Stoichiometry I MoleMole What is Stoichiometry The word








- Slides: 13

Stoichiometry I Mole-Mole


What is Stoichiometry? The word stoichiometry derives from two Greek words: stoicheion (meaning "element") and metron (meaning "measure"). +

What you Should Expect… The most common stoichiometric problem will present you with a certain amount of a reactant and then ask how much of a product can be formed.

Why do we use stoichiometry? Chemists use stoichiometry to determine quantities of chemicals needed, and to predict the quantities that will be produced for any chemical reaction.

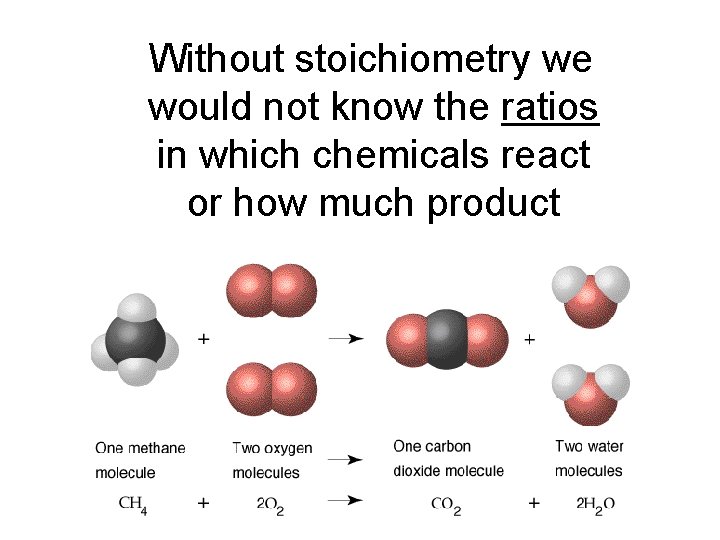
Without stoichiometry we would not know the ratios in which chemicals react or how much product they will produce.

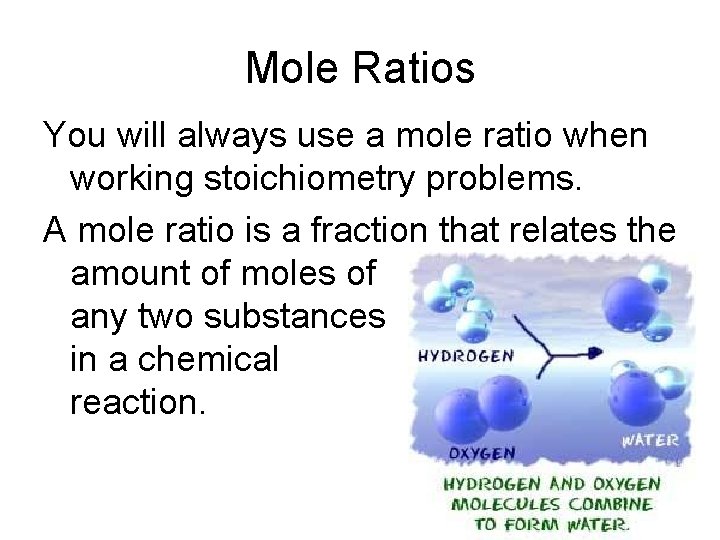
Mole Ratios You will always use a mole ratio when working stoichiometry problems. A mole ratio is a fraction that relates the amount of moles of any two substances in a chemical reaction.

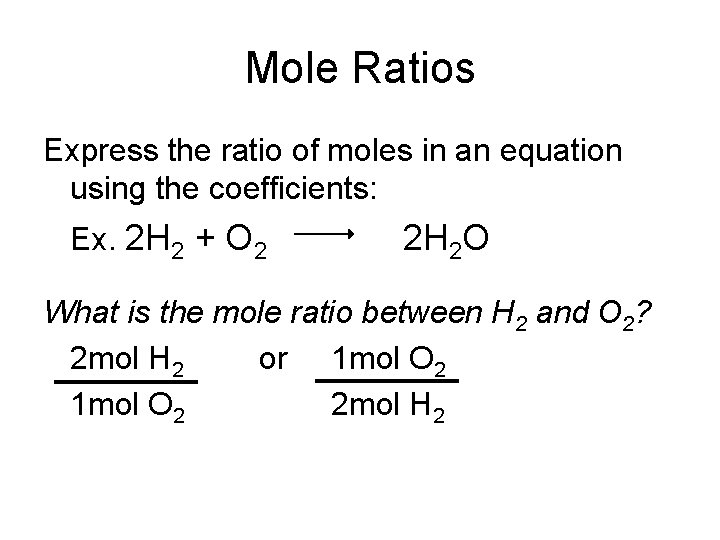
Mole Ratios Express the ratio of moles in an equation using the coefficients: Ex. 2 H 2 + O 2 2 H 2 O What is the mole ratio between H 2 and O 2? 2 mol H 2 or 1 mol O 2 2 mol H 2

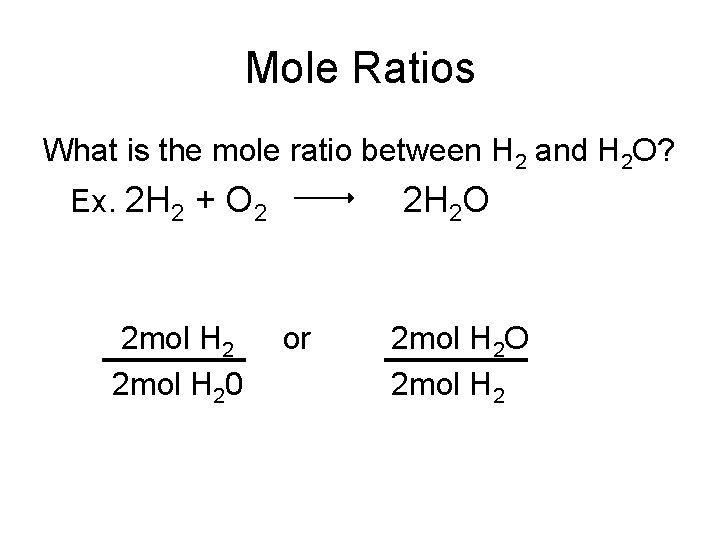
Mole Ratios What is the mole ratio between H 2 and H 2 O? Ex. 2 H 2 + O 2 2 mol H 20 2 H 2 O or 2 mol H 2 O 2 mol H 2

2 O 3 3 O 2 What is the mole ratio between O 3 and O 2? 3 mol O 2 2 mol O 3 or 2 mol O 3 3 mol O 2

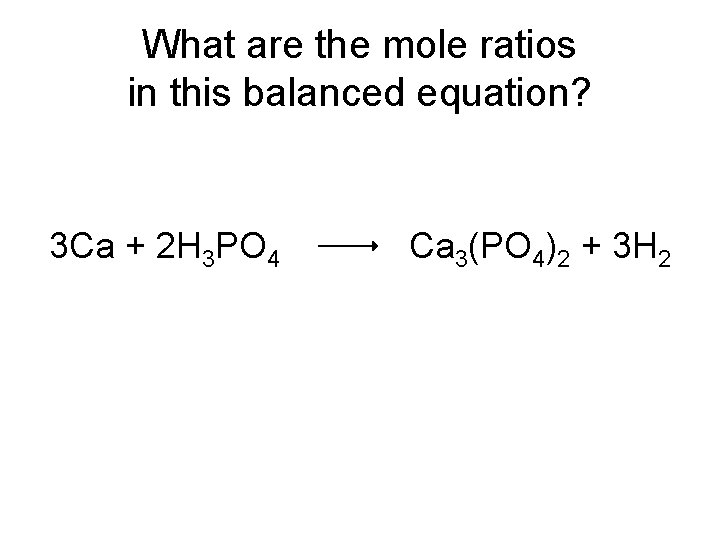
What are the mole ratios in this balanced equation? 3 Ca + 2 H 3 PO 4 Ca 3(PO 4)2 + 3 H 2

Mole to Mole Problems: 1. Write correct chemical formulas 2. Write and balance the equation 3. Convert everything to moles (sometimes it is already in moles) 4. Use mole ratio to solve for what you are trying to find 5. Convert everything into the required unit (if needed).

For Example How many moles of calcium chloride will be produced if you start with 5 moles of HCl? Ca(OH)2 + HCl Ca. Cl 2 + H 2 O