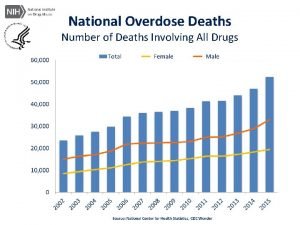
Cancer Cancer accounted for 7 1 million deaths






- Slides: 38

Cancer �Cancer accounted for 7. 1 million deaths world-wide (12. 5%). �Ranks as 3 of the top 10 leading causes of death world wide. � 11 million are diagnosed with cancer each year and by 2020 the World health organisation expects this rise to 16 million. � Second cause of death in the West (after cardiovascular diseases). Sources: WHO and Cancer Research UK

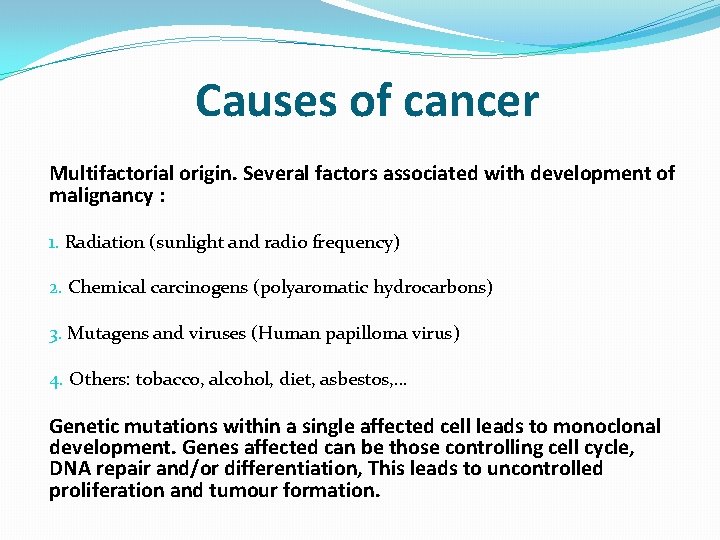
Causes of cancer Multifactorial origin. Several factors associated with development of malignancy : 1. Radiation (sunlight and radio frequency) 2. Chemical carcinogens (polyaromatic hydrocarbons) 3. Mutagens and viruses (Human papilloma virus) 4. Others: tobacco, alcohol, diet, asbestos, … Genetic mutations within a single affected cell leads to monoclonal development. Genes affected can be those controlling cell cycle, DNA repair and/or differentiation, This leads to uncontrolled proliferation and tumour formation.

Causes of cancer � 30% of cancer is due to smoking. � 30% of cancer cases is diet related. � 15% of cases are viral related infections: �Papilloma virus… sexually transmitted… cause cervical cancer. �Hepatitis-B is the cause of 80% of liver cancer. �Some are bacteria related: �H. pylori…. Leads to stomach cancer.

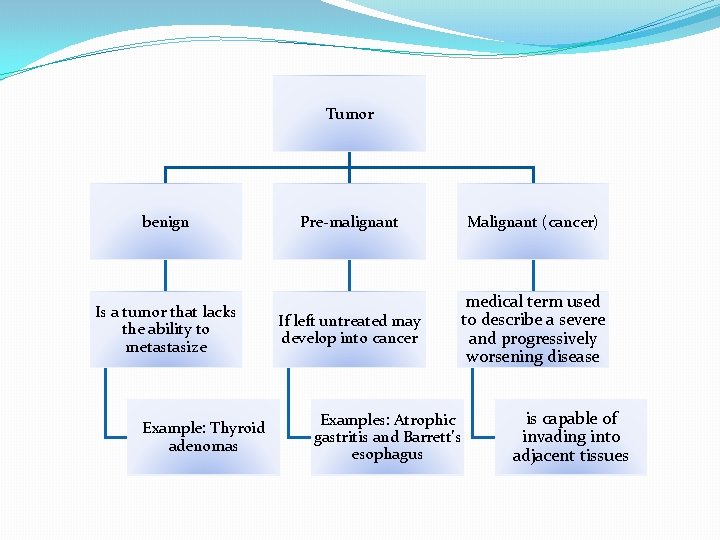
Tumor benign Is a tumor that lacks the ability to metastasize Example: Thyroid adenomas Pre-malignant Malignant (cancer) If left untreated may develop into cancer medical term used to describe a severe and progressively worsening disease Examples: Atrophic gastritis and Barrett’s esophagus is capable of invading into adjacent tissues

Terminology – Cancer types • Leukaemias are cancers of the blood or bone marrow. • Sarcomas are cancers of the connective or supportive tissue (bone, cartilage, fat, muscle, blood vessels) and soft tissue. • Carcinomas are cancers that arises from epithelial cells. These include breast, liver, lung, stomach etc. • Cancer metastasis is the spread of cancer from the primary location to a secondary location.

Terminology – Side effects • Neutropenia (or neutropaenia is a hematological disorder characterized by an abnormally low number of neutrophil granulocytes (a type of white blood cell). • Myelosuppression is a decrease in the production of blood cells. (Red blood cells and platelets). • Ototoxicity is damage of the ear, specifically the cochlea or auditory nerve. • Nephrotoxicity is kidney damage. Results in decreased kidney functions.

Terminology – Side effects • Hepatotoxicity is liver damage. Results in decreased liver function. • Neuropathy is usually short for peripheral neuropathy, and means a damage to peripheral nerve(s). • Hypomagnesaemia is an abnormally low level of magnesium in blood serum.

Treatment �Course of treatment will depend on the type of cancer, progress of the disease, available treatment options and patients choice: • Surgery • Radiation • Chemotherapy (including combination therapy) • Gene therapy • Natural products/herbal

Problems with chemotherapy �Treatments are non-specific, attack healthy cells as well as normal cells since cancer cells are derived from normal cells. �Cancers can develop resistance: for example with platinum-drugs, cancer cells became resistant by many ways: � Decreased drug uptake/increased efflux � Enhanced tolerance of DNA adducts � Enhanced repair of DNA adducts � Increased drug deactivation by intracellular glutathione

Cancer treatment �By surgery. �By radiation. �By anticancer drugs (cytotoxic agents): v Cytotoxic drugs of plant origin v Cytotoxic drugs of microbial origin v Antimetabolites v Alkylating agents v Platinum-based compounds

Ideal cytotoxic drugs should: �Selectively target cancer cells without causing damage to normal cells. �Reduce size of tumors + minimize risks of metastases. v unfortunately, most of the available agents are not selective, they also affect rapidly-proliferating normal tissues (bone marrow, gastro intestinal epithelium, hair cells, …), causing serious side-effects (bone marrow suppression, nausea, vomiting, …).

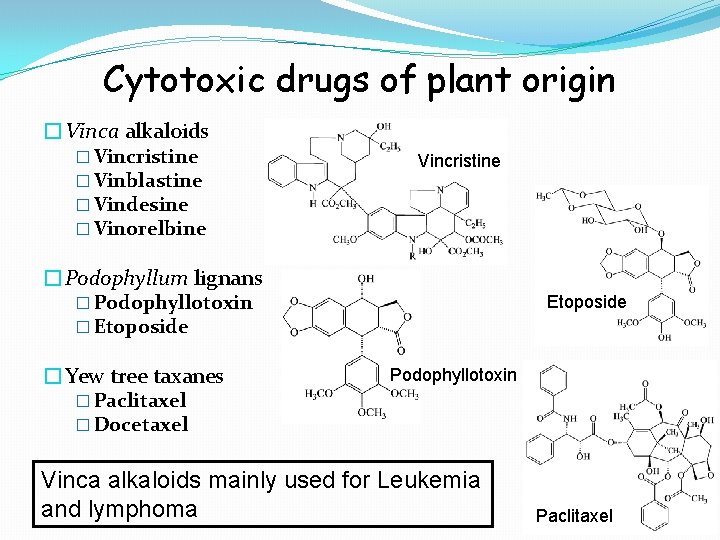
Cytotoxic drugs of plant origin �Vinca alkaloids � Vincristine � Vinblastine � Vindesine � Vinorelbine Vincristine �Podophyllum lignans � Podophyllotoxin � Etoposide �Yew tree taxanes Etoposide Podophyllotoxin � Paclitaxel � Docetaxel Vinca alkaloids mainly used for Leukemia and lymphoma Paclitaxel

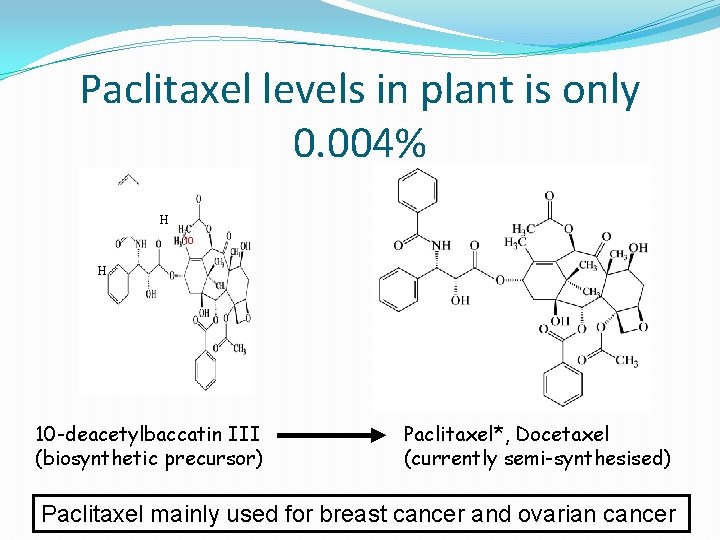
Paclitaxel levels in plant is only 0. 004% H 10 -deacetylbaccatin III (biosynthetic precursor) Paclitaxel*, Docetaxel (currently semi-synthesised) Paclitaxel mainly used for breast cancer and ovarian cancer

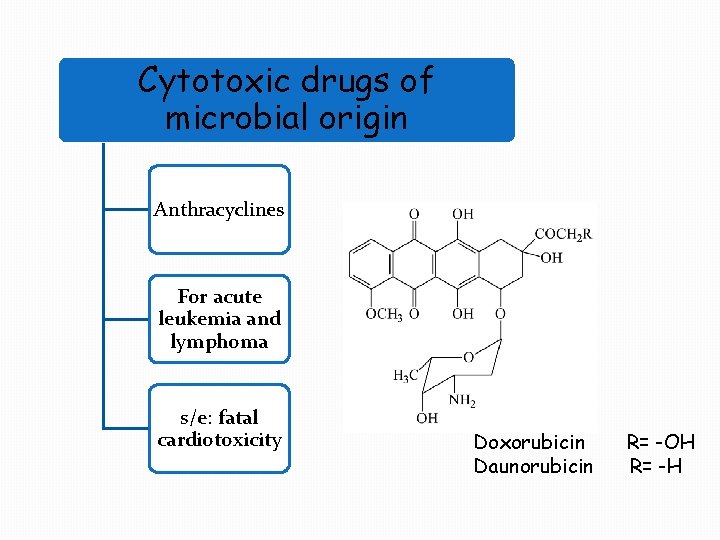
Cytotoxic drugs of microbial origin Anthracyclines For acute leukemia and lymphoma s/e: fatal cardiotoxicity Doxorubicin Daunorubicin R= -OH R= -H

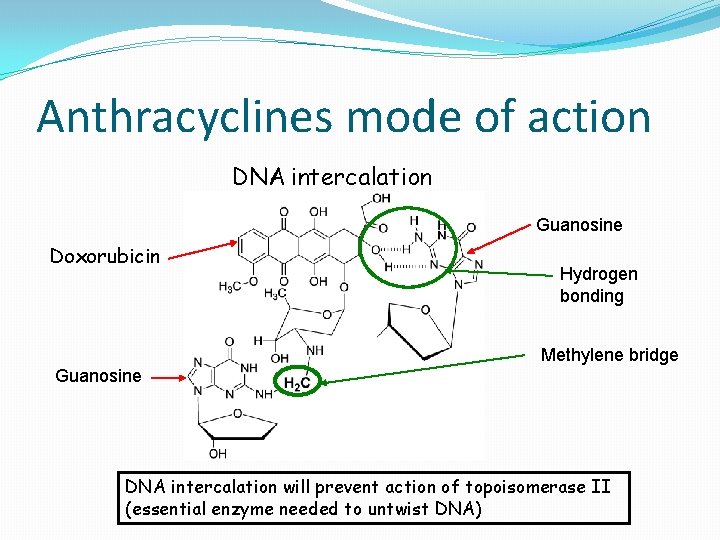
Anthracyclines mode of action DNA intercalation Guanosine Doxorubicin Hydrogen bonding Methylene bridge Guanosine DNA intercalation will prevent action of topoisomerase II (essential enzyme needed to untwist DNA)

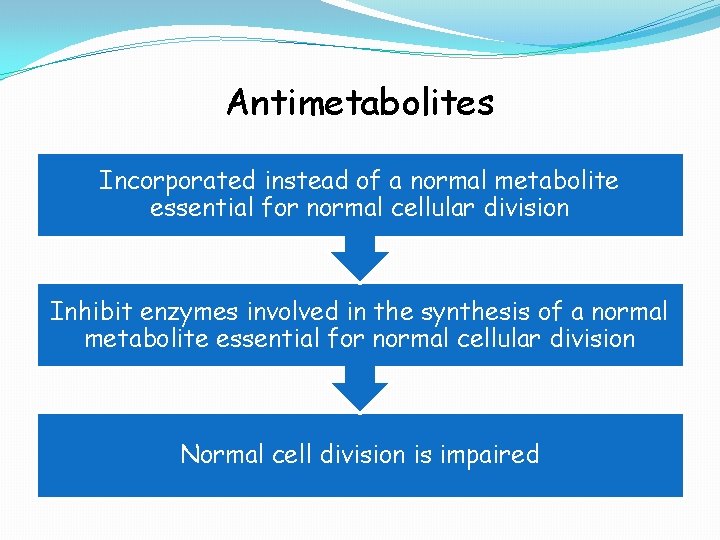
Antimetabolites Incorporated instead of a normal metabolite essential for normal cellular division Inhibit enzymes involved in the synthesis of a normal metabolite essential for normal cellular division Normal cell division is impaired

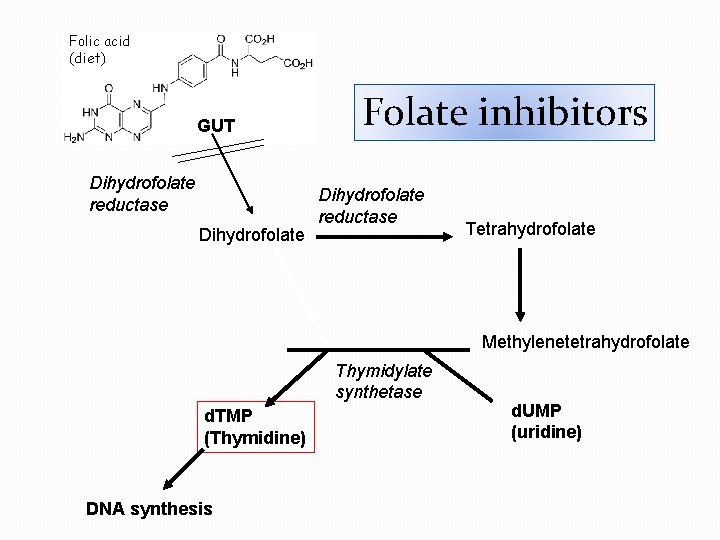
Folic acid (diet) GUT Dihydrofolate reductase Dihydrofolate Folate inhibitors Dihydrofolate reductase Tetrahydrofolate Methylenetetrahydrofolate Thymidylate synthetase d. TMP (Thymidine) DNA synthesis d. UMP (uridine)

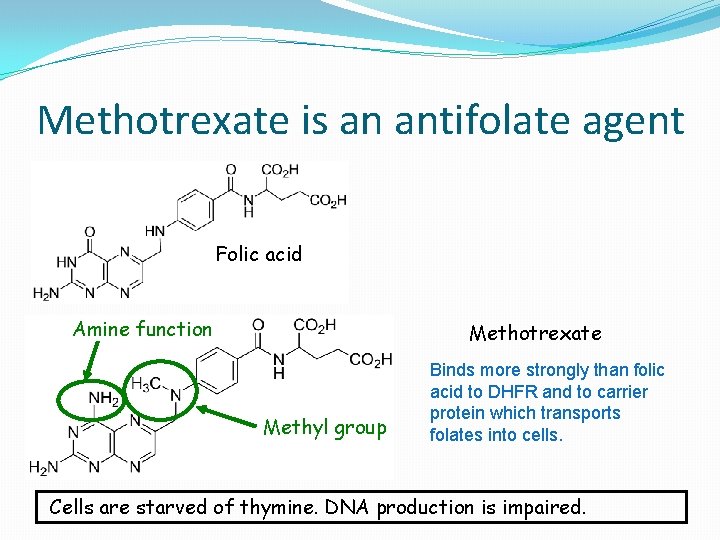
Methotrexate is an antifolate agent Folic acid Amine function Methotrexate Methyl group Binds more strongly than folic acid to DHFR and to carrier protein which transports folates into cells. Cells are starved of thymine. DNA production is impaired.

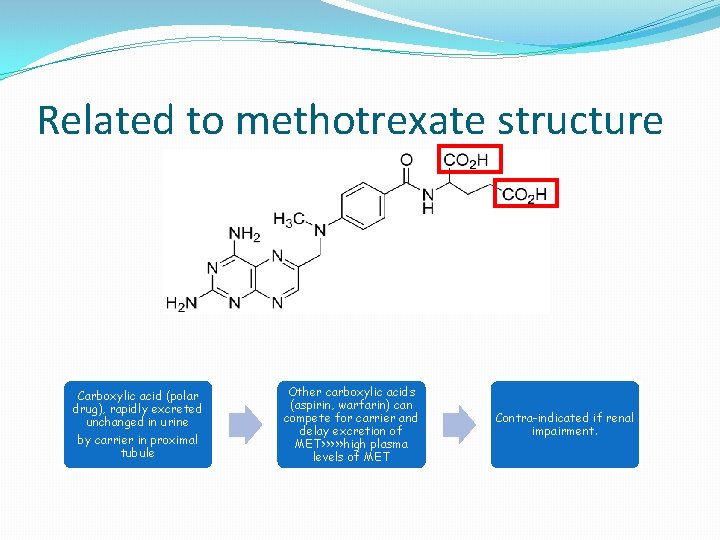
Related to methotrexate structure Carboxylic acid (polar drug), rapidly excreted unchanged in urine by carrier in proximal tubule Other carboxylic acids (aspirin, warfarin) can compete for carrier and delay excretion of MET>>>>>high plasma levels of MET Contra-indicated if renal impairment.

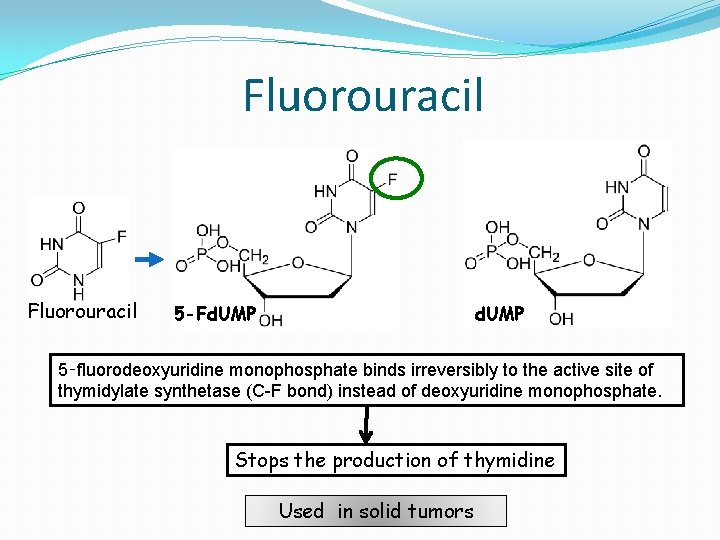
Fluorouracil d. UMP 5 -Fd. UMP 5‑fluorodeoxyuridine monophosphate binds irreversibly to the active site of thymidylate synthetase (C-F bond) instead of deoxyuridine monophosphate. Stops the production of thymidine Used in solid tumors

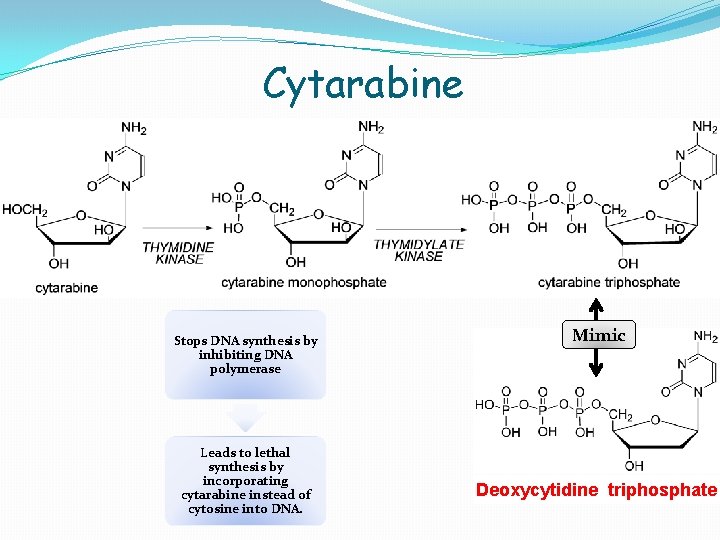
Cytarabine Stops DNA synthesis by inhibiting DNA polymerase Leads to lethal synthesis by incorporating cytarabine instead of cytosine into DNA. Mimic Deoxycytidine triphosphate

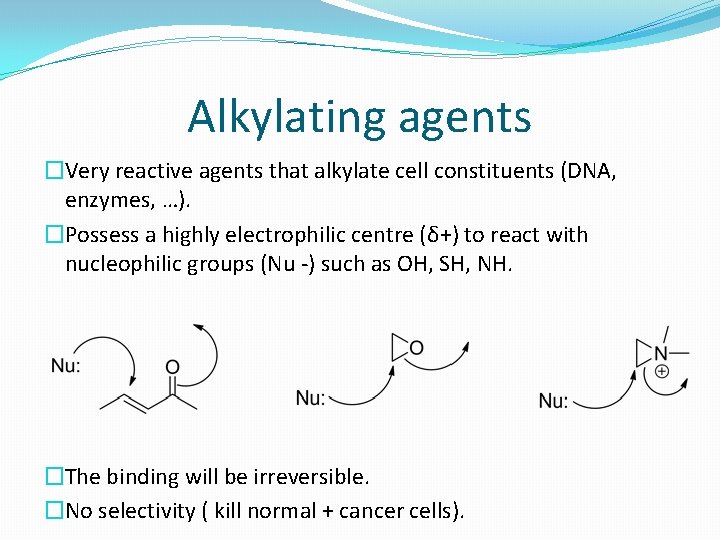
Alkylating agents �Very reactive agents that alkylate cell constituents (DNA, enzymes, …). �Possess a highly electrophilic centre (δ+) to react with nucleophilic groups (Nu -) such as OH, SH, NH. �The binding will be irreversible. �No selectivity ( kill normal + cancer cells).

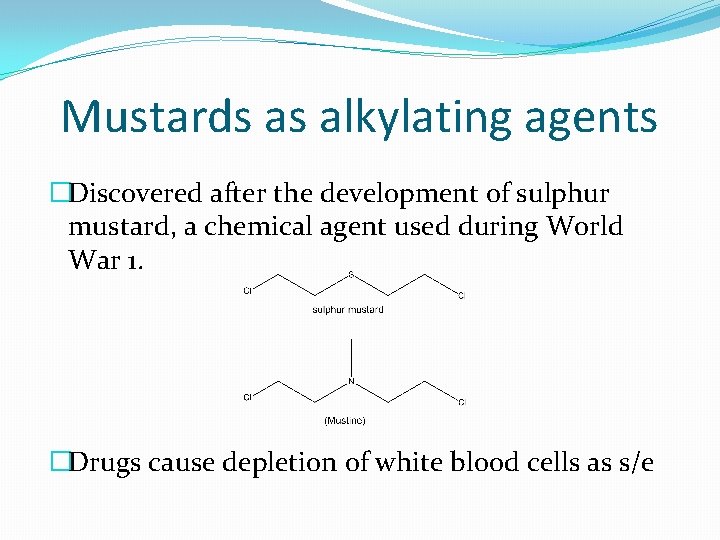
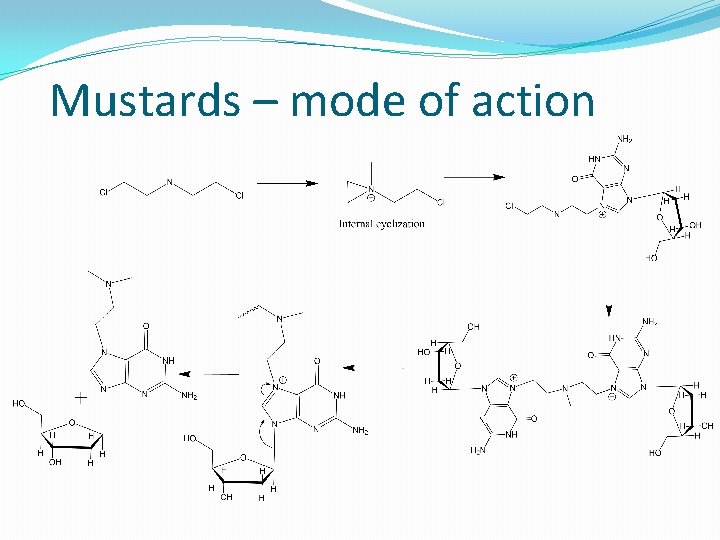
Mustards as alkylating agents �Discovered after the development of sulphur mustard, a chemical agent used during World War 1. �Drugs cause depletion of white blood cells as s/e

Mustards – mode of action

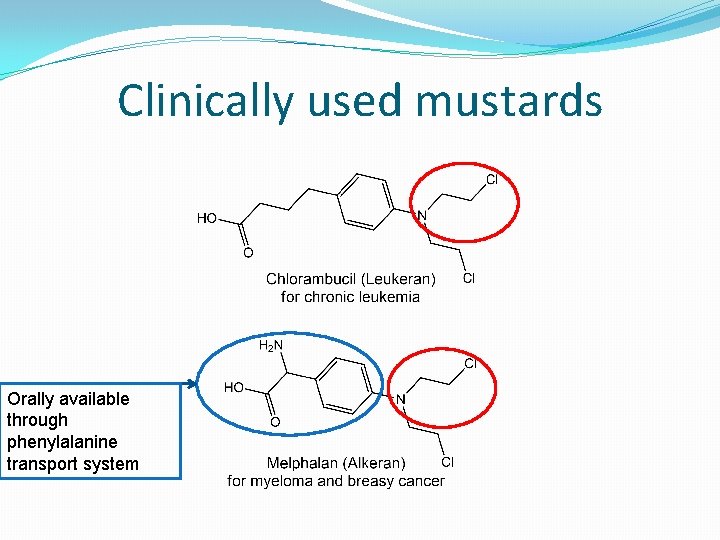
Clinically used mustards Orally available through phenylalanine transport system

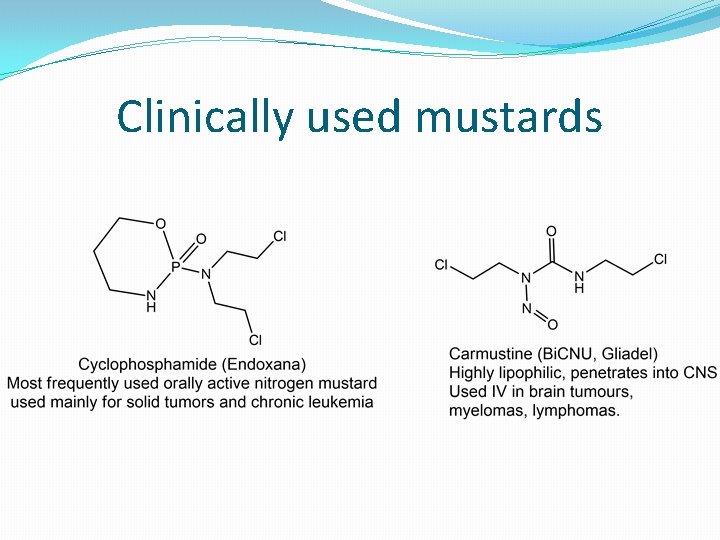
Clinically used mustards

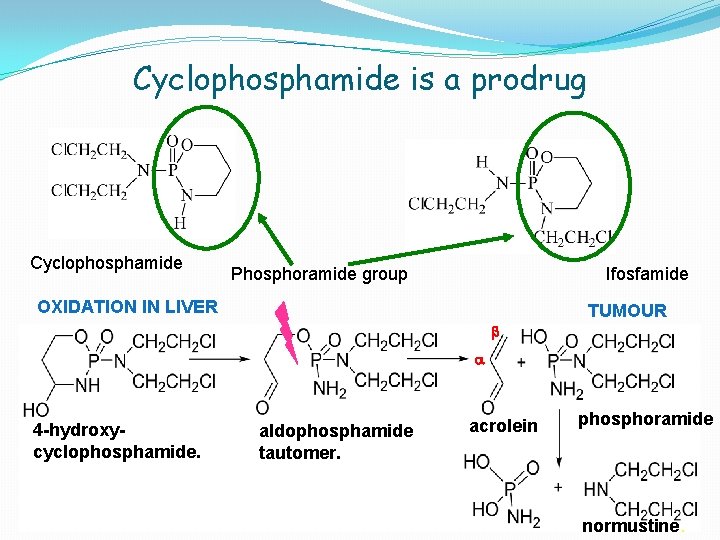
Cyclophosphamide is a prodrug Cyclophosphamide Phosphoramide group Ifosfamide OXIDATION IN LIVER TUMOUR b a 4 -hydroxycyclophosphamide. aldophosphamide tautomer. acrolein phosphoramide normustine.

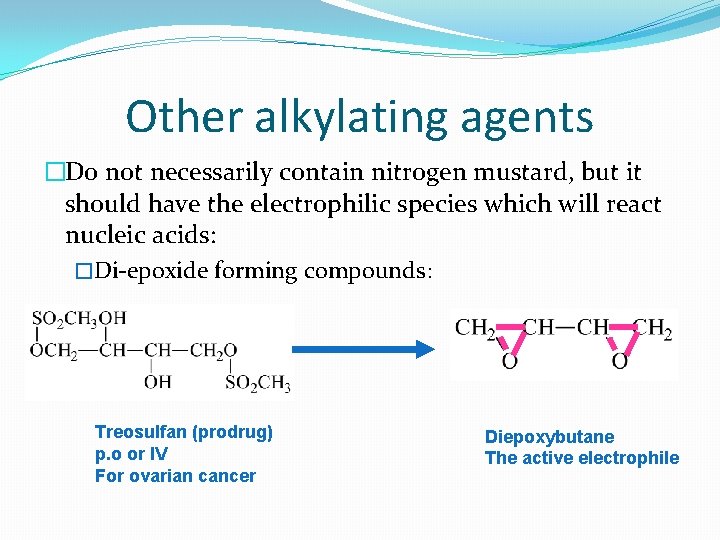
Other alkylating agents �Do not necessarily contain nitrogen mustard, but it should have the electrophilic species which will react nucleic acids: �Di-epoxide forming compounds: Treosulfan (prodrug) p. o or IV For ovarian cancer Diepoxybutane The active electrophile

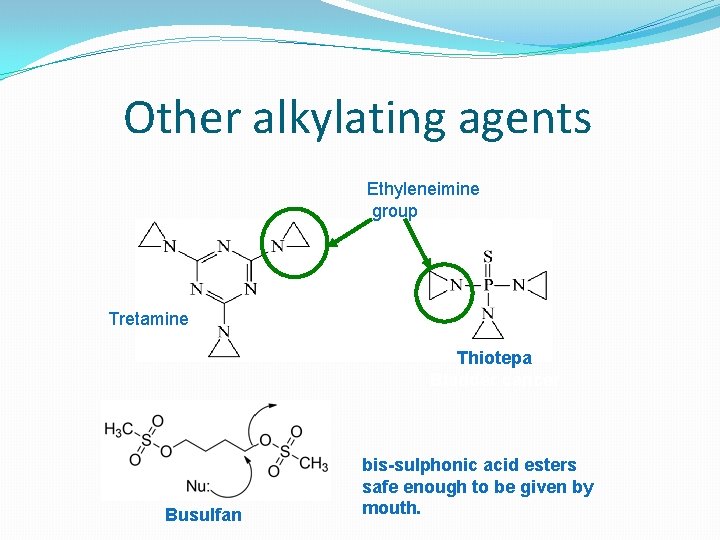
Other alkylating agents Ethyleneimine group Tretamine Thiotepa Bladder cancer Busulfan bis-sulphonic acid esters safe enough to be given by mouth.

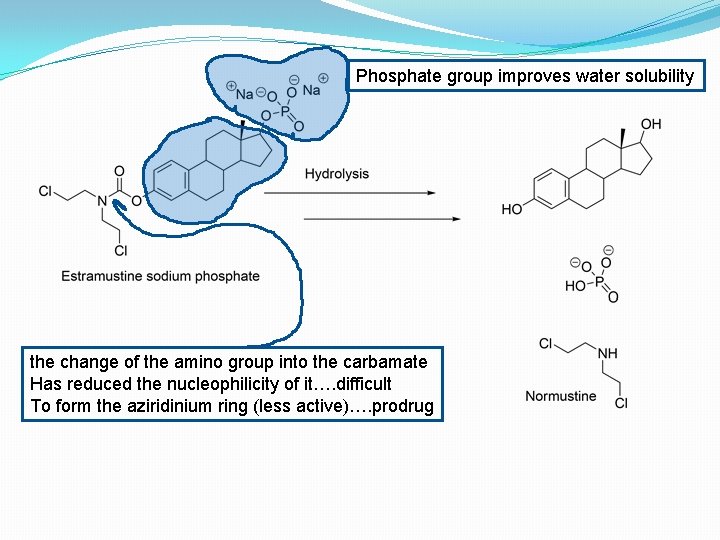
site-specific delivery of cytotoxic Mustine alkylating agents �Prostate tumour cells have abundant oestrogen receptor sites, and complexation of an Estradiol carrier with normustine enables accumulation of the complex within the tumour. The phosphate group provides water solubility, and the Carbamate linkage between the Mustine and the Estradiol deactivates the nitrogen lone pair through resonance stabilization, rendering the alkylating agent inactive when in the complex. Enzymatic hydrolysis in the tumour cell releases the active drug at the site of action.

Phosphate group improves water solubility the change of the amino group into the carbamate Has reduced the nucleophilicity of it…. difficult To form the aziridinium ring (less active)…. prodrug

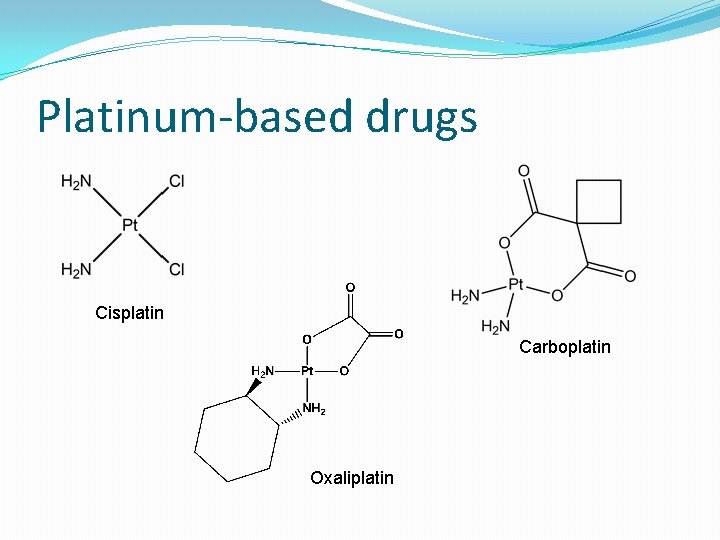
Platinum-based drugs Cisplatin Carboplatin Oxaliplatin

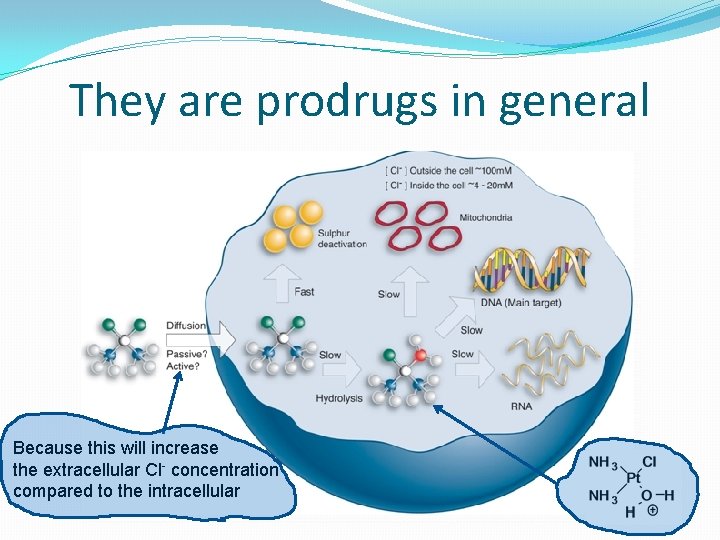
They are prodrugs in general Because this will increase the extracellular Cl- concentration compared to the intracellular

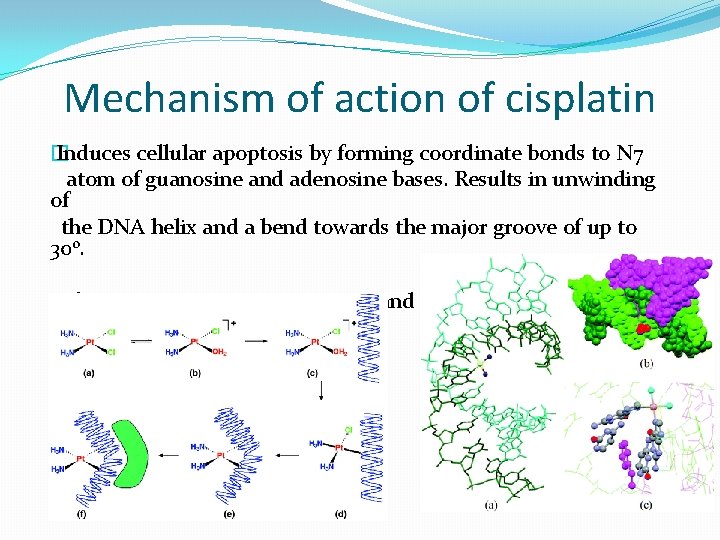
Mechanism of action of cisplatin � Induces cellular apoptosis by forming coordinate bonds to N 7 atom of guanosine and adenosine bases. Results in unwinding of the DNA helix and a bend towards the major groove of up to 30 o. �This prevents DNA transcription and Replication.

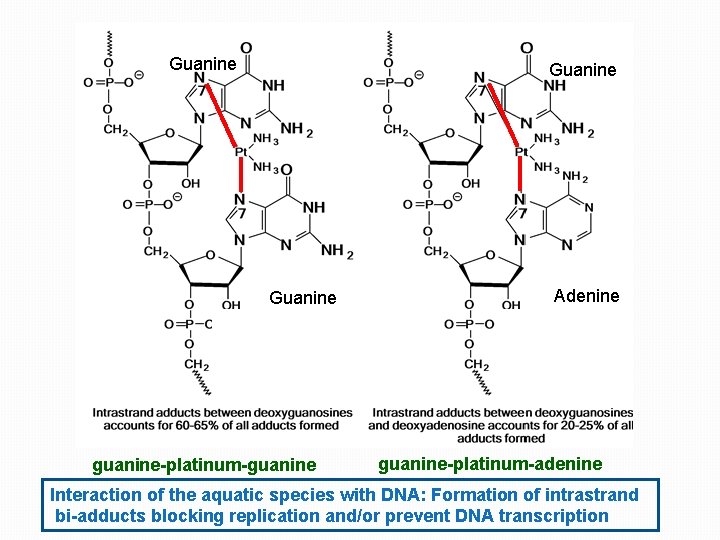
Guanine guanine-platinum-guanine Adenine guanine-platinum-adenine Interaction of the aquatic species with DNA: Formation of intrastrand bi-adducts blocking replication and/or prevent DNA transcription

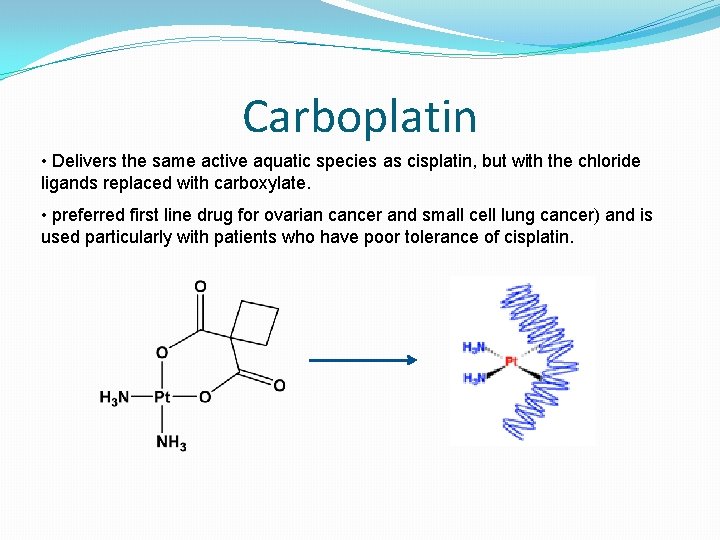
Carboplatin • Delivers the same active aquatic species as cisplatin, but with the chloride ligands replaced with carboxylate. • preferred first line drug for ovarian cancer and small cell lung cancer) and is used particularly with patients who have poor tolerance of cisplatin.

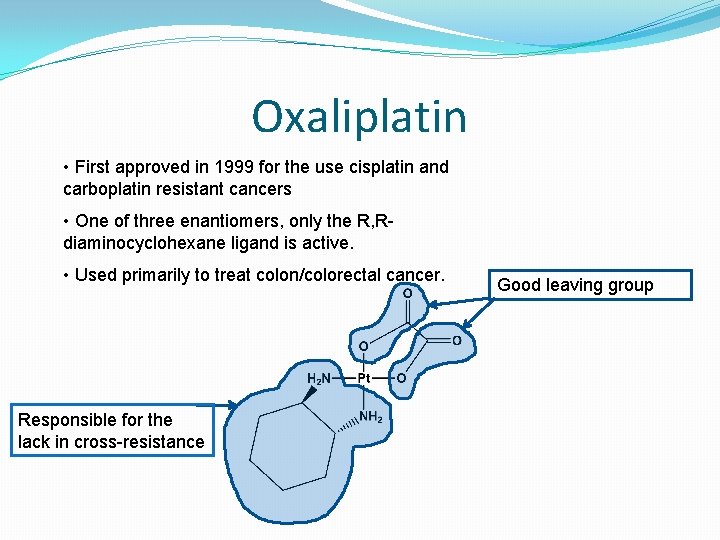
Oxaliplatin • First approved in 1999 for the use cisplatin and carboplatin resistant cancers • One of three enantiomers, only the R, Rdiaminocyclohexane ligand is active. • Used primarily to treat colon/colorectal cancer. Responsible for the lack in cross-resistance Good leaving group

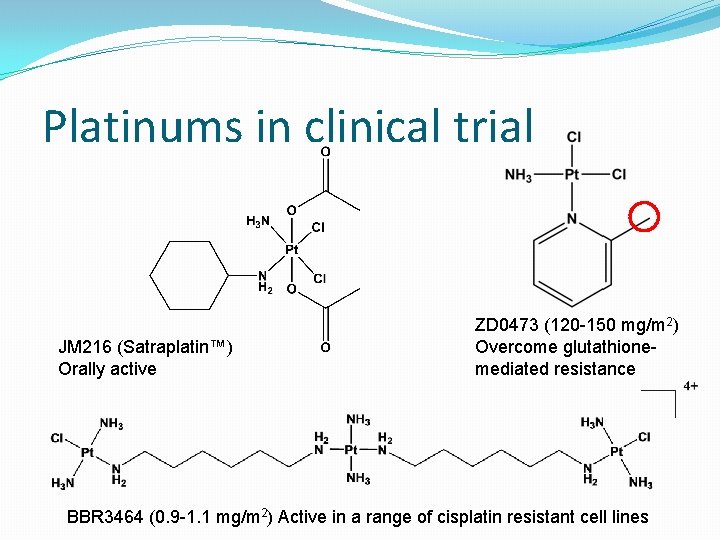
Platinums in clinical trial JM 216 (Satraplatin™) Orally active ZD 0473 (120 -150 mg/m 2) Overcome glutathionemediated resistance BBR 3464 (0. 9 -1. 1 mg/m 2) Active in a range of cisplatin resistant cell lines
 World million deaths
World million deaths Tobacco causes _______ of cancer deaths around the world. *
Tobacco causes _______ of cancer deaths around the world. * Kolopskopi
Kolopskopi Costs of normal spoilage are usually accounted for as
Costs of normal spoilage are usually accounted for as Determining the cost of plant assets
Determining the cost of plant assets Equity method vs cost method
Equity method vs cost method Equity accounted investments
Equity accounted investments Heart of darkness deaths
Heart of darkness deaths Menengai crater deaths
Menengai crater deaths Deaths from smoking
Deaths from smoking West point graduate deaths
West point graduate deaths Heart of darkness deaths
Heart of darkness deaths Clydebank blitz deaths
Clydebank blitz deaths Dr abeer deaths
Dr abeer deaths Gary job corps deaths
Gary job corps deaths Lincoln interpreted his reelection as a mandate to
Lincoln interpreted his reelection as a mandate to Millennium biltmore hotel deaths
Millennium biltmore hotel deaths Who fought ww2
Who fought ww2 Births deaths and marriages tasmania
Births deaths and marriages tasmania 2020 motor vehicle deaths
2020 motor vehicle deaths Rutin för avvikelsehantering
Rutin för avvikelsehantering Bris för vuxna
Bris för vuxna Presentera för publik crossboss
Presentera för publik crossboss Trög för kemist
Trög för kemist Hur skriver man en debattartikel
Hur skriver man en debattartikel Var 1721 för stormaktssverige
Var 1721 för stormaktssverige Vad är densitet
Vad är densitet Tobinskatten för och nackdelar
Tobinskatten för och nackdelar Nationell inriktning för artificiell intelligens
Nationell inriktning för artificiell intelligens Referatmarkeringar
Referatmarkeringar Byggprocessen steg för steg
Byggprocessen steg för steg Gumman cirkel sång
Gumman cirkel sång Epiteltyper
Epiteltyper Enheter för massa
Enheter för massa Fuktmätningar i betong enlig rbk
Fuktmätningar i betong enlig rbk Kraft per area
Kraft per area Elektronik för barn
Elektronik för barn Underlag för särskild löneskatt på pensionskostnader
Underlag för särskild löneskatt på pensionskostnader Tack för att ni har lyssnat
Tack för att ni har lyssnat