Stoichiometry Calculations from Chemical Equations MoleMole Calculations MoleMass




- Slides: 17

Stoichiometry Calculations from Chemical Equations Mole-Mole Calculations Mole-Mass Calculations Mass-Mass Calculations Thursday, March 1 st, 2018 1

What is stoichiometry? Stoichiometry is the quantitative study of reactants and products in a chemical reaction.

What is stoichiometry?

What You Should Expect Given : Amount of reactants Question: how much of products can be formed. Example 2 A + 2 B 3 C Given 20. 0 grams of A and sufficient B, how many grams of C can be produced?

What do you need? i. iii. iv. You will need to use molar ratios, molar masses, balancing and interpreting equations, and conversions between grams and moles. Note: This type of problem is often called "mass-mass. "

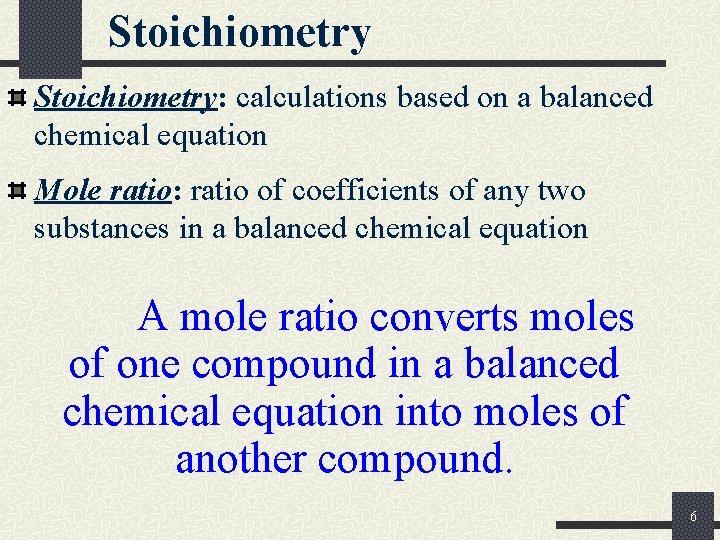
Stoichiometry: calculations based on a balanced chemical equation Mole ratio: ratio of coefficients of any two substances in a balanced chemical equation A mole ratio converts moles of one compound in a balanced chemical equation into moles of another compound. 6

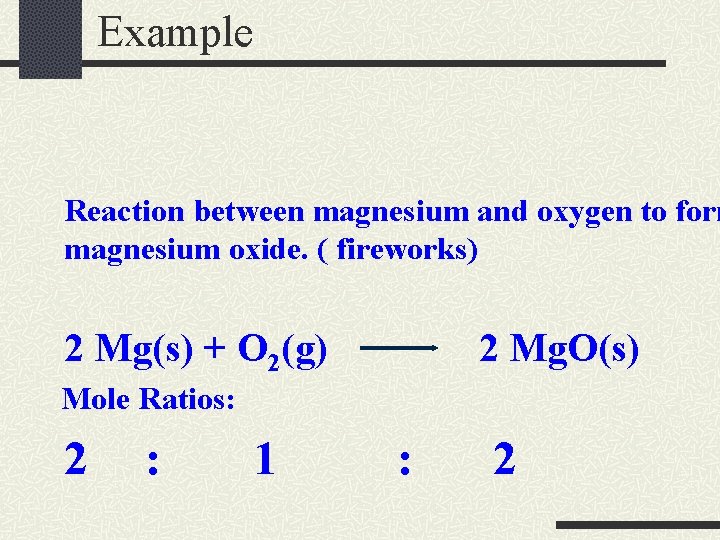
Example Reaction between magnesium and oxygen to form magnesium oxide. ( fireworks) 2 Mg(s) + O 2(g) 2 Mg. O(s) Mole Ratios: 2 : 1 : 2

The Goal The goal of stoichiometry is to perform conversions (changing between units) by cancelling out units until you end up with the units you want (the answer).

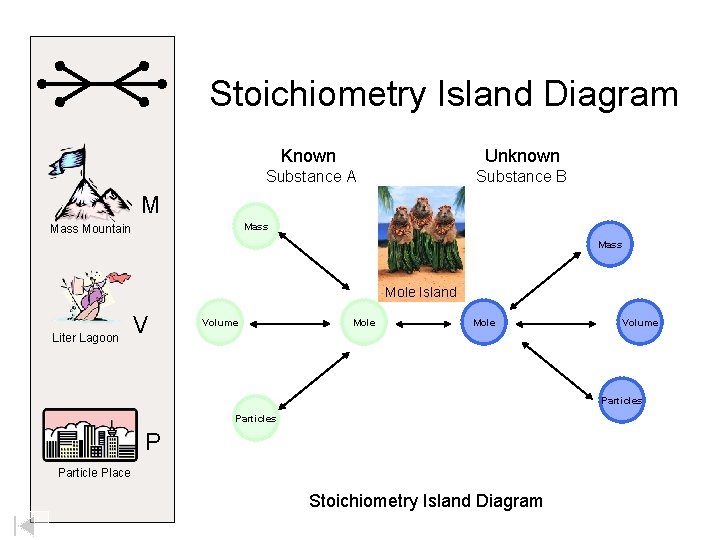
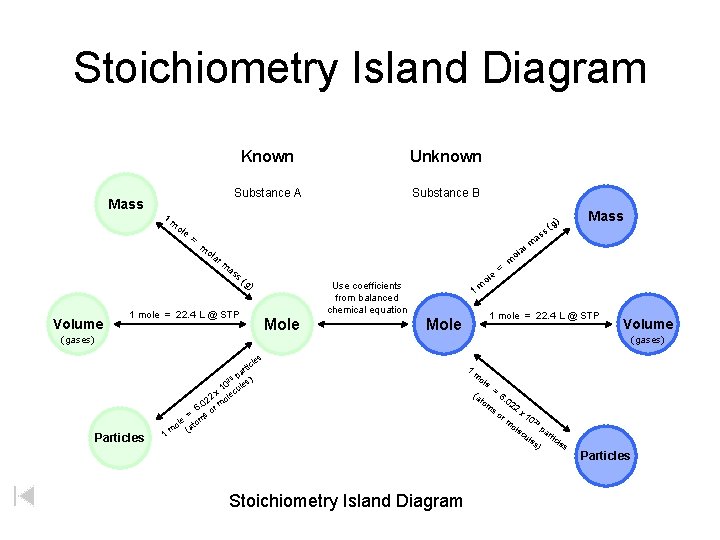
Welcome to Mole Island 1 mol = molar mass 1 mole = 22. 4 L @ STP 1 mol = 6. 02 x 1023 particles

Stoichiometry Island Diagram Known Unknown Substance A Substance B M Mass Mountain Mass Mole Island Liter Lagoon V Volume Mole Volume Particles P Particle Place Stoichiometry Island Diagram

Stoichiometry Island Diagram Mass 1 Known Unknown Substance A Substance B ole = Mass a rm m ola r a ol m as s( Volume ) (g ss m g) Use coefficients from balanced chemical equation 1 mole = 22. 4 L @ STP Mole e ol m = m 1 1 mole = 22. 4 L @ STP Mole Volume (gases) les Particles 1 c rti pa ) 23 0 les x 1 lecu 2 o 02 6. or m = s m ole (ato m Stoichiometry Island Diagram 1 m ole (a to m = 6. 02 so 2 x 1 0 23 ole pa cu r les ticle s ) rm Particles

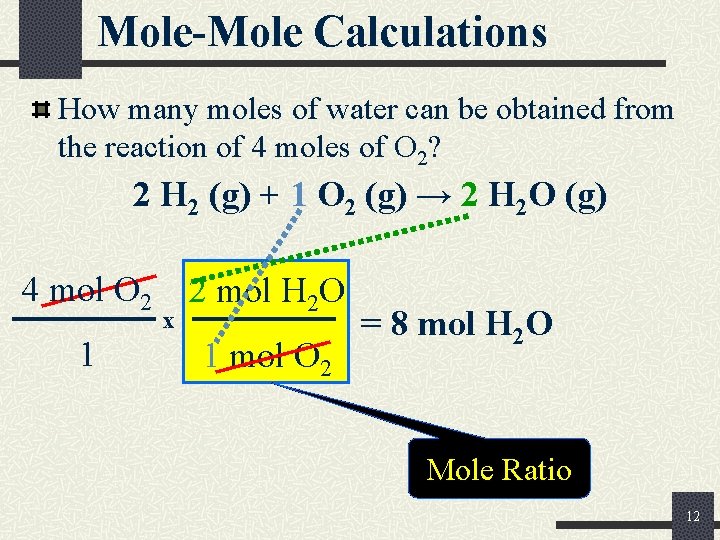
Mole-Mole Calculations How many moles of water can be obtained from the reaction of 4 moles of O 2? 2 H 2 (g) + 1 O 2 (g) → 2 H 2 O (g) 4 mol O 2 1 x 2 mol H 2 O 1 mol O 2 = 8 mol H 2 O Mole Ratio 12

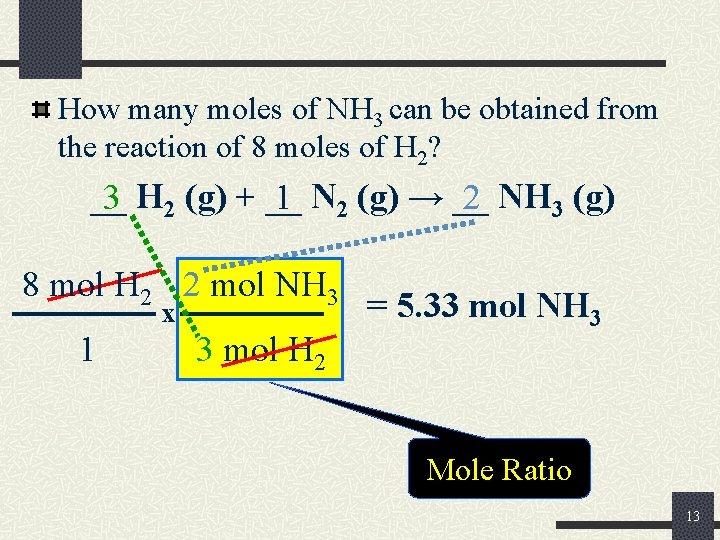
How many moles of NH 3 can be obtained from the reaction of 8 moles of H 2? __ 3 H 2 (g) + __ 1 N 2 (g) → __ 2 NH 3 (g) 8 mol H 2 2 mol NH 3 x 1 3 mol H 2 = 5. 33 mol NH 3 Mole Ratio 13

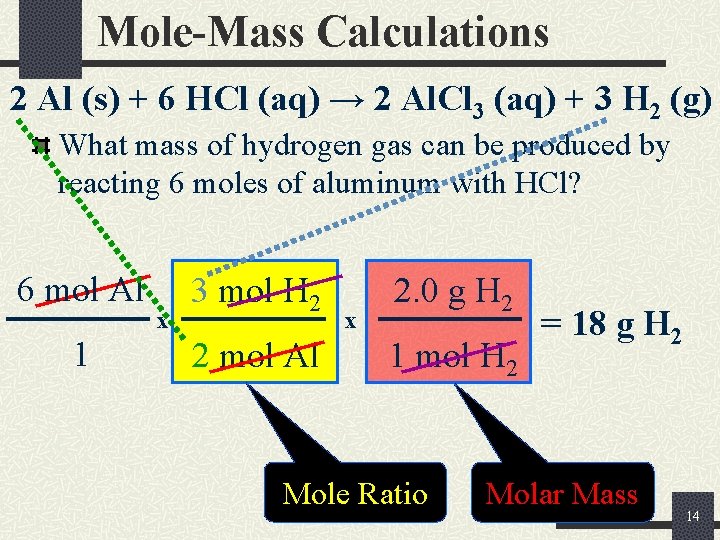
Mole-Mass Calculations 2 Al (s) + 6 HCl (aq) → 2 Al. Cl 3 (aq) + 3 H 2 (g) What mass of hydrogen gas can be produced by reacting 6 moles of aluminum with HCl? 6 mol Al 1 x 3 mol H 2 2 mol Al x 2. 0 g H 2 1 mol H 2 Mole Ratio = 18 g H 2 Molar Mass 14

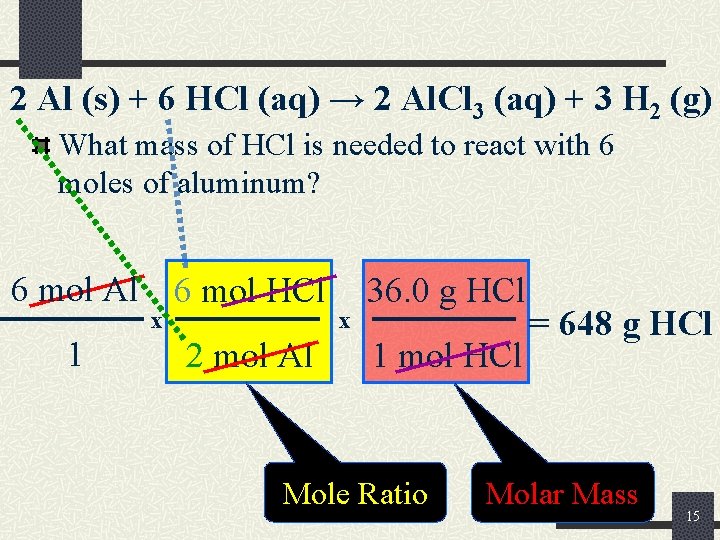
2 Al (s) + 6 HCl (aq) → 2 Al. Cl 3 (aq) + 3 H 2 (g) What mass of HCl is needed to react with 6 moles of aluminum? 6 mol Al 6 mol HCl x 1 2 mol Al x 36. 0 g HCl 1 mol HCl Mole Ratio = 648 g HCl Molar Mass 15

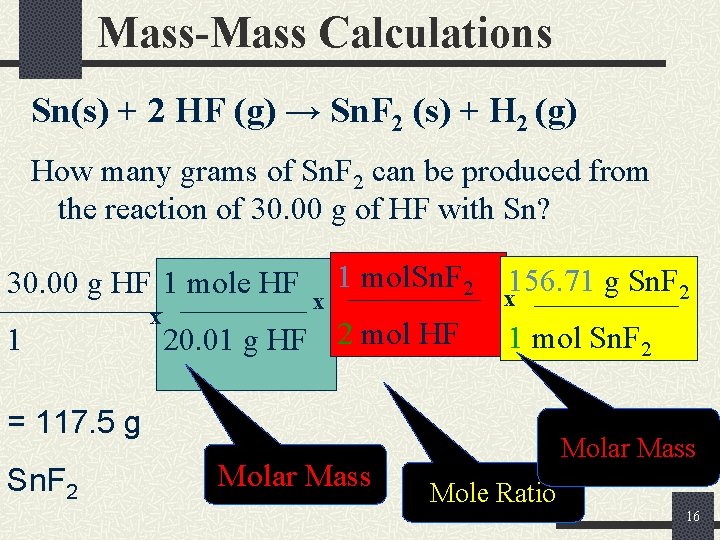
Mass-Mass Calculations Sn(s) + 2 HF (g) → Sn. F 2 (s) + H 2 (g) How many grams of Sn. F 2 can be produced from the reaction of 30. 00 g of HF with Sn? 30. 00 g HF 1 mole HF 1 x x 1 mol. Sn. F 2 20. 01 g HF 2 mol HF 156. 71 g Sn. F 2 x 1 mol Sn. F 2 = 117. 5 g Sn. F 2 Molar Mass Mole Ratio 16

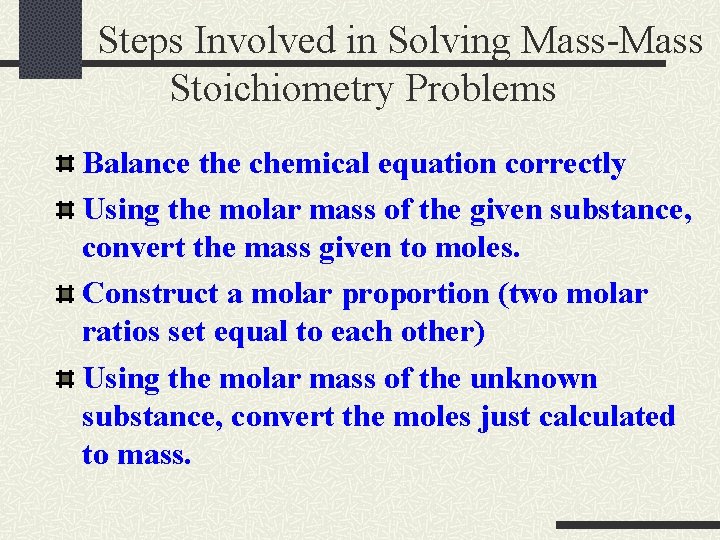
Steps Involved in Solving Mass-Mass Stoichiometry Problems Balance the chemical equation correctly Using the molar mass of the given substance, convert the mass given to moles. Construct a molar proportion (two molar ratios set equal to each other) Using the molar mass of the unknown substance, convert the moles just calculated to mass.
 Mole ratio definition
Mole ratio definition Molemass
Molemass Stoichiometric calculations
Stoichiometric calculations Are kc and kp equal
Are kc and kp equal Translate word equations to chemical equations
Translate word equations to chemical equations Structural steel connection calculations calculations
Structural steel connection calculations calculations Chemical rxns/balancing equ./stoichiometry
Chemical rxns/balancing equ./stoichiometry In a chemical reaction, stoichiometry refers to
In a chemical reaction, stoichiometry refers to Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Chemical accounting stoichiometry
Chemical accounting stoichiometry Chemical accounting stoichiometry
Chemical accounting stoichiometry Stoichiometry map for chemical reactions
Stoichiometry map for chemical reactions Definition of fermentation
Definition of fermentation The calculation of quantities in chemical equations
The calculation of quantities in chemical equations Kwl chart of chemical formula
Kwl chart of chemical formula Calculations using balanced equations are called
Calculations using balanced equations are called Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers