ATOMS IONS AND ISOTOPES What is an atom





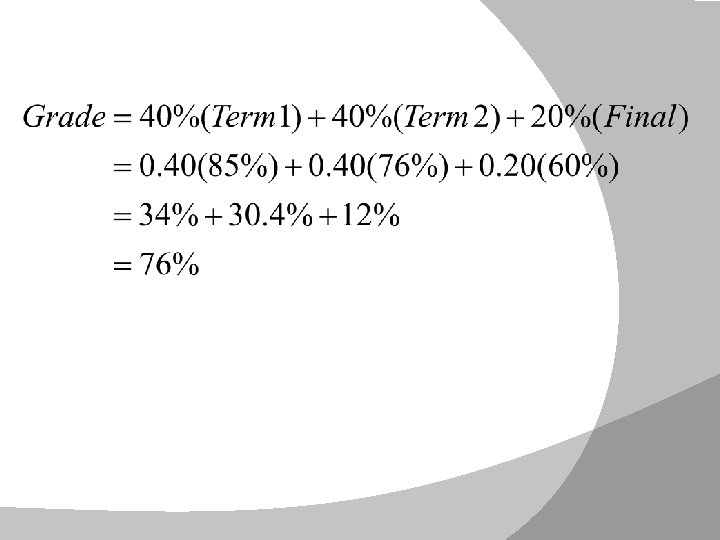

- Slides: 19

ATOMS, IONS AND ISOTOPES !

What is an atom? � Atoms: are the smallest unit of an element that still maintains the chemical properties of that element. � Eg. An Oxygen atom

The Atom … old school…

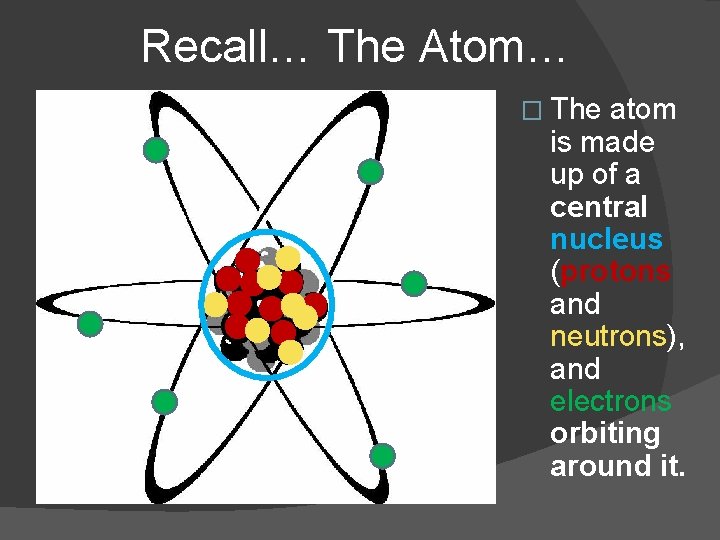
Recall… The Atom… � The atom is made up of a central nucleus (protons and neutrons), and electrons orbiting around it.

Another way to look at the Atom!!

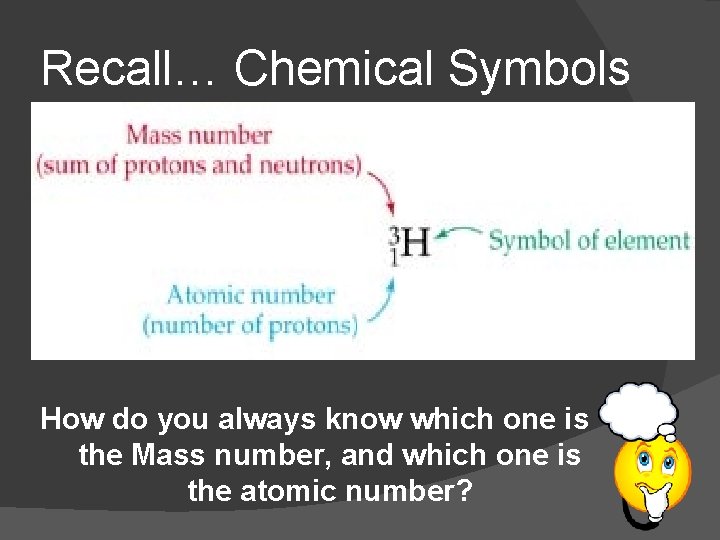
Recall… Chemical Symbols How do you always know which one is the Mass number, and which one is the atomic number?

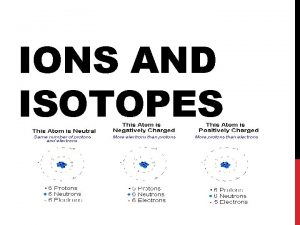
Part I: Atomic Number and Mass # 1. Recall that the atomic number tells us the number of protons in an atom. If an atom is neutral – this is also the number of Electrons (Atom = Neutral, Ion = Not Neutral) a. Since the atom is neutral we know that the number of protons (p+) must equal the number of electrons (e-). e. g. Mercury’s atomic number is 80 so it has 80 p+ and 80 e-.

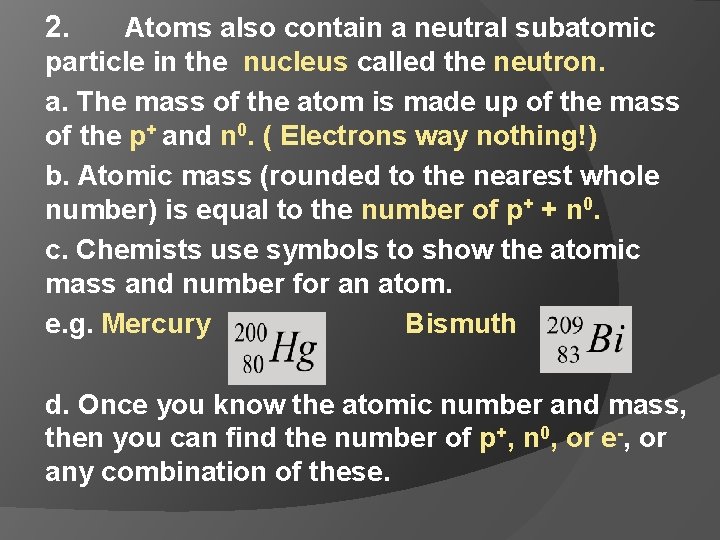
2. Atoms also contain a neutral subatomic particle in the nucleus called the neutron. a. The mass of the atom is made up of the mass of the p+ and n 0. ( Electrons way nothing!) b. Atomic mass (rounded to the nearest whole number) is equal to the number of p+ + n 0. c. Chemists use symbols to show the atomic mass and number for an atom. e. g. Mercury Bismuth d. Once you know the atomic number and mass, then you can find the number of p+, n 0, or e-, or any combination of these.

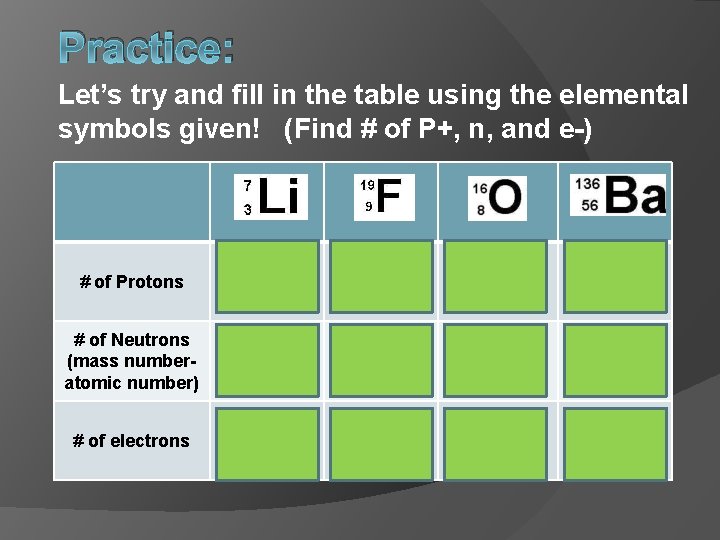
Practice: Let’s try and fill in the table using the elemental symbols given! (Find # of P+, n, and e-) # of Protons 3 9 8 56 # of Neutrons (mass numberatomic number) 4 10 8 80 # of electrons 3 9 8 56


Part II: Ions are formed when atoms gain or lose electrons during a chemical reaction (and therefore have a + or – charge on them. 2. This can happen when an atom is heated to high temperatures, when exposed to high voltage, or when ionic compounds are put in water. 3. Metals tend to lose(give away) electrons and form positive ions because they will have more p+ than e-. Non-metals tend to gain (take) electrons and form negative ions because they have more e- than p+. 1.

So…. If you see and element has a charge of 2+ - what did the atom do to get that charge? Gave away 2 electrons If you see a charge of 3 -, what did the atom do to get that charge? Gained 3 electrons Why do atoms want to be ions? They all want to look like Noble Gases because Noble Gases are extremely stable (have a full valence shell of electrons). Every atom wants to be stable, so they will give away, take, or share electrons to give the appearance of a Noble Gas.

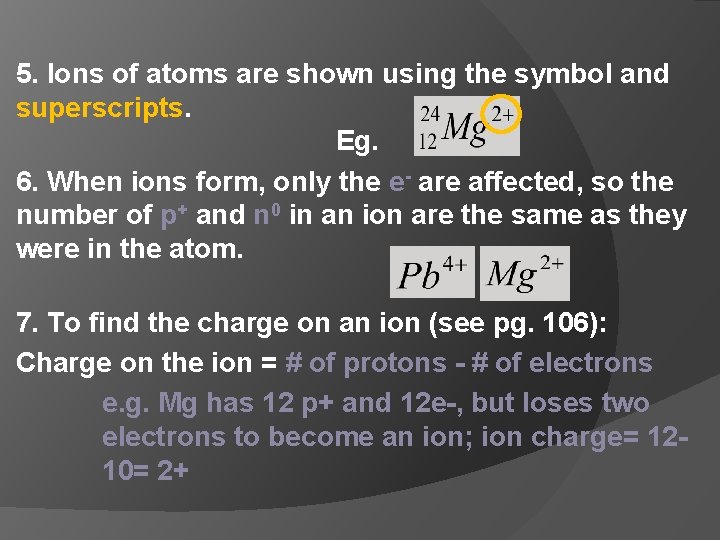
5. Ions of atoms are shown using the symbol and superscripts. Eg. 6. When ions form, only the e- are affected, so the number of p+ and n 0 in an ion are the same as they were in the atom. 7. To find the charge on an ion (see pg. 106): Charge on the ion = # of protons - # of electrons e. g. Mg has 12 p+ and 12 e-, but loses two electrons to become an ion; ion charge= 1210= 2+

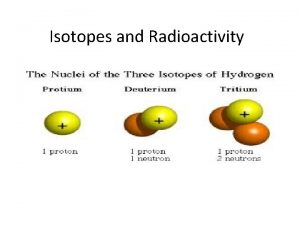
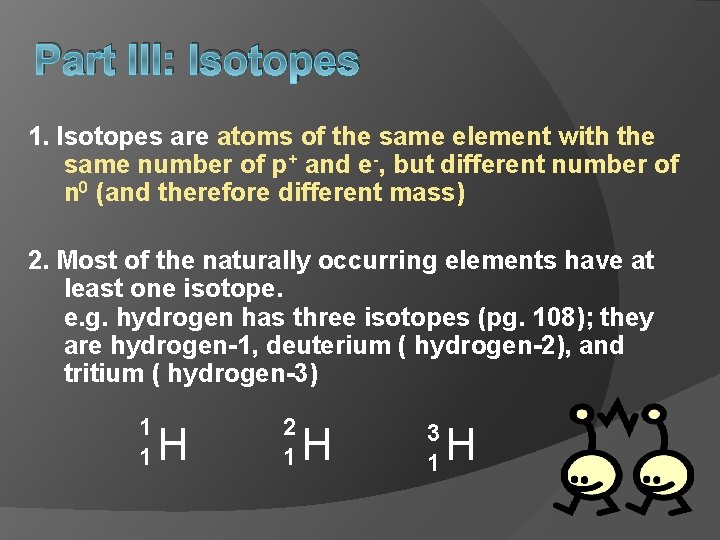
Part III: Isotopes 1. Isotopes are atoms of the same element with the same number of p+ and e-, but different number of n 0 (and therefore different mass) 2. Most of the naturally occurring elements have at least one isotope. e. g. hydrogen has three isotopes (pg. 108); they are hydrogen-1, deuterium ( hydrogen-2), and tritium ( hydrogen-3) 1 1 H 2 1 H 3 1 H

Isotopes: Video Clip

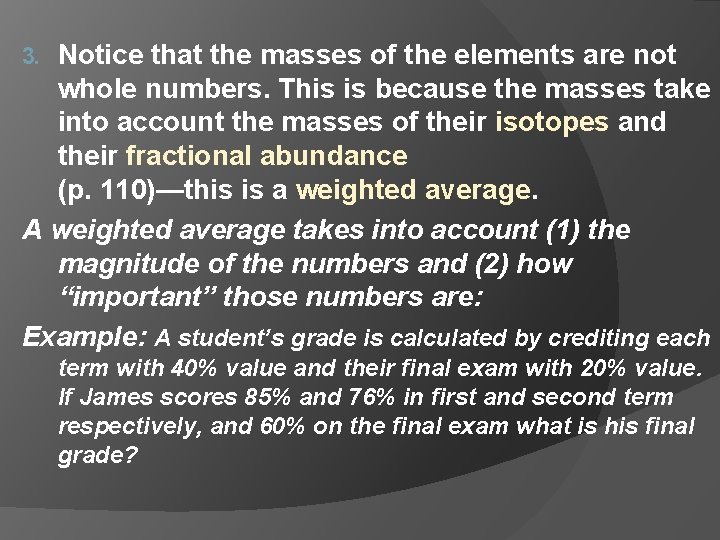
Notice that the masses of the elements are not whole numbers. This is because the masses take into account the masses of their isotopes and their fractional abundance (p. 110)—this is a weighted average. A weighted average takes into account (1) the magnitude of the numbers and (2) how “important” those numbers are: Example: A student’s grade is calculated by crediting each 3. term with 40% value and their final exam with 20% value. If James scores 85% and 76% in first and second term respectively, and 60% on the final exam what is his final grade?
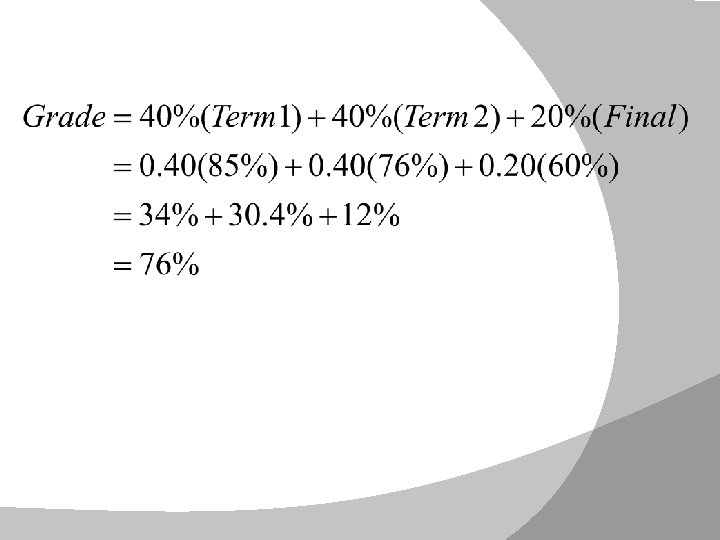

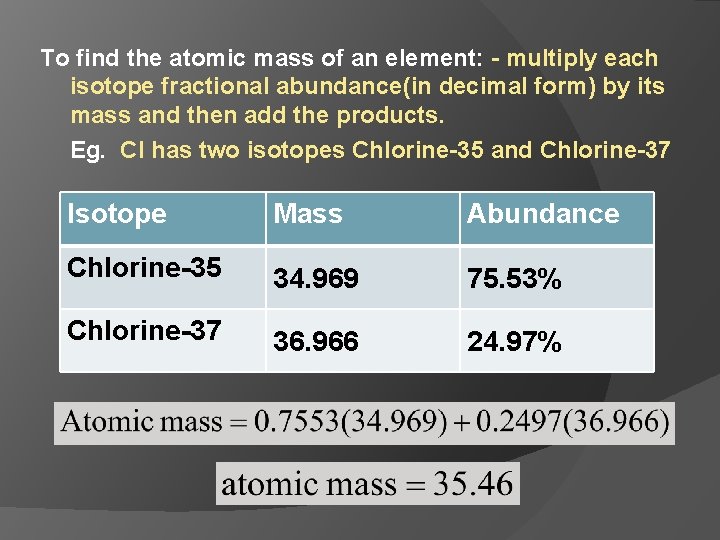
To find the atomic mass of an element: - multiply each isotope fractional abundance(in decimal form) by its mass and then add the products. Eg. Cl has two isotopes Chlorine-35 and Chlorine-37 Isotope Mass Abundance Chlorine-35 34. 969 75. 53% Chlorine-37 36. 966 24. 97%

Try: 3 -3 Practice Problems and 3 -3 Apply Worksheet
 Isotopes pogil
Isotopes pogil Carbon trichloride
Carbon trichloride Atoms molecules and ions
Atoms molecules and ions Atoms molecules and ions
Atoms molecules and ions Atoms molecules and ions
Atoms molecules and ions Atoms molecules and ions
Atoms molecules and ions Atoms ions and molecules
Atoms ions and molecules Atoms ions and molecules
Atoms ions and molecules States that atoms ions and molecules must collide to react
States that atoms ions and molecules must collide to react Chapter 2 atoms molecules and ions
Chapter 2 atoms molecules and ions Positive ions are atoms that have
Positive ions are atoms that have Atoms or ions are considered isoelectronic if
Atoms or ions are considered isoelectronic if Regents periodic table
Regents periodic table Electrons in atoms section 2 quantum theory and the atom
Electrons in atoms section 2 quantum theory and the atom Electrons in atoms section 2 quantum theory and the atom
Electrons in atoms section 2 quantum theory and the atom Isotopes stable and unstable
Isotopes stable and unstable The structure of the atom section 2 defining the atom
The structure of the atom section 2 defining the atom Gambar atom democritus
Gambar atom democritus Fertile isotopes
Fertile isotopes How to find percent abundance given atomic mass
How to find percent abundance given atomic mass