Ch 9 Calculations from Chemical Equations Stoichiometry MoleMole

- Slides: 11

Ch. 9: Calculations from Chemical Equations Stoichiometry Mole-Mole Calculations Mole-Mass Calculations Mass-Mass Calculations 1

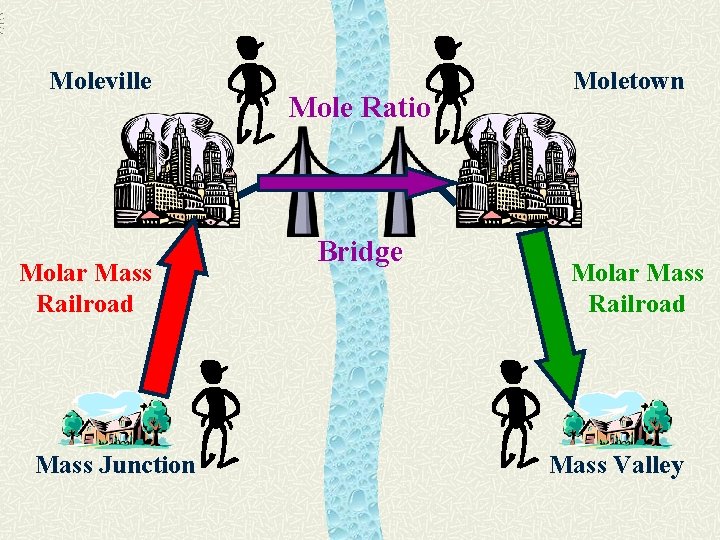
Moleville Molar Mass Railroad Mass Junction Mole Ratio Bridge Moletown Molar Mass Railroad Mass Valley

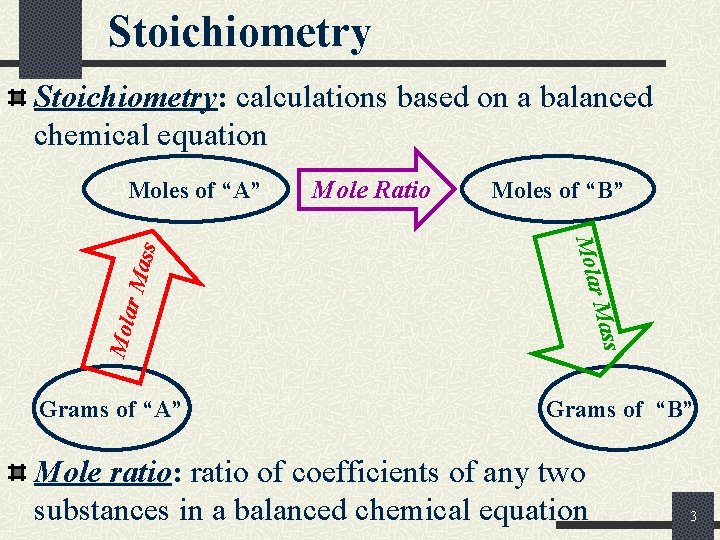
Stoichiometry: calculations based on a balanced chemical equation s r Ma Moles of “B” r Mas Grams of “A” Mole Ratio Mola ss Moles of “A” Grams of “B” Mole ratio: ratio of coefficients of any two substances in a balanced chemical equation 3

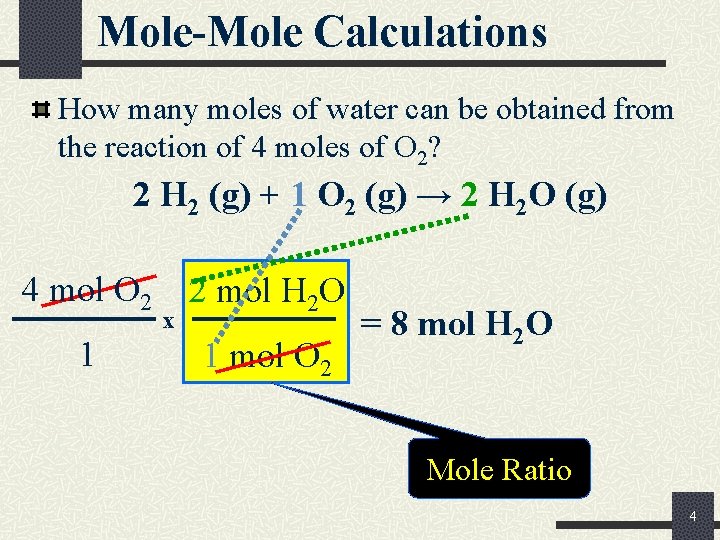
Mole-Mole Calculations How many moles of water can be obtained from the reaction of 4 moles of O 2? 2 H 2 (g) + 1 O 2 (g) → 2 H 2 O (g) 4 mol O 2 1 x 2 mol H 2 O 1 mol O 2 = 8 mol H 2 O Mole Ratio 4

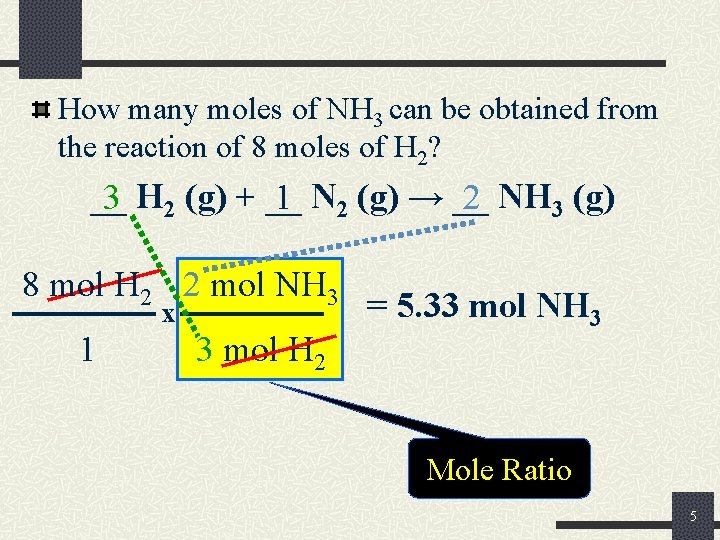
How many moles of NH 3 can be obtained from the reaction of 8 moles of H 2? __ 3 H 2 (g) + __ 1 N 2 (g) → __ 2 NH 3 (g) 8 mol H 2 2 mol NH 3 x 1 3 mol H 2 = 5. 33 mol NH 3 Mole Ratio 5

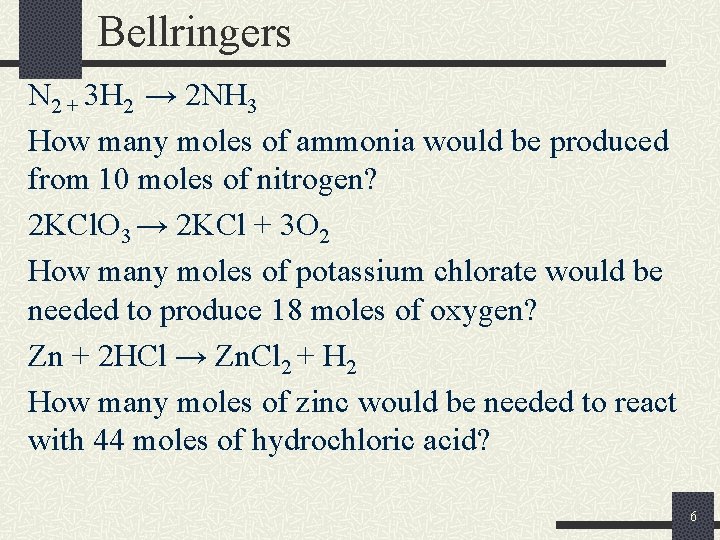
Bellringers N 2 + 3 H 2 → 2 NH 3 How many moles of ammonia would be produced from 10 moles of nitrogen? 2 KCl. O 3 → 2 KCl + 3 O 2 How many moles of potassium chlorate would be needed to produce 18 moles of oxygen? Zn + 2 HCl → Zn. Cl 2 + H 2 How many moles of zinc would be needed to react with 44 moles of hydrochloric acid? 6

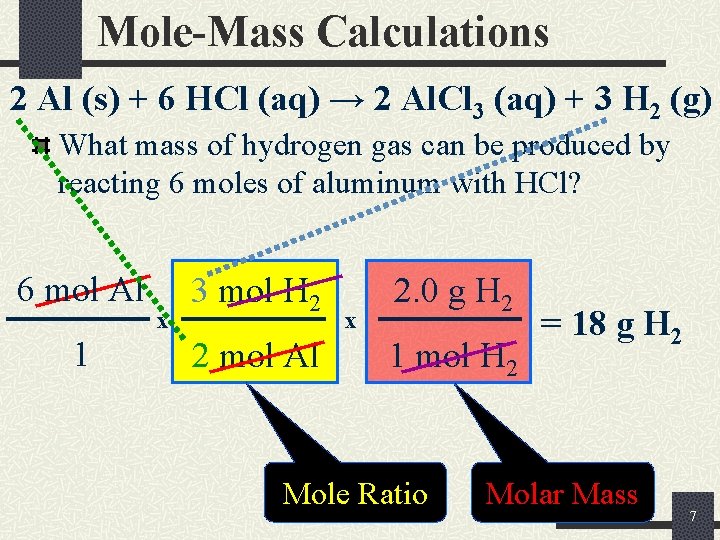
Mole-Mass Calculations 2 Al (s) + 6 HCl (aq) → 2 Al. Cl 3 (aq) + 3 H 2 (g) What mass of hydrogen gas can be produced by reacting 6 moles of aluminum with HCl? 6 mol Al 1 x 3 mol H 2 2 mol Al x 2. 0 g H 2 1 mol H 2 Mole Ratio = 18 g H 2 Molar Mass 7

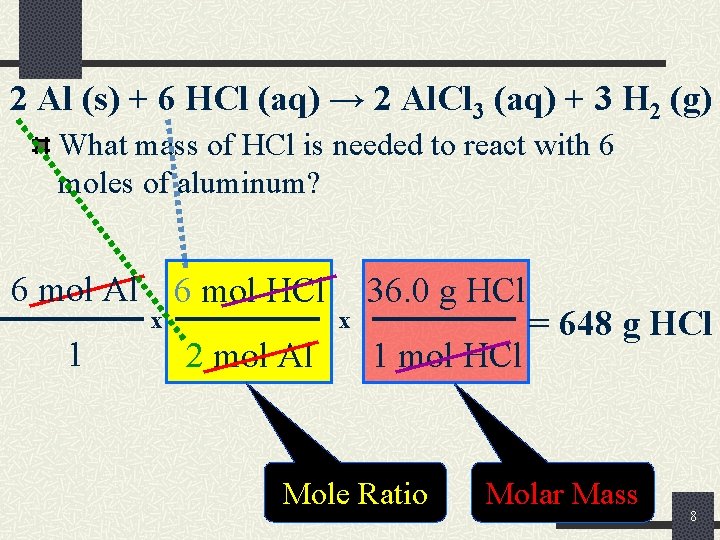
2 Al (s) + 6 HCl (aq) → 2 Al. Cl 3 (aq) + 3 H 2 (g) What mass of HCl is needed to react with 6 moles of aluminum? 6 mol Al 6 mol HCl x 1 2 mol Al x 36. 0 g HCl 1 mol HCl Mole Ratio = 648 g HCl Molar Mass 8

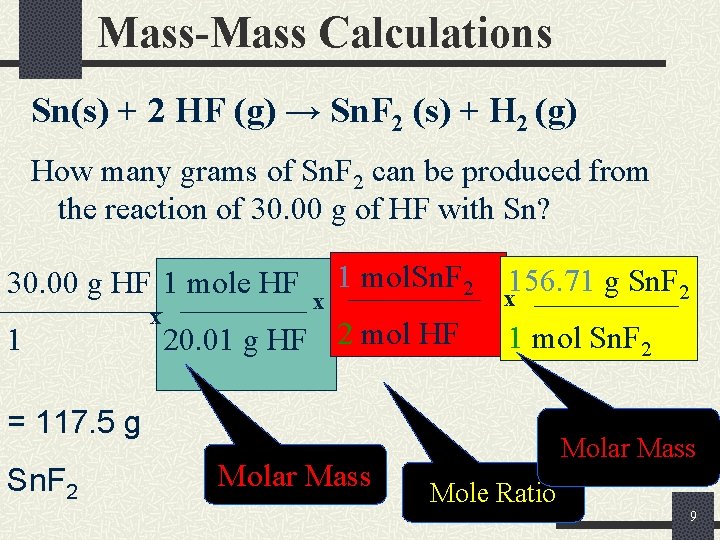
Mass-Mass Calculations Sn(s) + 2 HF (g) → Sn. F 2 (s) + H 2 (g) How many grams of Sn. F 2 can be produced from the reaction of 30. 00 g of HF with Sn? 30. 00 g HF 1 mole HF 1 x x 1 mol. Sn. F 2 20. 01 g HF 2 mol HF 156. 71 g Sn. F 2 x 1 mol Sn. F 2 = 117. 5 g Sn. F 2 Molar Mass Mole Ratio 9

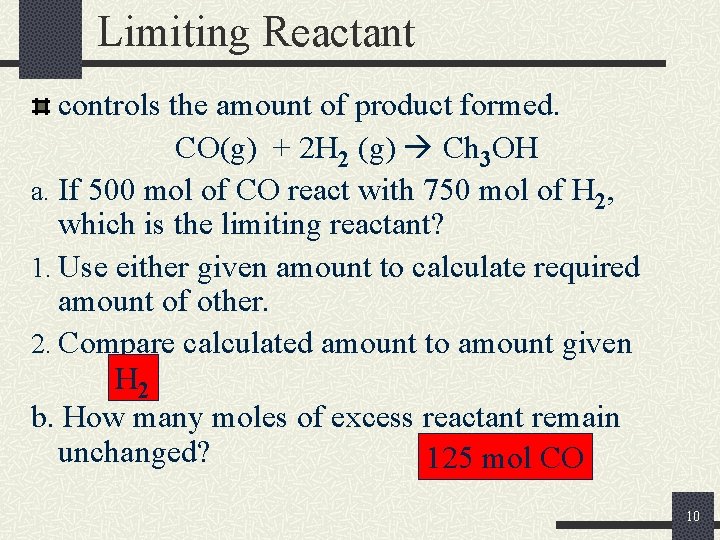
Limiting Reactant controls the amount of product formed. CO(g) + 2 H 2 (g) Ch 3 OH a. If 500 mol of CO react with 750 mol of H 2, which is the limiting reactant? 1. Use either given amount to calculate required amount of other. 2. Compare calculated amount to amount given H 2 b. How many moles of excess reactant remain unchanged? 125 mol CO 10

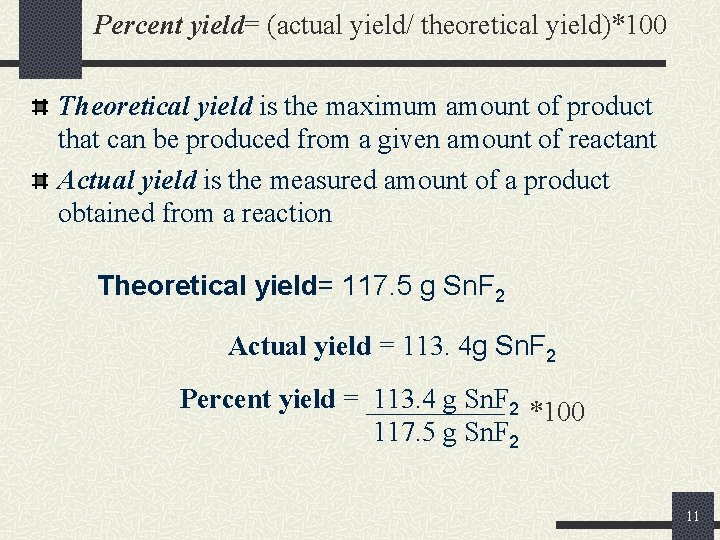
Percent yield= (actual yield/ theoretical yield)*100 Theoretical yield is the maximum amount of product that can be produced from a given amount of reactant Actual yield is the measured amount of a product obtained from a reaction Theoretical yield= 117. 5 g Sn. F 2 Actual yield = 113. 4 g Sn. F 2 Percent yield = 113. 4 g Sn. F 2 *100 117. 5 g Sn. F 2 11