Mole Calculations 1 Chemical Calculations Atoms and molecules









- Slides: 16

Mole Calculations 1

Chemical Calculations Atoms and molecules are extremely small. If they are so small and so light, how can we weigh them? We weigh large numbers of them.

Avogadro took 1. 00 g of the smallest atom (H) and determined how many H atoms there are in 1. 00 g of H. He found that: 1. 00 g H = 6. 02 x 1023 atoms = 1. 00 mole This is called Avogadro’s number

1 dozen donuts = 12 donuts 1 century 1 millennium 1. 00 mole = 100 years = 1000 years = 6. 02 x 1023 particles

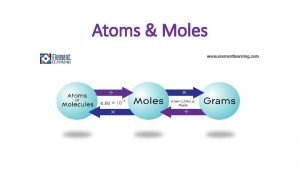
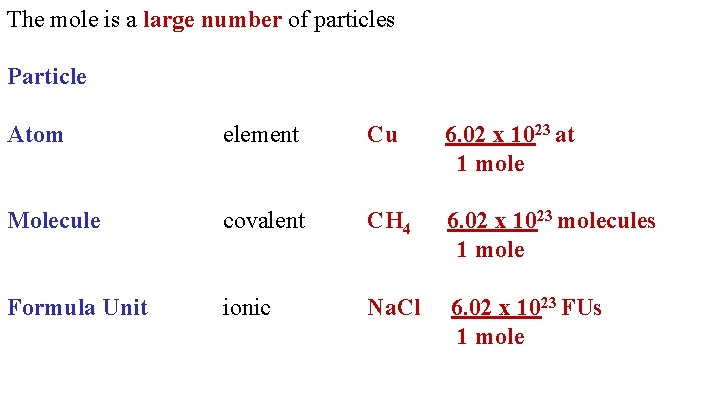
The mole is a large number of particles Particle Atom element Cu 6. 02 x 1023 at 1 mole Molecule covalent CH 4 6. 02 x 1023 molecules 1 mole Formula Unit ionic Na. Cl 6. 02 x 1023 FUs 1 mole

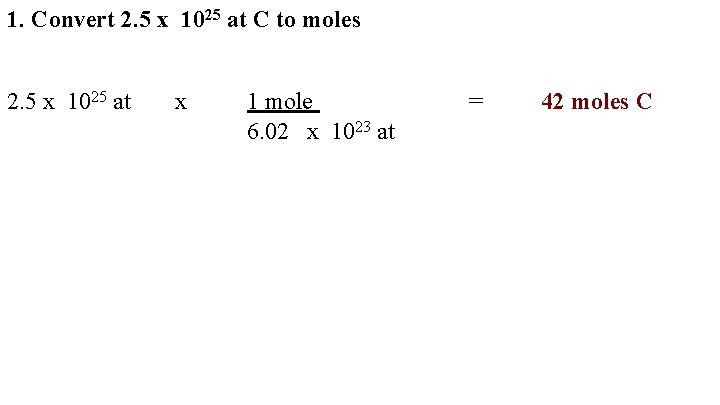
1. Convert 2. 5 x 1025 at C to moles 2. 5 x 1025 at x 1 mole 6. 02 x 1023 at = 42 moles C

2. Convert 16. 3 moles CO 2 to molecules 16. 3 moles x 6. 02 x 1023 molecules 1 mole = 9. 81 x 1024 molecules

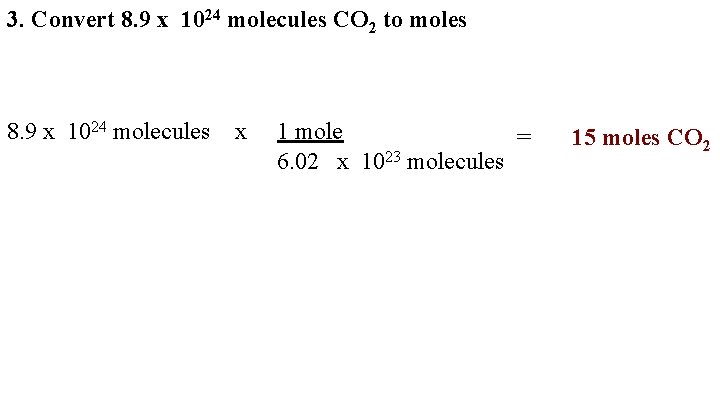
3. Convert 8. 9 x 1024 molecules CO 2 to moles 8. 9 x 1024 molecules x 1 mole = 6. 02 x 1023 molecules 15 moles CO 2

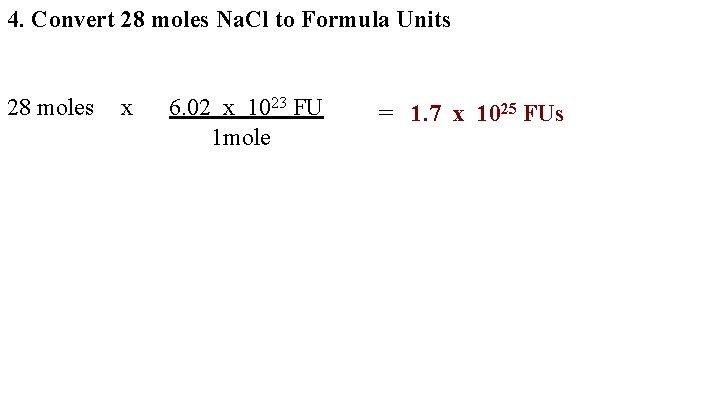
4. Convert 28 moles Na. Cl to Formula Units 28 moles x 6. 02 x 1023 FU 1 mole = 1. 7 x 1025 FUs

Determining Avogadro’s Number

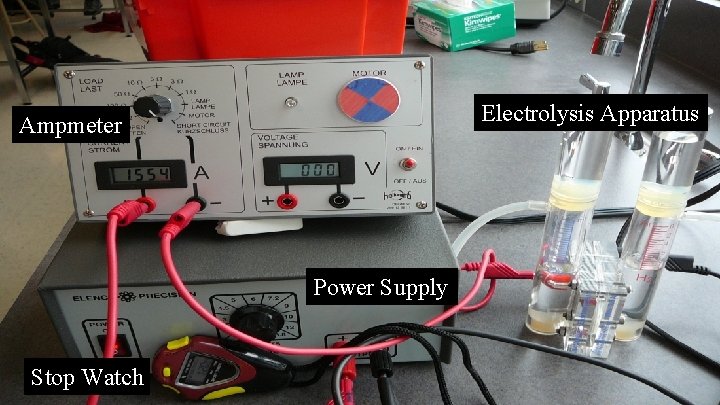
Electrolysis Apparatus Ampmeter Power Supply Stop Watch

Determining Avogadro’s Number Produce a volume of hydrogen gas while measuring the time and electrical current. Volume of H 2 Time Current 10. 0 m. L 80. 7 s 0. 913 amp

Background information 1 amp is defined as the number of coulombs per second. There are 6. 24 x 1018 electrons in a coulomb. The density of H 2 is 0. 07871 g/L. It takes 1 electron to make 1 H atom

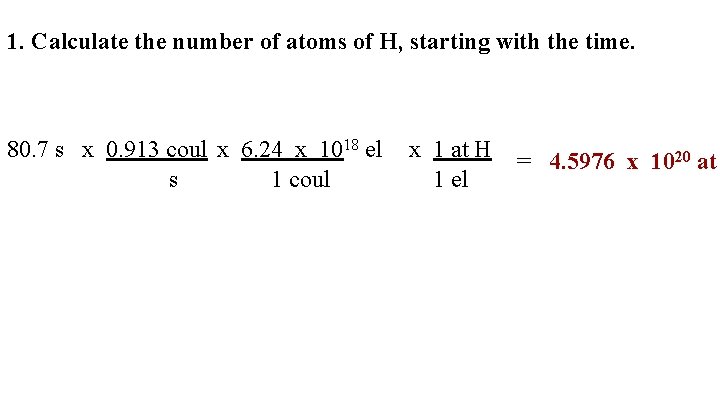
1. Calculate the number of atoms of H, starting with the time. 80. 7 s x 0. 913 coul x 6. 24 x 1018 el s 1 coul x 1 at H 1 el = 4. 5976 x 1020 at

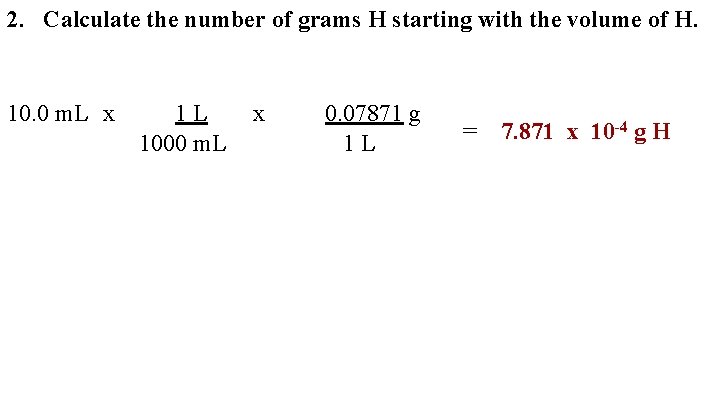
2. Calculate the number of grams H starting with the volume of H. 10. 0 m. L x 1 L 1000 m. L x 0. 07871 g 1 L = 7. 871 x 10 -4 g H

3. Divide the atoms of H by the grams of H to get the number of H atoms in a gram which is Avogadro’s number. 4. 5976 x 1020 at 7. 871 x 10 -4 g H = 5. 84 x 1023 at/ 1 gram H = 5. 84 x 1023 at/ 1 mole The Mole Song Avogadro Facts
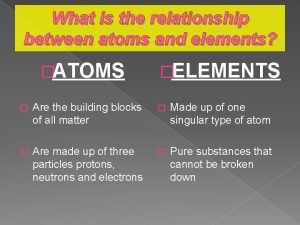
 What is the relationship between atoms and elements
What is the relationship between atoms and elements Mixture of elements
Mixture of elements 3bacl2 counting atoms
3bacl2 counting atoms Ion chapter 11
Ion chapter 11 Atoms molecules and ions
Atoms molecules and ions Atoms molecules and ions
Atoms molecules and ions Atoms molecules and ions
Atoms molecules and ions Atoms ions and molecules
Atoms ions and molecules Atoms ions and molecules
Atoms ions and molecules Collision theory states that
Collision theory states that Chapter 2 atoms molecules and ions
Chapter 2 atoms molecules and ions Organic molecules vs inorganic molecules
Organic molecules vs inorganic molecules Mole mass and mole volume relationships
Mole mass and mole volume relationships Why do atoms combine to form molecules
Why do atoms combine to form molecules Compared to atoms of metals, atoms of nonmetals generally
Compared to atoms of metals, atoms of nonmetals generally Atoms to mole
Atoms to mole Stoichiometry: mole-mole problems
Stoichiometry: mole-mole problems