AP Chemistry Reactions in Solution solution a homogeneous










- Slides: 16

AP Chemistry Reactions in Solution

solution: a homogeneous mixture of two or more substances -- The ______ solvent is present in greatest quantity. -- Any other substance present is solute called a ______. aqueous solutions: solutions in which water is the dissolving medium (i. e. , the solvent) electrolyte: any substance whose aqueous solution will conduct electricity e. g. , HCl, Na. Cl, KOH -- as opposed to a nonelectrolyte, e. g. , any sugar (C 6 H 12 O 6, C 12 H 22 O 11) or any alcohol (CH 3 OH, CH 3 CH 2 OH)

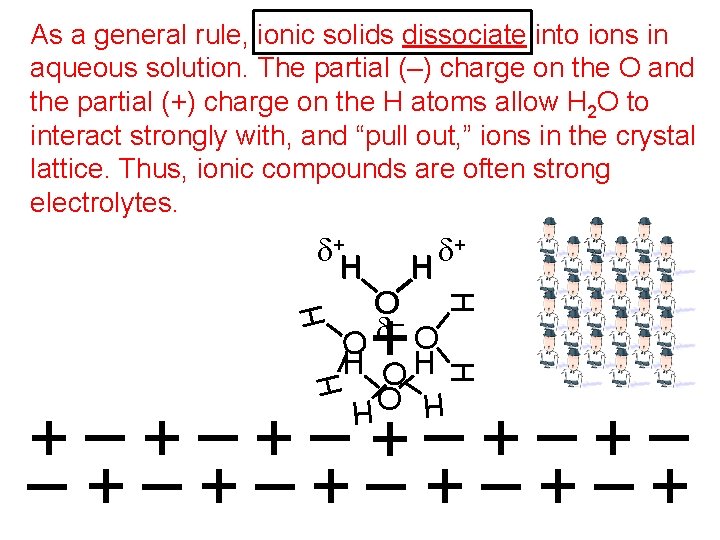
As a general rule, ionic solids dissociate into ions in aqueous solution. The partial (–) charge on the O and the partial (+) charge on the H atoms allow H 2 O to interact strongly with, and “pull out, ” ions in the crystal lattice. Thus, ionic compounds are often strong electrolytes. d+ H H O d– H d+ H H H O O H H O +–+–+– –+–+–+ H

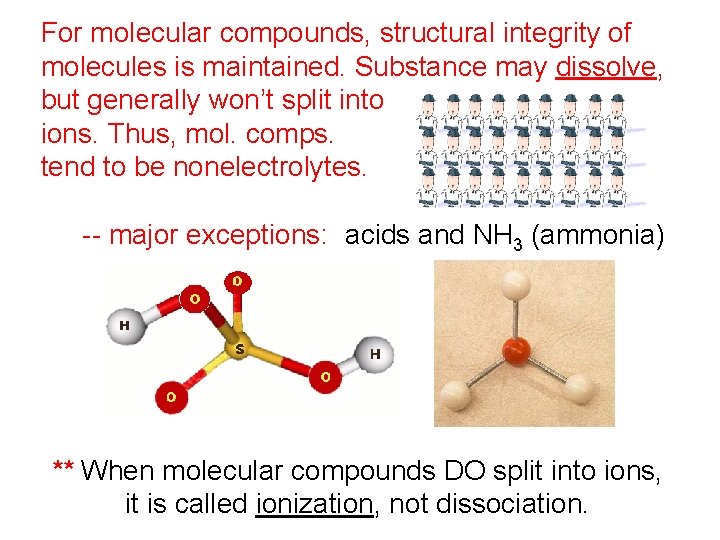
For molecular compounds, structural integrity of molecules is maintained. Substance may dissolve, but generally won’t split into ions. Thus, mol. comps. tend to be nonelectrolytes. -- major exceptions: acids and NH 3 (ammonia) ** When molecular compounds DO split into ions, it is called ionization, not dissociation.

Strong electrolytes exist almost completely as ions in aqueous solution. e. g. , HCl(aq) H+(aq) + Cl–(aq) KCl(aq) K+(aq) + Cl–(aq) lots of product (note the one-sided arrow) Weak electrolytes produce only a small concentration of ions in reaching equilibrium. e. g. , CH 3 COOH(aq) HF(aq) lots of reactant CH 3 COO–(aq) + H+(aq) + F–(aq) (note the double arrow)

Some of the strong electrolytes are the strong acids and strong bases. STRONG ACIDS STRONG BASES the hydroxides of. . . hydrochloric, HCl hydrobromic, HBr hydroiodic, HI chloric, HCl. O 3 perchloric, HCl. O 4 nitric, HNO 3 sulfuric, H 2 SO 4 Li, Na, K, Rb, Cs, Ca, Sr, Ba “strong base cations”

Be careful to distinguish between dissolution and dissociation/ionization in regard to strongs or weaks. For example, CH 3 COOH dissolves completely, but ionizes only slightly; it is therefore a weak electrolyte. On the other hand, Ba(OH)2 dissolves very little, but the amount that does dissolve dissociates almost completely. Ba(OH)2 is a strong electrolyte. The question is: Of the amount that dissolves, what fraction dissociates/ionizes? If… “most” If… “not much” STRONG WEAK

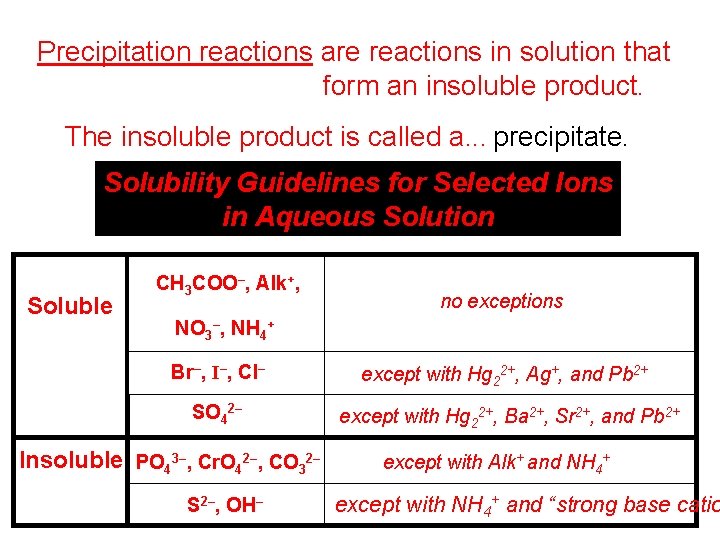
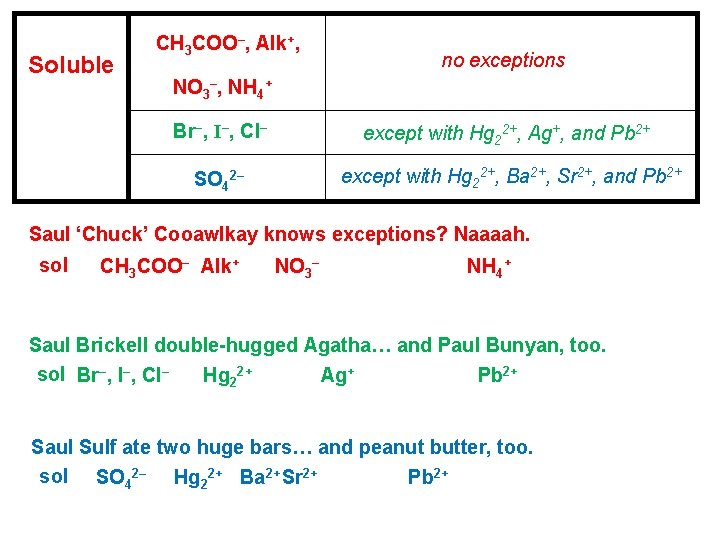
Precipitation reactions are reactions in solution that form an insoluble product. The insoluble product is called a. . . precipitate. Solubility Guidelines for Selected Ions in Aqueous Solution Soluble CH 3 COO–, Alk+, no exceptions NO 3–, NH 4+ Br–, I–, Cl– except with Hg 22+, Ag+, and Pb 2+ SO 42– except with Hg 22+, Ba 2+, Sr 2+, and Pb 2+ Insoluble PO 43–, Cr. O 42–, CO 32– S 2–, OH– except with Alk+ and NH 4+ except with NH 4+ and “strong base catio

Soluble CH 3 COO–, Alk+, no exceptions NO 3–, NH 4+ Br–, I–, Cl– except with Hg 22+, Ag+, and Pb 2+ SO 42– except with Hg 22+, Ba 2+, Sr 2+, and Pb 2+ Saul ‘Chuck’ Cooawlkay knows exceptions? Naaaah. sol CH 3 COO– Alk+ NO 3– NH 4+ Saul Brickell double-hugged Agatha… and Paul Bunyan, too. sol Br–, I–, Cl– Hg 22+ Ag+ Pb 2+ Saul Sulf ate two huge bars… and peanut butter, too. sol SO 42– Hg 22+ Ba 2+ Sr 2+ Pb 2+

Insoluble PO 43–, Cr. O 42–, CO 32– S 2–, OH– except with Alk+ and NH 4+ except with NH 4+ and “strong base catio “The poor crow was cold; he huddled with everyone, but Al K. said, PO 43– Cr. O 42– CO 32– Alk+ NH 4+ “Soooooo… You two are always combined. ” “Naaaaht when we’re stro S 2– OH– NH 4+ SBCs

Saul ‘Chuck’ Cooawlkay knows exceptions? N sol CH 3 COO– Alk+ NO 3– NH 4+ the honorable, no-nonsense judge: Saul ‘Chuck’ C

Saul Brickell double-hugged Agatha… and Paul Buny sol Br–, I–, Cl– Hg 22+ Ag+ Pb 2+ Saul Brickell double-hugging Agatha Saul Brickell double-huggin the Paul Bunyan trophy

Saul Sulf ate two huge bars… and peanut butt sol SO 42– Hg 22+ Ba 2+ Sr 2+ Pb 2+ the slightly sickened two HUGE, “I’m-going- peanut Saul Sulf into-a-sugar-coma” bars butter

The poor crow was cold; he huddled with everyone, but Al K. said PO 43– Cr. O 42– CO 32– the poor crow who was cold Alk+ NH 4+ the poor crow the somewhat aloof who was cold Al K. politely attempting to declining the huddle with crow’s overtures everyone to huddle

‘Soooooo… You two are always combined. ’ “Naaaaht when we’re str S 2– OH– NH 4+ SBCs “OH!”

Suppose you mix solutions of lead(II) nitrate and sodium iodide. Pb(NO 3)2 Na. I The ions present are. . . Pb 2+, NO 3–, Na+, I– Write the overall ionic equation… Pb 2+ + 2 NO 3– + 2 Na+ + 2 I– (aq) Cancel the spectator ions to get the net ionic equation… Pb 2+ + 2 I– (aq) Pb. I 2 (ppt) Pb. I 2 + 2 NO 3– + 2 Na+ (ppt) (aq)