et dona ferentes Draw segment sr the bisector of the vertex angle prq
Draw segment sr the bisector of the vertex angle prq Option greeks wikipedia
Option greeks wikipedia History alive the ancient world chapter 8
History alive the ancient world chapter 8 History alive chapter 14
History alive chapter 14 From hunters and gatherers to farmers chapter 3
From hunters and gatherers to farmers chapter 3 Early cpr and early defibrillation can: *
Early cpr and early defibrillation can: * Chapter 9 lesson 1 early civilizations
Chapter 9 lesson 1 early civilizations Early empires in the ancient near east
Early empires in the ancient near east Ancient rome outcomes geography and early republic
Ancient rome outcomes geography and early republic Ancient ways of communication
Ancient ways of communication Ancient india vs ancient china
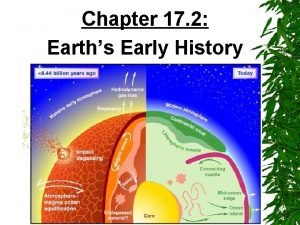
Ancient india vs ancient china Section 17-2 earth's early history
Section 17-2 earth's early history 17-2 earth's early history
17-2 earth's early history Section 17-2 earth's early history
Section 17-2 earth's early history 2700 bc popcorn
2700 bc popcorn What do historians call the early period of human history
What do historians call the early period of human history Ancient roman plays
Ancient roman plays Medieval netherlands
Medieval netherlands Tasc ancient history
Tasc ancient history Physical feature of punjab
Physical feature of punjab Ancient greek theatre history
Ancient greek theatre history Kalasiris
Kalasiris Ap world jeopardy
Ap world jeopardy Ancient civilizations through the renaissance
Ancient civilizations through the renaissance Also history physical
Also history physical Types of early childhood programs activity a chapter 2
Types of early childhood programs activity a chapter 2 Chapter 7 early childhood ages 3 through 5 answer key
Chapter 7 early childhood ages 3 through 5 answer key Chapter 9 lesson 2 early challenges answers
Chapter 9 lesson 2 early challenges answers A vygotskian classroom promotes ________.
A vygotskian classroom promotes ________.