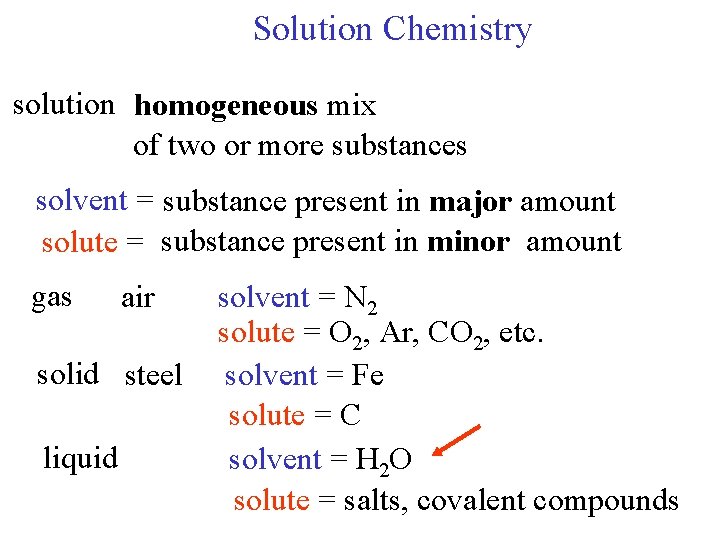
Solution Chemistry solution homogeneous mix of two or

![Solution Composition molarity concentration = amount of solute = mol = [ M ] Solution Composition molarity concentration = amount of solute = mol = [ M ]](https://slidetodoc.com/presentation_image_h2/06dd054bdc4a08c3bd63241e2bbddf1c/image-11.jpg)
![Solution Composition concentration = amount of solute = mol = [ M ] volume Solution Composition concentration = amount of solute = mol = [ M ] volume](https://slidetodoc.com/presentation_image_h2/06dd054bdc4a08c3bd63241e2bbddf1c/image-12.jpg)
![Solution Composition concentration = amount of solute = mol = [ M ] volume Solution Composition concentration = amount of solute = mol = [ M ] volume](https://slidetodoc.com/presentation_image_h2/06dd054bdc4a08c3bd63241e2bbddf1c/image-13.jpg)
![Solution Composition concentration = amount of solute = mol = [ M ] volume Solution Composition concentration = amount of solute = mol = [ M ] volume](https://slidetodoc.com/presentation_image_h2/06dd054bdc4a08c3bd63241e2bbddf1c/image-14.jpg)

- Slides: 15

Solution Chemistry solution homogeneous mix of two or more substances solvent = substance present in major amount solute = substance present in minor amount gas air solid steel liquid solvent = N 2 solute = O 2, Ar, CO 2, etc. solvent = Fe solute = C solvent = H 2 O solute = salts, covalent compounds

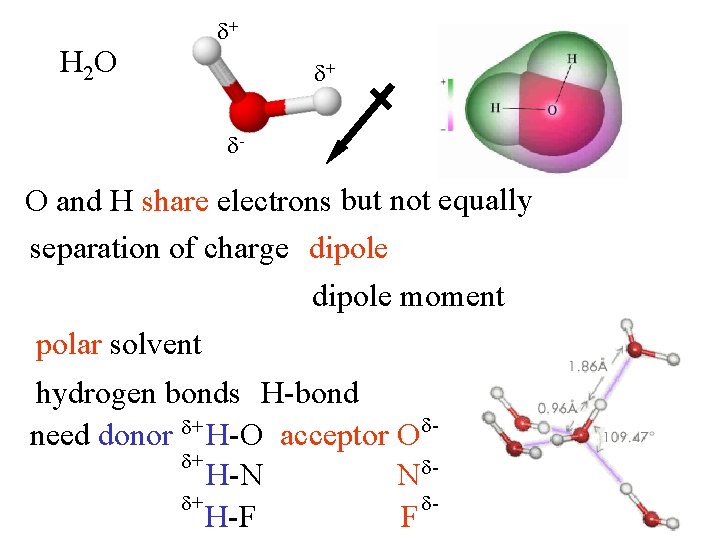
H 2 O + + - O and H share electrons but not equally separation of charge dipole moment polar solvent hydrogen bonds H-bond + need donor H-O acceptor O + H-N N + H-F F

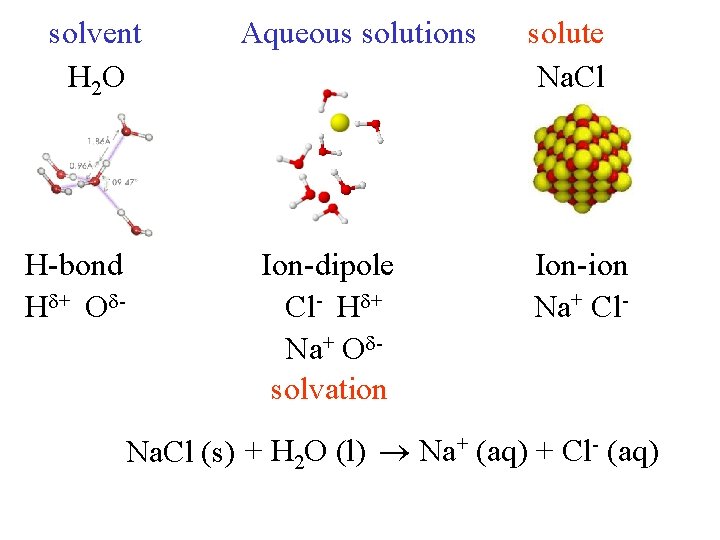
solvent H 2 O H-bond H + O - Aqueous solutions Ion-dipole Cl- H + Na+ O solvation solute Na. Cl Ion-ion Na+ Cl- Na. Cl (s) + H 2 O (l) Na+ (aq) + Cl- (aq)

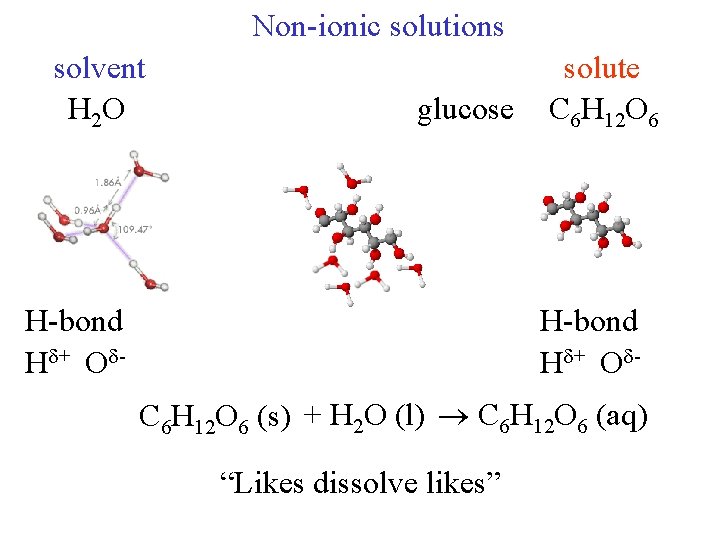
Non-ionic solutions solvent H 2 O glucose H-bond H + O - solute C 6 H 12 O 6 H-bond H + O C 6 H 12 O 6 (s) + H 2 O (l) C 6 H 12 O 6 (aq) “Likes dissolve likes”

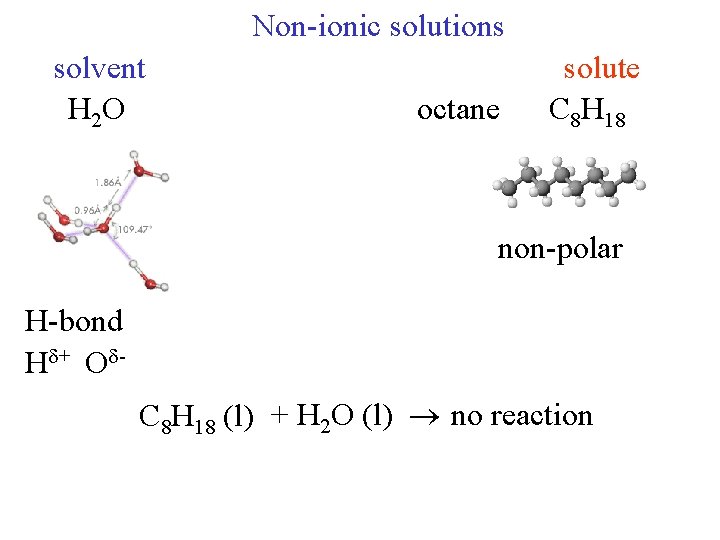
Non-ionic solutions solvent H 2 O octane solute C 8 H 18 non-polar H-bond H + O C 8 H 18 (l) + H 2 O (l) no reaction

Properties of aqueous solutions ionic covalent conduct electricity do not conduct electricity Na. Cl C 6 H 12 O 6 electrolytes produce ions non-electrolytes mobile, charged salts produce other anions and cations bases produce OH- in aqueous solutions acids produce H+ in aqueous solutions

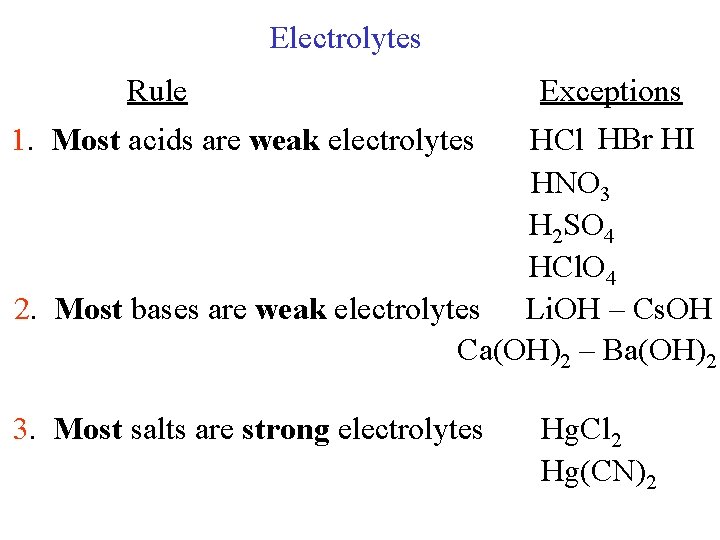
Electrolytes Rule Exceptions 1. Most acids are weak electrolytes HCl HBr HI HNO 3 H 2 SO 4 HCl. O 4 2. Most bases are weak electrolytes Li. OH – Cs. OH Ca(OH)2 – Ba(OH)2 3. Most salts are strong electrolytes Hg. Cl 2 Hg(CN)2

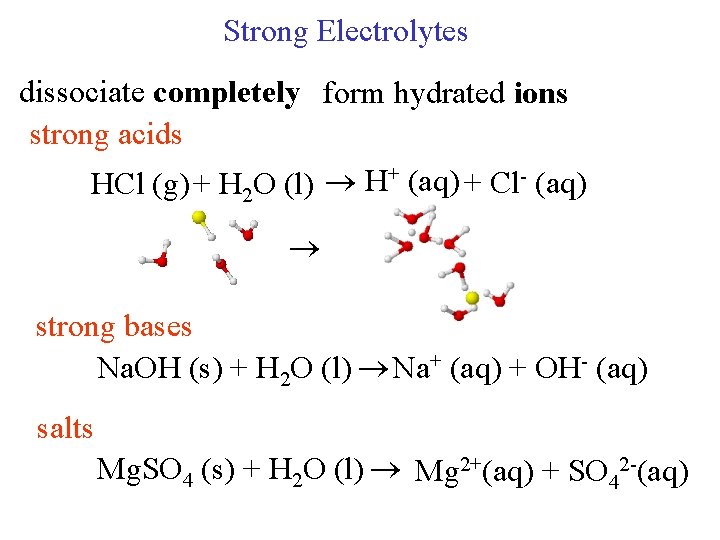
Strong Electrolytes dissociate completely form hydrated ions strong acids HCl (g) + H 2 O (l) H+ (aq) + Cl- (aq) strong bases Na. OH (s) + H 2 O (l) Na+ (aq) + OH- (aq) salts Mg. SO 4 (s) + H 2 O (l) Mg 2+(aq) + SO 42 -(aq)

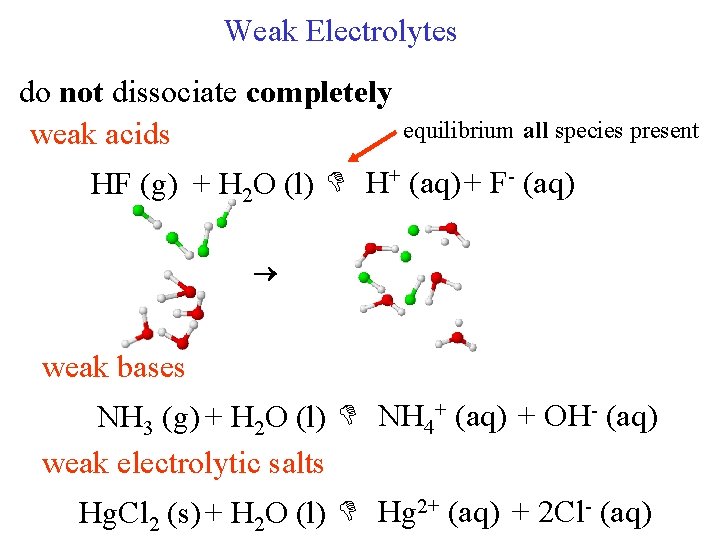
Weak Electrolytes do not dissociate completely equilibrium all species present weak acids HF (g) + H 2 O (l) H+ (aq) + F- (aq) weak bases NH 3 (g) + H 2 O (l) NH 4+ (aq) + OH- (aq) weak electrolytic salts Hg. Cl 2 (s) + H 2 O (l) Hg 2+ (aq) + 2 Cl- (aq)

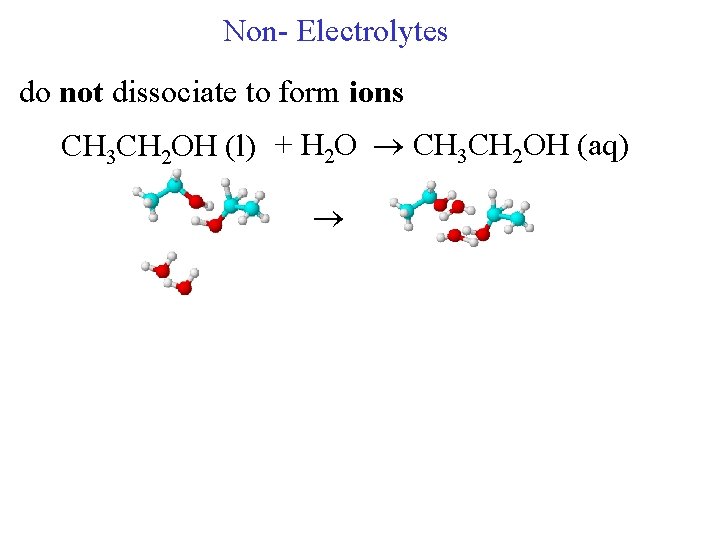
Non- Electrolytes do not dissociate to form ions CH 3 CH 2 OH (l) + H 2 O CH 3 CH 2 OH (aq)
![Solution Composition molarity concentration amount of solute mol M Solution Composition molarity concentration = amount of solute = mol = [ M ]](https://slidetodoc.com/presentation_image_h2/06dd054bdc4a08c3bd63241e2bbddf1c/image-11.jpg)
Solution Composition molarity concentration = amount of solute = mol = [ M ] volume of solution L What is the molarity of a solution prepared by dissolving 23. 4 g sodium sulfate in enough water to give 125 m. L of solution? 23. 4 g Na 2 SO 4 1 mol Na 2 SO 4 = 0. 165 mol Na 2 SO 4 142. 0 g Na 2 SO 4 125 m. L 1 L =. 125 L 1000 m. L M = 0. 165 mol Na 2 SO 4 = 1. 32 M 0. 125 L [Na 2 SO 4] = 1. 32 M
![Solution Composition concentration amount of solute mol M volume Solution Composition concentration = amount of solute = mol = [ M ] volume](https://slidetodoc.com/presentation_image_h2/06dd054bdc4a08c3bd63241e2bbddf1c/image-12.jpg)
Solution Composition concentration = amount of solute = mol = [ M ] volume of solution L How many moles of HNO 3 are present in 2. 0 L of 0. 200 M HNO 3 solution? 0. 200 mol HNO 3 2. 0 L = 0. 40 mol HNO 3 L
![Solution Composition concentration amount of solute mol M volume Solution Composition concentration = amount of solute = mol = [ M ] volume](https://slidetodoc.com/presentation_image_h2/06dd054bdc4a08c3bd63241e2bbddf1c/image-13.jpg)
Solution Composition concentration = amount of solute = mol = [ M ] volume of solution L How many grams of Na 2 SO 4 are required to make 350 m. L of 0. 500 M Na 2 SO 4? 0. 500 mol Na 2 SO 4 0. 350 L 142. 0 g = 24. 9 g Na 2 SO 4 1 mol Na 2 SO 4 L
![Solution Composition concentration amount of solute mol M volume Solution Composition concentration = amount of solute = mol = [ M ] volume](https://slidetodoc.com/presentation_image_h2/06dd054bdc4a08c3bd63241e2bbddf1c/image-14.jpg)
Solution Composition concentration = amount of solute = mol = [ M ] volume of solution L stock solution HCl = 12. 0 M moles solute before dilution = moles solute after dilution How would you prepare 1. 5 L of a 0. 10 M HCl solution? 0. 10 mol HCl 1. 5 L = 0. 15 mol HCl L 0. 15 mol HCl = 12. 0 mol HCl (x) L L moles after dilution moles before dilution (x) = 0. 0125 L 12. 5 m. L of 12. 0 M HCl + 1. 4875 L H 2 O = 1. 50 L 0. 10 M HCl

How would you prepare 1. 5 L of a 0. 10 M HCl solution, using a 12. 0 M stock solution? moles of solute before dilution = moles of solute after dilution Mi (mol/L) x Vi (L) = Mf x Vf 12. 0 M HCl x Vi = 0. 10 M HCl x 1. 5 L Vi = 0. 0125 L then add H 2 O to get to Vf =1. 37 L H 2 O