Ch E 201 Chemical Engineering Calculations I Fall

























- Slides: 38

Ch. E 201 Chemical Engineering Calculations I Fall 2005/2006 Course Description: System of units and dimensions. Stoichiometry. Ideal and non-ideal gases, critical properties and compressibility charts. Vapor-Liquid equilibria. Material balance calculations for steady and unsteady state processes with and without chemical reaction.

My name is Dr. Faisal Iskanderani Instructor: Dr. Faisal Iskanderani Tutorial assistance: Engr Sayed Hammoda Office: Main Library, Dean’s Office Hours: Sat 9: 00 -10. 30 Mon 12: 00 -1: 30

Ch. E 201 Chemical Engineering Calculations I Fall 2005/2006 Course Description: . Recycle, by-pass and purge calculations. Process flow sheeting with computer applications Text Book: David Himmelblau, Basic Principles and Calculations in Chemical Engineering, Prentice Hall PTR, 7 th Edition 2003.

Reference: Fedler, Elementary Principles of Chemical Processes, John Wiley, 1998 Course Goals: The overall goal of this course is to introduce students to the fundamentals of chemical engineering, material balance Specific instructional goals • Demonstrate the ability to apply knowledge of mathematics and freshman chemistry to solve material balance problems.

Reference: Fedler, Elementary Principles of Chemical Processes, John Wiley, 1998 Specific instructional goals • Function on teams to solve problems. • Use computers to solve material balance problems.

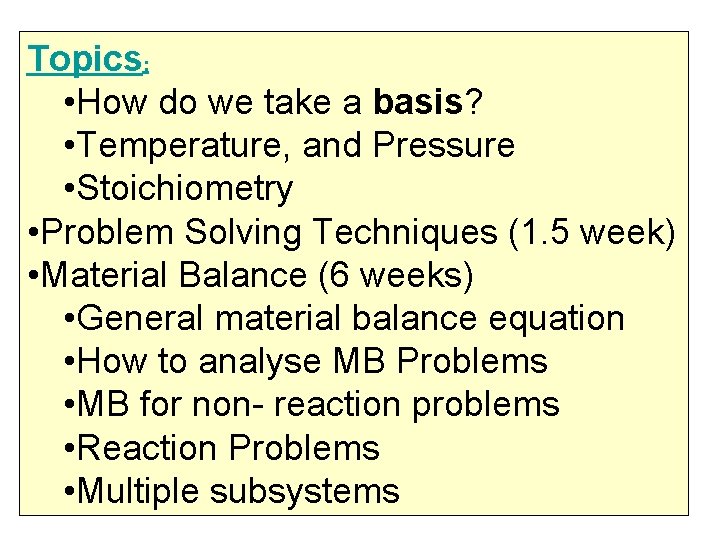
Topics: • Introduction (3 weeks) • Units & Dimensions • Dimensional consistency • The mole unit • Density, Specific gravity, sp. Volume, mole fraction, mass fraction, Volume fraction • Analyses of mixtures : mole %, mass %, volume %, • Concentrations of solutions, mass/unit volume, PPM, molar, molal , etc

Topics: • How do we take a basis? • Temperature, and Pressure • Stoichiometry • Problem Solving Techniques (1. 5 week) • Material Balance (6 weeks) • General material balance equation • How to analyse MB Problems • MB for non- reaction problems • Reaction Problems • Multiple subsystems

Topics: • Recycle, Bypass and Purge • Material Balance (MB) for Gases, Vapors, and Solids (4. 5 weeks) • Ideal gas law for a single gas • Ideal gas law for gas mixtures • Material Balance for gases • Real gas Relationships • Vapor pressure and liquids • Saturation • Equilibria

Topics: • Equilibria • Partial saturation • MB involving Condensation and Vaporization

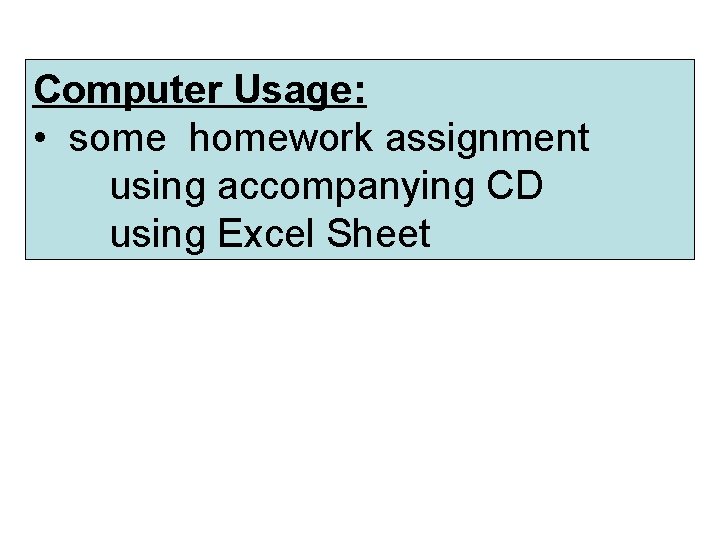
Computer Usage: • some homework assignment using accompanying CD using Excel Sheet

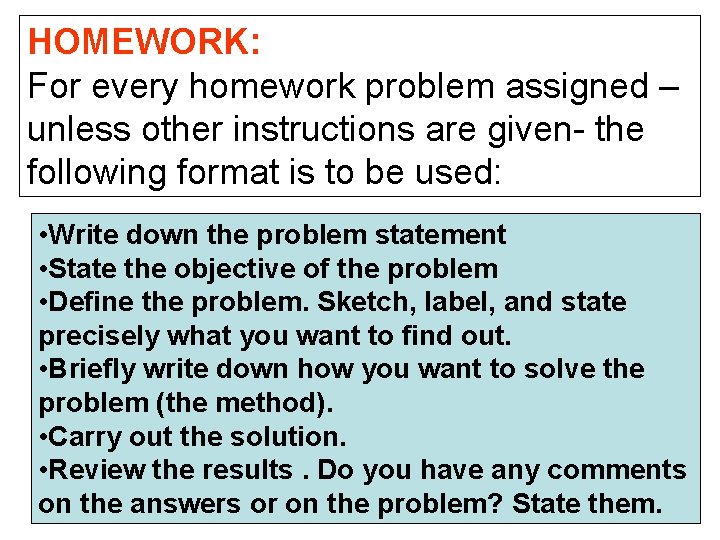
HOMEWORK: For every homework problem assigned – unless other instructions are given- the following format is to be used: • Write down the problem statement • State the objective of the problem • Define the problem. Sketch, label, and state precisely what you want to find out. • Briefly write down how you want to solve the problem (the method). • Carry out the solution. • Review the results. Do you have any comments on the answers or on the problem? State them.

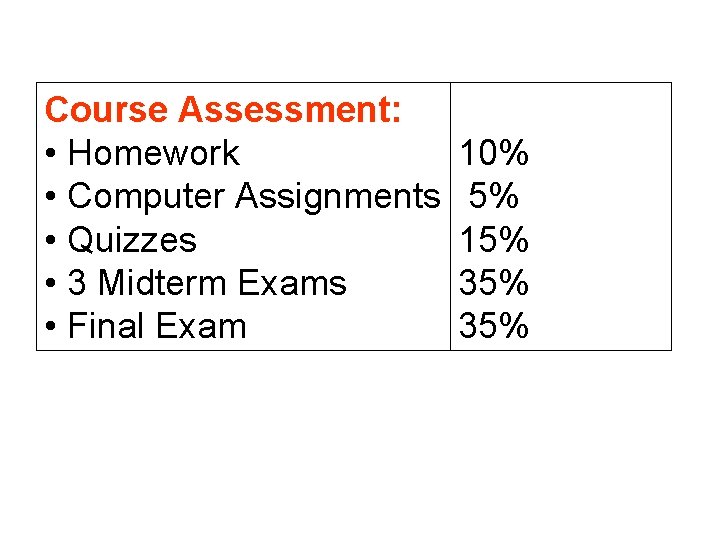
Course Assessment: • Homework 10% • Computer Assignments 5% • Quizzes 15% • 3 Midterm Exams 35% • Final Exam 35%

Attendance: 1. 25% absence will result into a DN grade. 25% of (2 lectures + 1 tutorial) x 15 = 11 2. Maximum lateness allowed is 5 minutes. If you come more than 5 minutes late, you will be allowed to attend class, but you will be considered late. Each 2 late attendances will be considered as 1 absence.

Course Conduct Codes : In all aspects of the course honesty and trust are a must. Any violations will be severely confronted.

You will be working in class as follows: • individually • As a team For homework, quizzes and exams you must work individually LET US NOW CONSTRUCT THE TEAMS EACH ONE MUST DO HIS DUTY

INTRODUCTION Individually: Take 5 minutes and write down “ What do you know about Chemical Engineering, what processes does chemical engineering involve?

INTRODUCTION As a team: Take 5 minutes and write down “ What do you know about Chemical Engineering, what processes does chemical engineering involve?

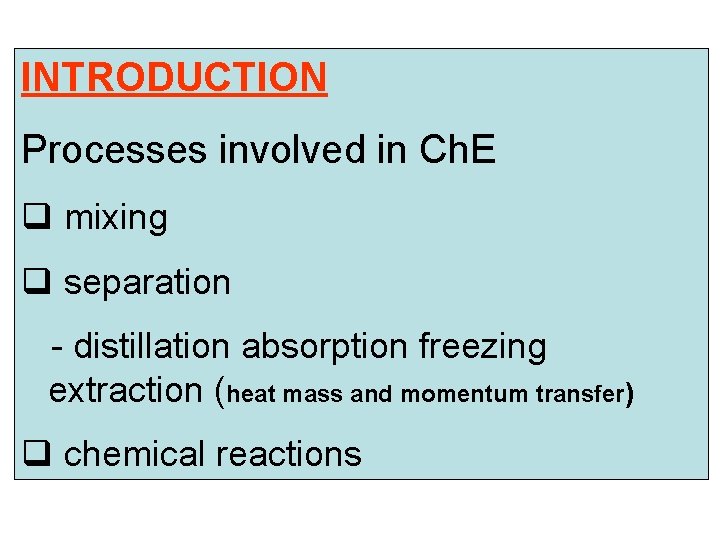
INTRODUCTION Processes involved in Ch. E q mixing q separation - distillation absorption freezing extraction (heat mass and momentum transfer) q chemical reactions

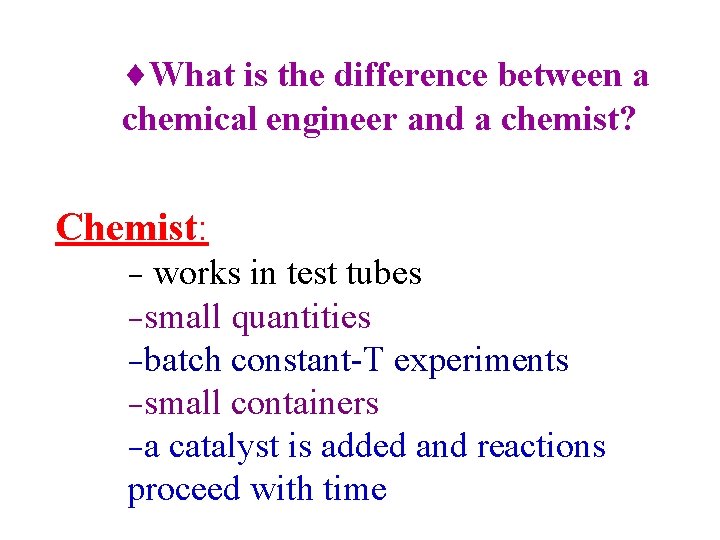
¨What is the difference between a chemical engineer and a chemist? Chemist: - works in test tubes -small quantities -batch constant-T experiments -small containers -a catalyst is added and reactions proceed with time

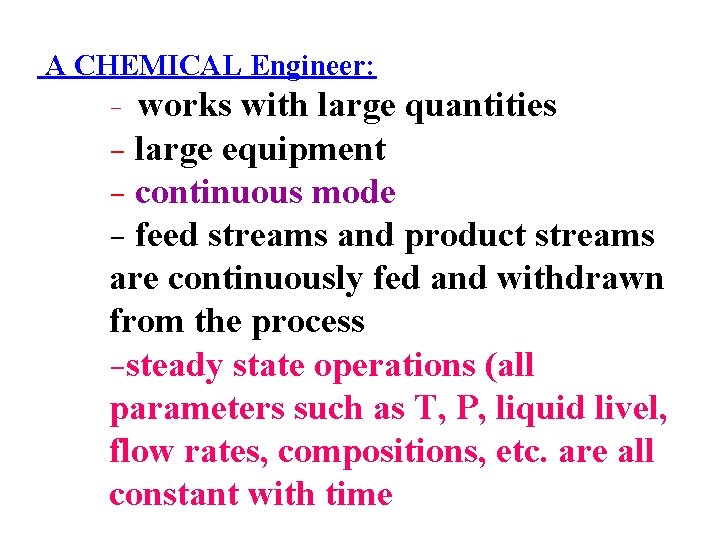
A CHEMICAL Engineer: - works with large quantities - large equipment - continuous mode - feed streams and product streams are continuously fed and withdrawn from the process -steady state operations (all parameters such as T, P, liquid livel, flow rates, compositions, etc. are all constant with time

CHEMICAL Engineer: -scaling up - works closely with mechanical, electrical, civil, and metallurgical engineers in order to design and operate the physical equipment in a plant ( such as: ? )

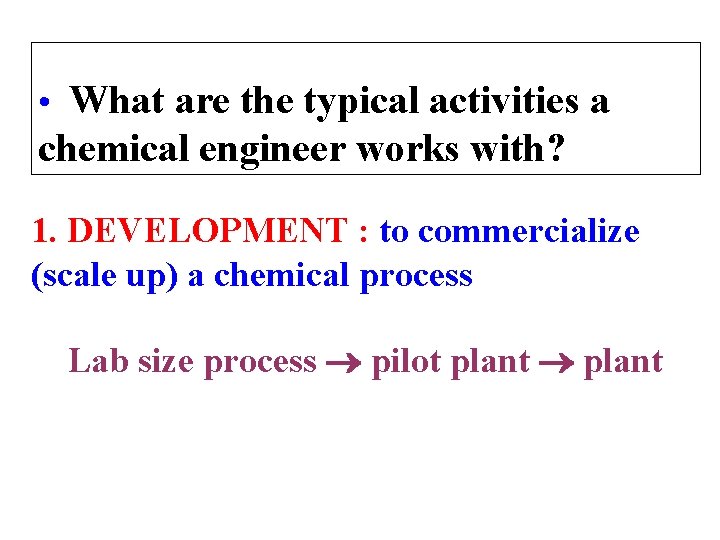
• What are the typical activities a chemical engineer works with? 1. DEVELOPMENT : to commercialize (scale up) a chemical process Lab size process pilot plant

2. DESIGN : A team of engineers design the commercial plant, based on experience and data obtained from the Lab size process and the pilot plant. The Chem E specifies: q. Process flow rates and conditions q. Equipment types and sizes q. Materials of constructions q. Process configuration q. Control systems q. Safety systems q. Other

3. CONSTRUCTION : Assembling of all components into a complete plant 4. MANUFACTURING : running the plant or operations and production. Things that are important and relevant: qefficiency qsafety qdesign modifications qreduce costs qimprove product quality qreduce pollution

5. TECHNICAL SALES 6. RESEARCH





18.








Homework due next Saturday 13/8/1426 (17/9/2005) • Read Chapter 1 • Solve Problems 1, 5, 9, 14, 18, 23, 28, 33, 38, 43, 48, 52, 55