AP Chemistry Reactions in Solution solution a homogeneous

























- Slides: 68

AP Chemistry Reactions in Solution

solution: a homogeneous mixture of two or more substances -The ______ solvent is present in greatest quantity. -- Any other substance present is called a ______. solute aqueous solutions: solutions in which water is the dissolving medium (i. e. , the solvent) electrolyte: any substance whose aqueous solution will conduct electricity e. g. , HCl, Na. Cl, KOH -- as opposed to a nonelectrolyte, a substance in aqueous solution that will NOT conduct electricity e. g. , any sugar (C 6 H 12 O 6, C 12 H 22 O 11) or any alcohol (CH 3 OH, CH 3 CH 2 OH)

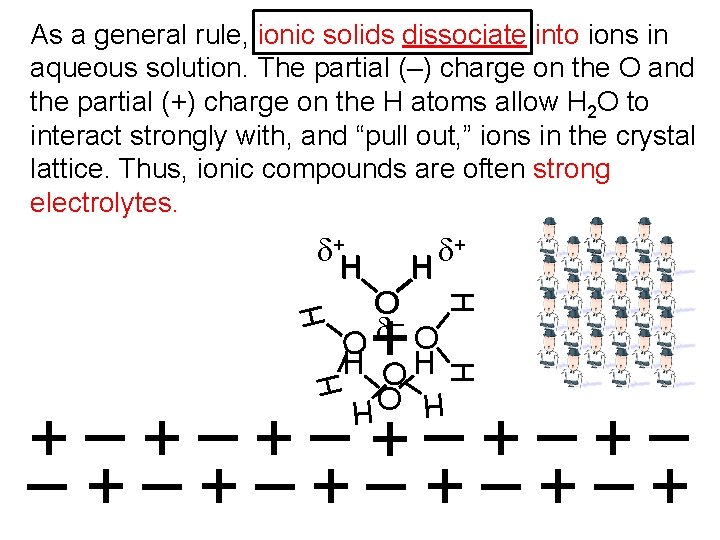
As a general rule, ionic solids dissociate into ions in aqueous solution. The partial (–) charge on the O and the partial (+) charge on the H atoms allow H 2 O to interact strongly with, and “pull out, ” ions in the crystal lattice. Thus, ionic compounds are often strong electrolytes. d+ H H O d– H d+ H H H O O H H O +–+–+– –+–+–+ H

For molecular compounds, structural integrity of molecules is maintained. Substance may dissolve, but generally won’t split into ions. Thus, mol. comps. tend to be nonelectrolytes. -- major exceptions: acids and NH 3 (ammonia) ** When molecular compounds DO split into ions, it is called ionization, not dissociation.

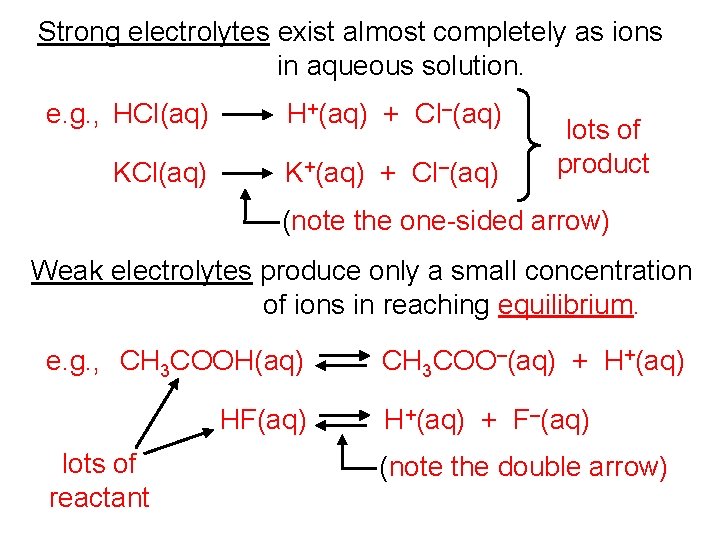
Strong electrolytes exist almost completely as ions in aqueous solution. e. g. , HCl(aq) H+(aq) + Cl–(aq) KCl(aq) K+(aq) + Cl–(aq) lots of product (note the one-sided arrow) Weak electrolytes produce only a small concentration of ions in reaching equilibrium. e. g. , CH 3 COOH(aq) HF(aq) lots of reactant CH 3 COO–(aq) + H+(aq) + F–(aq) (note the double arrow)

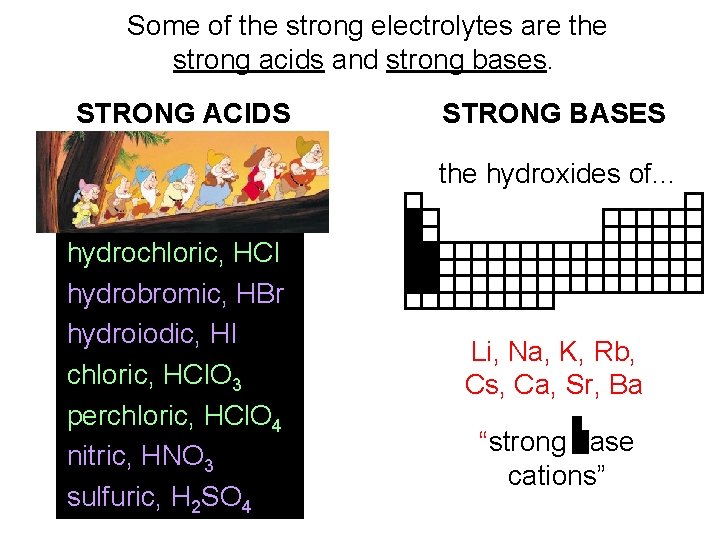
Some of the strong electrolytes are the strong acids and strong bases. STRONG ACIDS STRONG BASES the hydroxides of. . . hydrochloric, HCl hydrobromic, HBr hydroiodic, HI chloric, HCl. O 3 perchloric, HCl. O 4 nitric, HNO 3 sulfuric, H 2 SO 4 Li, Na, K, Rb, Cs, Ca, Sr, Ba “strong base cations”

Be careful to distinguish between dissolution and dissociation/ionization in regard to strongs or weaks. For example, CH 3 COOH dissolves completely, but ionizes only slightly; it is therefore a weak electrolyte. On the other hand, Ba(OH)2 dissolves very little, but the amount that does dissolve dissociates almost completely. Ba(OH)2 is a strong electrolyte. The question is: Of the amount that dissolves, what fraction dissociates/ionizes? If… “most” If… “not much” STRONG WEAK

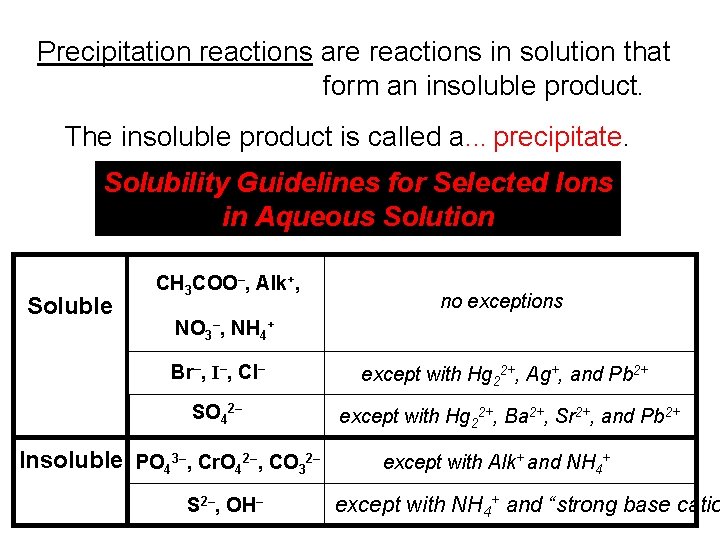
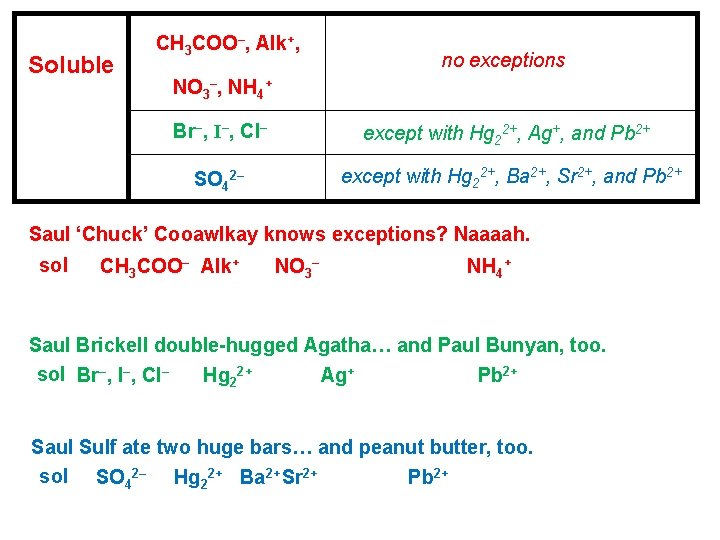
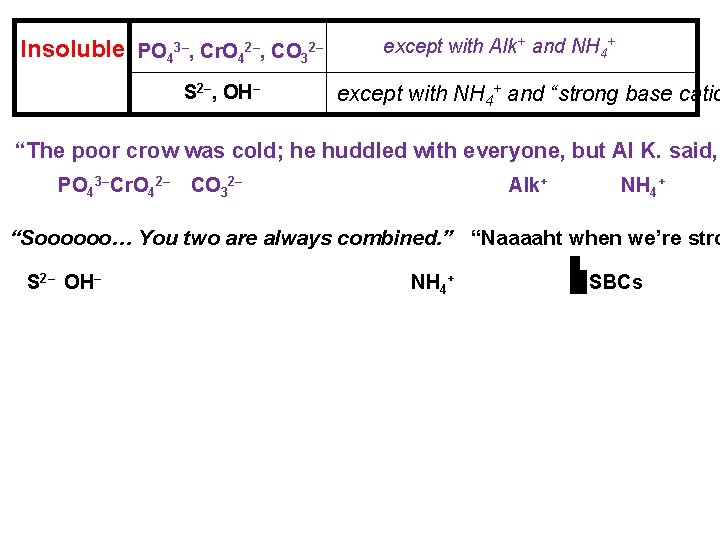
Precipitation reactions are reactions in solution that form an insoluble product. The insoluble product is called a. . . precipitate. Solubility Guidelines for Selected Ions in Aqueous Solution Soluble CH 3 COO–, Alk+, no exceptions NO 3–, NH 4+ Br–, I–, Cl– except with Hg 22+, Ag+, and Pb 2+ SO 42– except with Hg 22+, Ba 2+, Sr 2+, and Pb 2+ Insoluble PO 43–, Cr. O 42–, CO 32– S 2–, OH– except with Alk+ and NH 4+ except with NH 4+ and “strong base catio

Soluble CH 3 COO–, Alk+, no exceptions NO 3–, NH 4+ Br–, I–, Cl– except with Hg 22+, Ag+, and Pb 2+ SO 42– except with Hg 22+, Ba 2+, Sr 2+, and Pb 2+ Saul ‘Chuck’ Cooawlkay knows exceptions? Naaaah. sol CH 3 COO– Alk+ NO 3– NH 4+ Saul Brickell double-hugged Agatha… and Paul Bunyan, too. sol Br–, I–, Cl– Hg 22+ Ag+ Pb 2+ Saul Sulf ate two huge bars… and peanut butter, too. sol SO 42– Hg 22+ Ba 2+ Sr 2+ Pb 2+

Insoluble PO 43–, Cr. O 42–, CO 32– S 2–, OH– except with Alk+ and NH 4+ except with NH 4+ and “strong base catio “The poor crow was cold; he huddled with everyone, but Al K. said, PO 43– Cr. O 42– CO 32– Alk+ NH 4+ “Soooooo… You two are always combined. ” “Naaaaht when we’re stro S 2– OH– NH 4+ SBCs

Saul ‘Chuck’ Cooawlkay knows exceptions? N sol CH 3 COO– Alk+ NO 3– NH 4+ the honorable, no-nonsense judge: Saul ‘Chuck’ C

Saul Brickell double-hugged Agatha… and Paul Buny sol Br–, I–, Cl– Hg 22+ Ag+ Pb 2+ Saul Brickell double-hugging Agatha Saul Brickell double-huggin the Paul Bunyan trophy

Saul Sulf ate two huge bars… and peanut butt sol SO 42– Hg 22+ Ba 2+ Sr 2+ Pb 2+ the slightly sickened two HUGE, “I’m-going- peanut Saul Sulf into-a-sugar-coma” bars butter

The poor crow was cold; he huddled with everyone, but Al K. said PO 43– Cr. O 42– CO 32– the poor crow who was cold Alk+ NH 4+ the poor crow the somewhat aloof who was cold Al K. politely attempting to declining the huddle with crow’s overtures everyone to huddle

‘Soooooo… You two are always combined. ’ “Naaaaht when we’re str S 2– OH– NH 4+ SBCs “OH!”

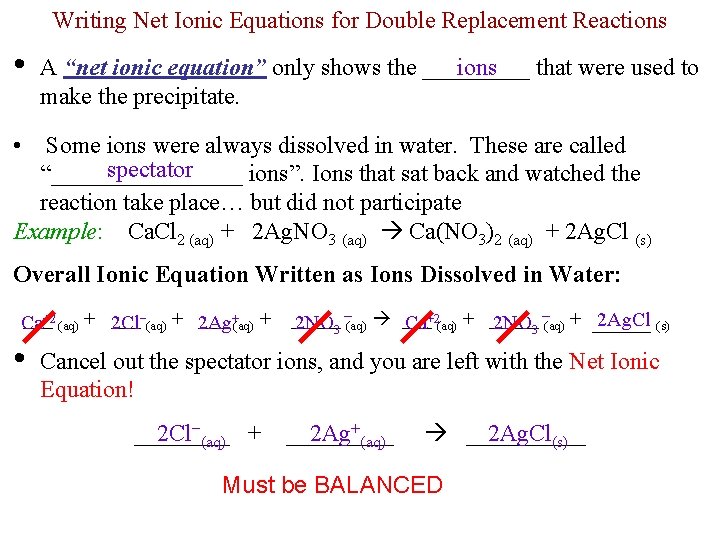
Writing Net Ionic Equations for Double Replacement Reactions • ions A “net ionic equation” only shows the _____ that were used to make the precipitate. • Some ions were always dissolved in water. These are called spectator “________ ions”. Ions that sat back and watched the reaction take place… but did not participate Example: Ca. Cl 2 (aq) + 2 Ag. NO 3 (aq) Ca(NO 3)2 (aq) + 2 Ag. Cl (s) Overall Ionic Equation Written as Ions Dissolved in Water: +2 ___ +(aq) + Ca 2 Cl−(aq) + 2 Ag (aq) + ___ • _____ 2 Ag. Cl (s) 2 NO 3 −(aq) ___ Ca+2(aq) + _____ 2 NO 3 −(aq) + _____ Cancel out the spectator ions, and you are left with the Net Ionic Equation! ____ 2 Cl−(aq) + _____ 2 Ag+(aq) _____ 2 Ag. Cl(s) Must be BALANCED

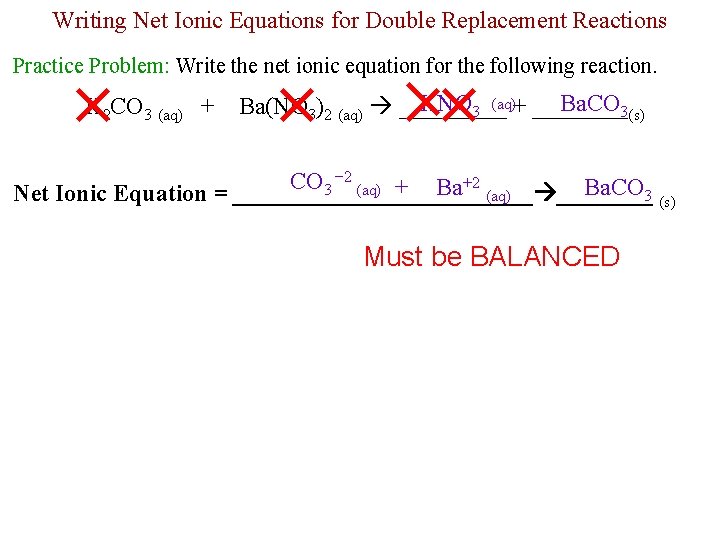
Writing Net Ionic Equations for Double Replacement Reactions Practice Problem: Write the net ionic equation for the following reaction. K 2 CO 3 (aq) + KNO 3 (aq)+ ____ Ba. CO 3(s) Ba(NO 3)2 (aq) _____ CO 3 − 2 (aq) + Ba+2 (aq) Ba. CO 3 Net Ionic Equation = _____________ (s) Must be BALANCED

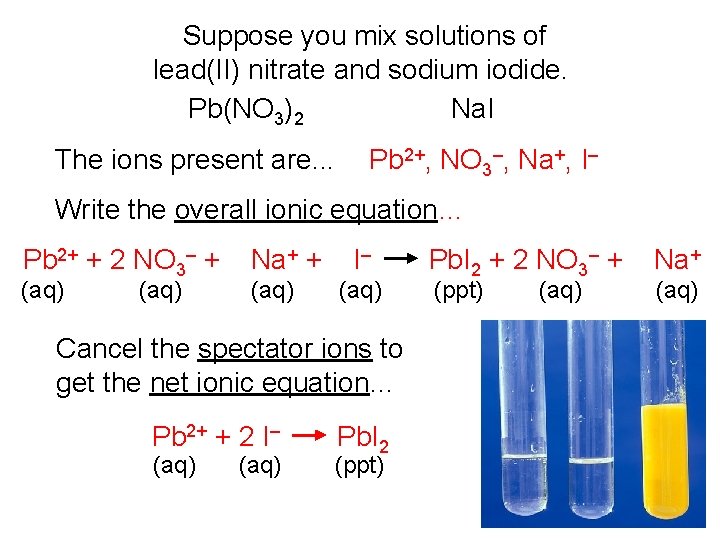
Suppose you mix solutions of lead(II) nitrate and sodium iodide. Pb(NO 3)2 Na. I The ions present are. . . Pb 2+, NO 3–, Na+, I– Write the overall ionic equation… Pb 2+ + 2 NO 3– + 2 Na+ + 2 I– (aq) Cancel the spectator ions to get the net ionic equation… Pb 2+ + 2 I– (aq) Pb. I 2 (ppt) Pb. I 2 + 2 NO 3– + 2 Na+ (ppt) (aq)

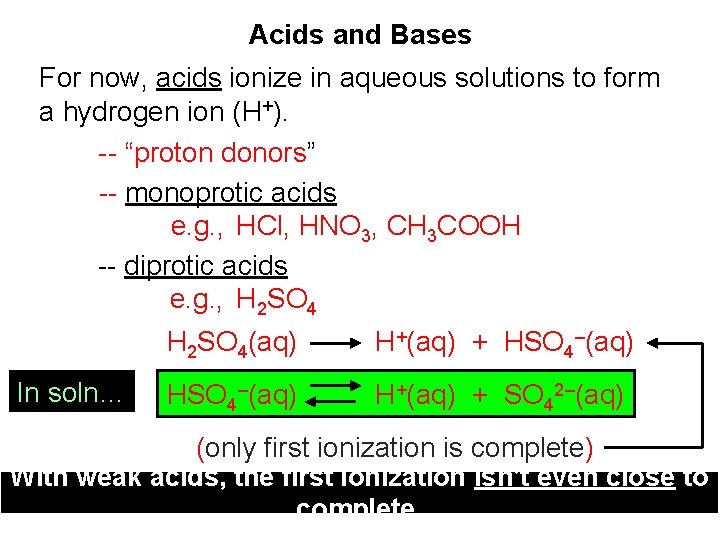
Acids and Bases For now, acids ionize in aqueous solutions to form a hydrogen ion (H+). -- “proton donors” -- monoprotic acids e. g. , HCl, HNO 3, CH 3 COOH -- diprotic acids e. g. , H 2 SO 4 In soln… H 2 SO 4(aq) H+(aq) + HSO 4–(aq) H+(aq) + SO 42–(aq) (only first ionization is complete) With weak acids, the first ionization isn’t even close to complete.

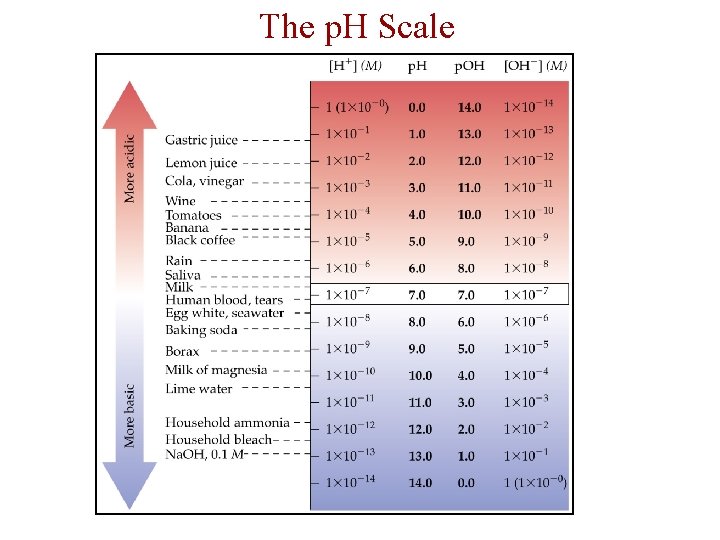
The p. H Scale

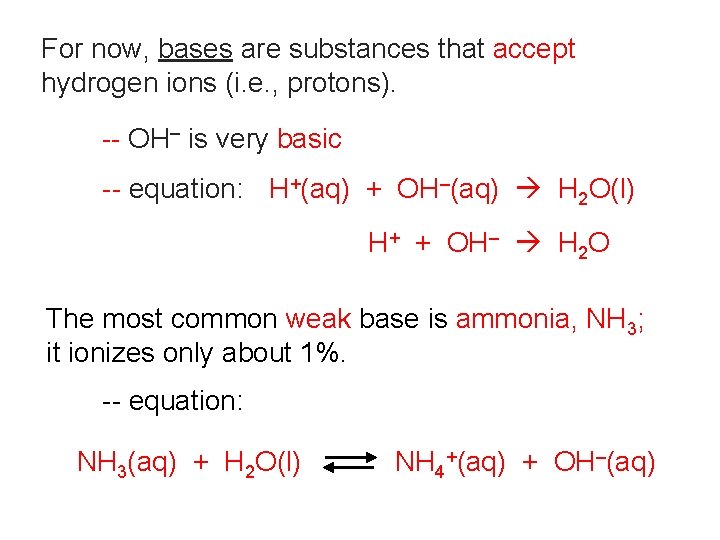
For now, bases are substances that accept hydrogen ions (i. e. , protons). -- OH– is very basic -- equation: H+(aq) + OH–(aq) H 2 O(l) H+ + OH– H 2 O The most common weak base is ammonia, NH 3; it ionizes only about 1%. -- equation: NH 3(aq) + H 2 O(l) NH 4+(aq) + OH–(aq)

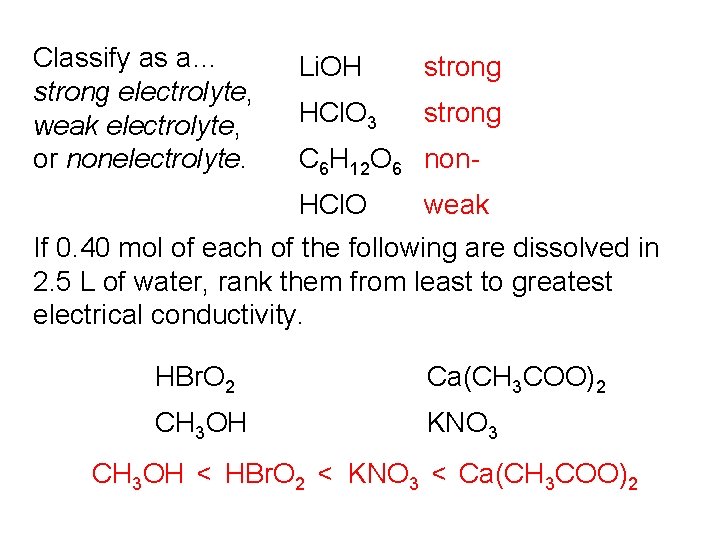
Classify as a… strong electrolyte, weak electrolyte, or nonelectrolyte. Li. OH strong HCl. O 3 strong C 6 H 12 O 6 non. HCl. O weak If 0. 40 mol of each of the following are dissolved in 2. 5 L of water, rank them from least to greatest electrical conductivity. HBr. O 2 Ca(CH 3 COO)2 CH 3 OH KNO 3 CH 3 OH < HBr. O 2 < KNO 3 < Ca(CH 3 COO)2

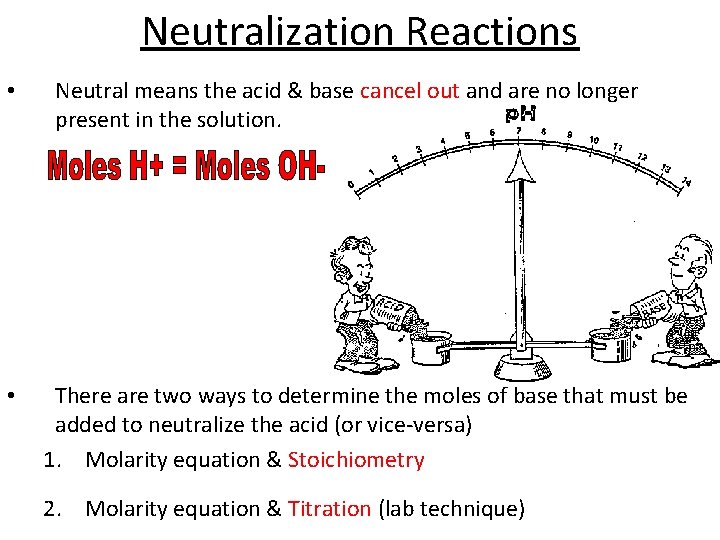
Neutralization Reactions • • Neutral means the acid & base cancel out and are no longer present in the solution. There are two ways to determine the moles of base that must be added to neutralize the acid (or vice-versa) 1. Molarity equation & Stoichiometry 2. Molarity equation & Titration (lab technique)

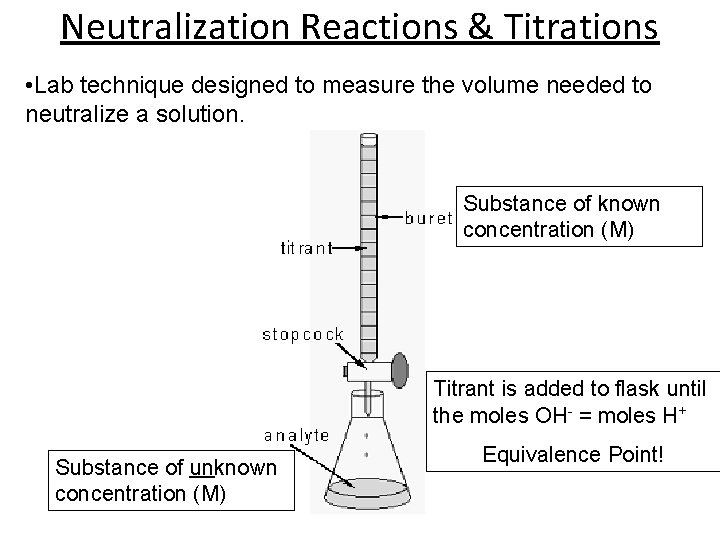
Neutralization Reactions & Titrations • Lab technique designed to measure the volume needed to neutralize a solution. Substance of known concentration (M) Titrant is added to flask until the moles OH- = moles H+ Substance of unknown concentration (M) Equivalence Point!

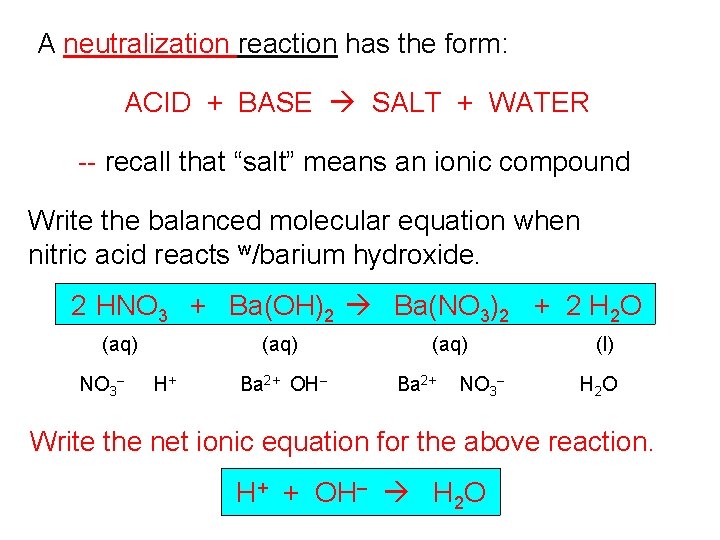
A neutralization reaction has the form: ACID + BASE SALT + WATER -- recall that “salt” means an ionic compound Write the balanced molecular equation when nitric acid reacts w/barium hydroxide. 2 HNO 3 + Ba(OH)2 Ba(NO 3)2 + 2 H 2 O (aq) NO 3– (aq) H+ Ba 2+ OH– (aq) Ba 2+ NO 3– (l) H 2 O Write the net ionic equation for the above reaction. H+ + OH– H 2 O

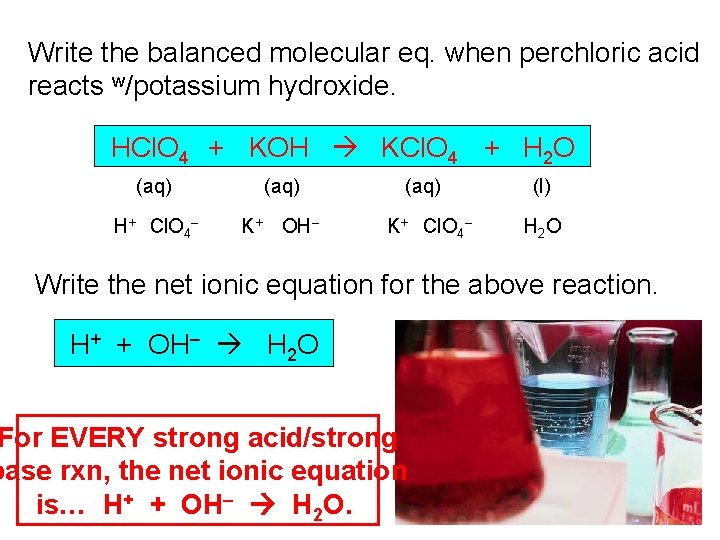
Write the balanced molecular eq. when perchloric acid reacts w/potassium hydroxide. HCl. O 4 + KOH KCl. O 4 + H 2 O (aq) H+ Cl. O 4– K+ OH– (aq) K+ Cl. O 4– (l) H 2 O Write the net ionic equation for the above reaction. H+ + OH– H 2 O For EVERY strong acid/strong base rxn, the net ionic equation is… H+ + OH– H 2 O.

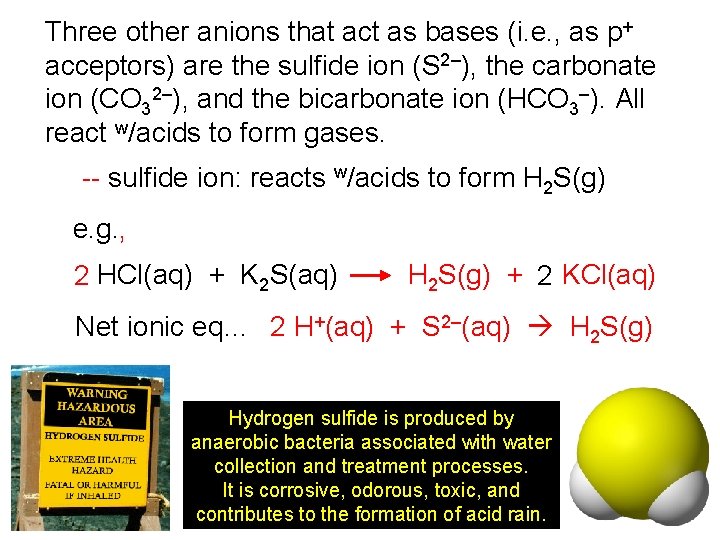
Three other anions that act as bases (i. e. , as p+ acceptors) are the sulfide ion (S 2–), the carbonate ion (CO 32–), and the bicarbonate ion (HCO 3–). All react w/acids to form gases. -- sulfide ion: reacts w/acids to form H 2 S(g) e. g. , 2 HCl(aq) + K 2 S(aq) H 2 S(g) + 2 KCl(aq) Net ionic eq… 2 H+(aq) + S 2–(aq) H 2 S(g) Hydrogen sulfide is produced by anaerobic bacteria associated with water collection and treatment processes. It is corrosive, odorous, toxic, and contributes to the formation of acid rain.

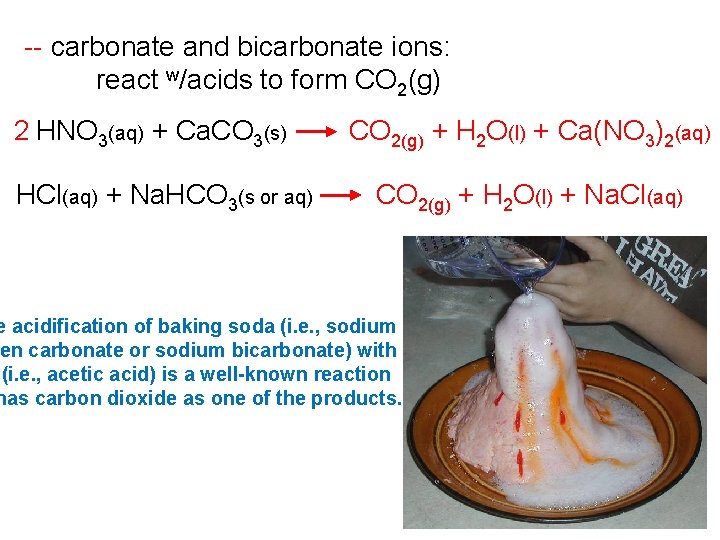
-- carbonate and bicarbonate ions: react w/acids to form CO 2(g) 2 HNO 3(aq) + Ca. CO 3(s) HCl(aq) + Na. HCO 3(s or aq) CO 2(g) + H 2 O(l) + Ca(NO 3)2(aq) CO 2(g) + H 2 O(l) + Na. Cl(aq) e acidification of baking soda (i. e. , sodium en carbonate or sodium bicarbonate) with (i. e. , acetic acid) is a well-known reaction has carbon dioxide as one of the products.

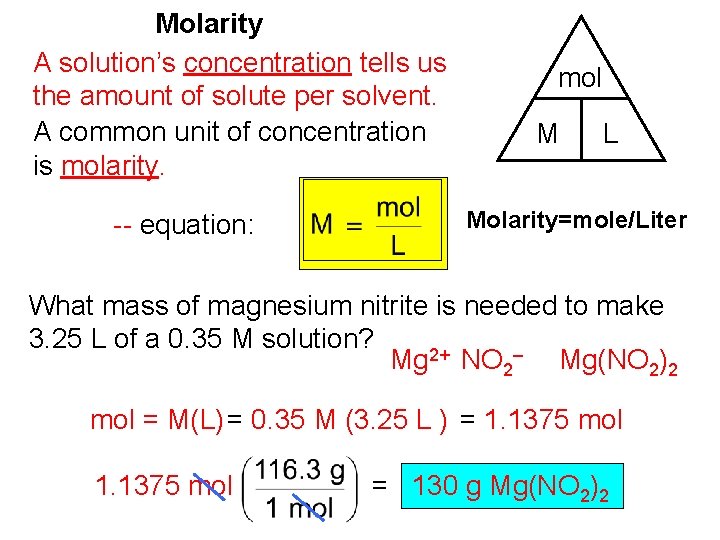
Molarity A solution’s concentration tells us the amount of solute per solvent. A common unit of concentration is molarity. -- equation: mol M L Molarity=mole/Liter What mass of magnesium nitrite is needed to make 3. 25 L of a 0. 35 M solution? Mg 2+ NO 2– Mg(NO 2)2 mol = M(L) = 0. 35 M (3. 25 L ) = 1. 1375 mol = 130 g Mg(NO 2)2

Steps for Properly Mixing an Aqueous Solution 1. Fill an appropriate container (e. g. , graduated cylinder or volumetric flask) mostly full of water (~80% full). This is an approximate technique and should take very little time. 2. Weigh out the proper amount of solute and mix it into the water from Step 1. 3. “Top off” the solution to the proper volume and mix. DONE.

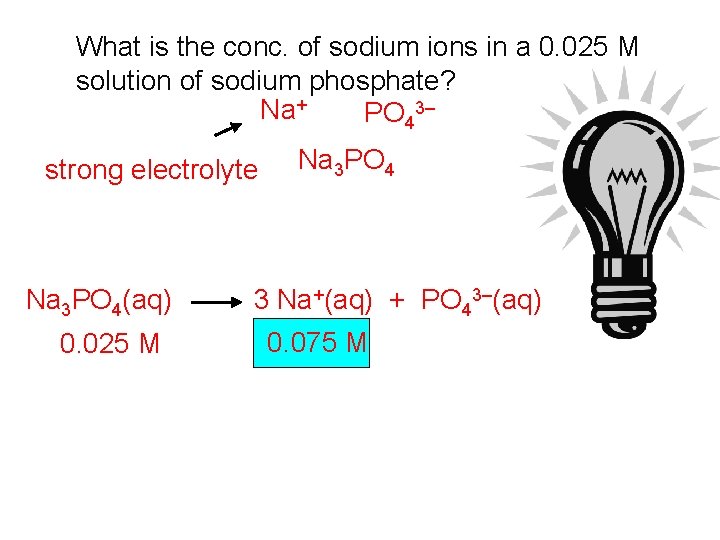
What is the conc. of sodium ions in a 0. 025 M solution of sodium phosphate? Na+ PO 43– strong electrolyte Na 3 PO 4(aq) 0. 025 M Na 3 PO 4 3 Na+(aq) + PO 43–(aq) 0. 075 M

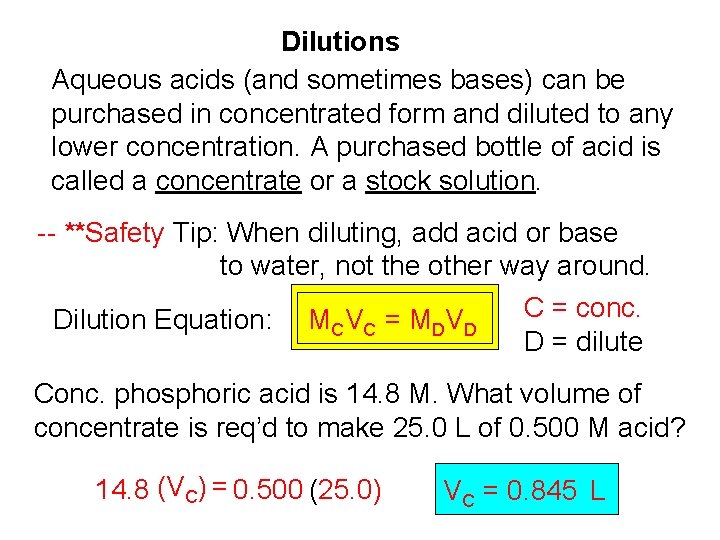
Dilutions Aqueous acids (and sometimes bases) can be purchased in concentrated form and diluted to any lower concentration. A purchased bottle of acid is called a concentrate or a stock solution. -- **Safety Tip: When diluting, add acid or base to water, not the other way around. Dilution Equation: MC V C = M D V D C = conc. D = dilute Conc. phosphoric acid is 14. 8 M. What volume of concentrate is req’d to make 25. 0 L of 0. 500 M acid? 14. 8 (VC) = 0. 500 (25. 0) VC = 0. 845 L

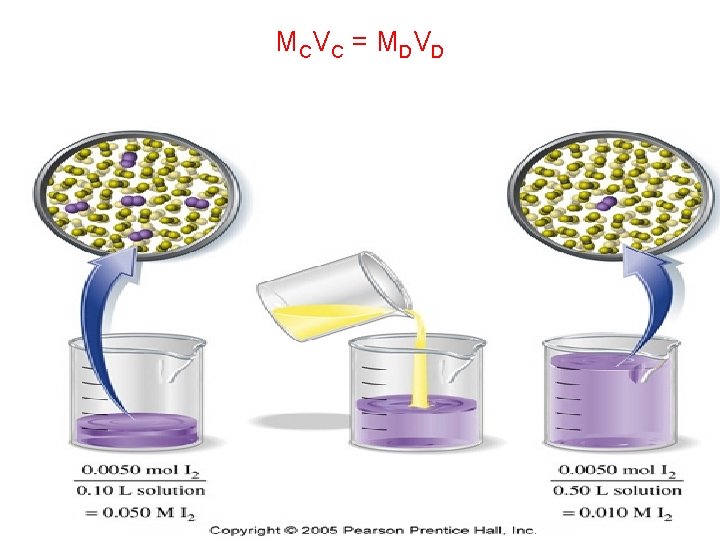
MC V C = M D V D

Serial Dilutions • Taking one concentrated solution and diluting it several times to get a series of solutions at various dilute concentrations!

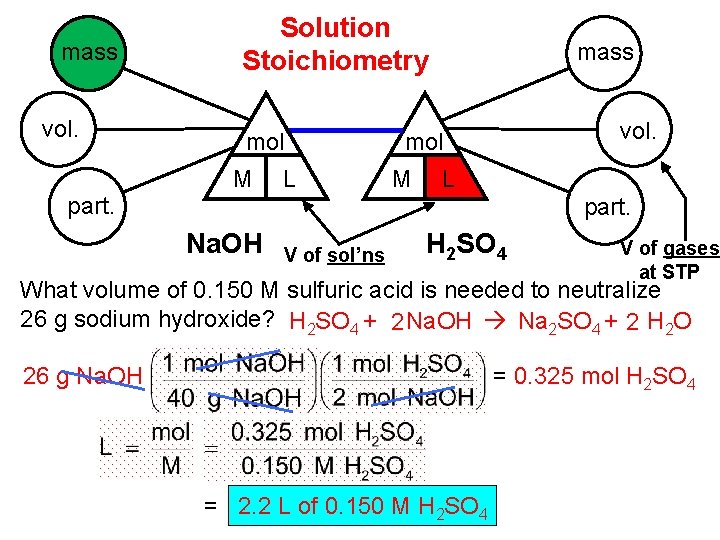
mass vol. Solution Stoichiometry mol M L mass vol. mol M L part. Na. OH V of sol’ns H 2 SO 4 V of gases at STP What volume of 0. 150 M sulfuric acid is needed to neutralize 26 g sodium hydroxide? H 2 SO 4 + 2 Na. OH Na 2 SO 4 + 2 H 2 O 26 g Na. OH = 0. 325 mol H 2 SO 4 = 2. 2 L of 0. 150 M H 2 SO 4

What mass of lead(II) nitrate will consume 85. 0 m. L of 0. 45 M sodium iodide? Pb(NO 3)2 + 2 Na. I Pb. I 2 + 2 Na. NO 3 mol Na. I = M(L) = 0. 45 M (0. 085 L ) = 0. 03825 mol Na. I = 6. 3 g Pb(NO 3)2

Titrations If we don’t know a solution’s concentration, we react a second solution of known concentration – called a standard solution –with the first. Based on the stoichiometry of the reaction, we can determine the unknown solution’s concentration. -- This procedure is called a titration.

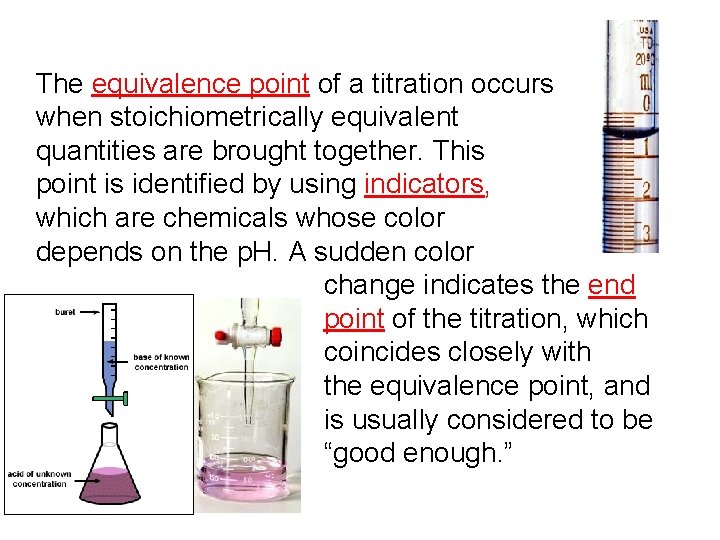
The equivalence point of a titration occurs when stoichiometrically equivalent quantities are brought together. This point is identified by using indicators, which are chemicals whose color depends on the p. H. A sudden color change indicates the end point of the titration, which coincides closely with the equivalence point, and is usually considered to be “good enough. ”

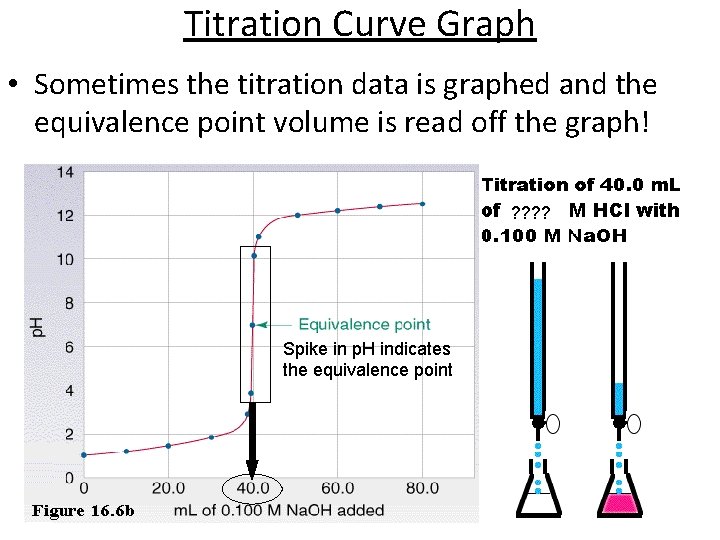
Titration Curve Graph • Sometimes the titration data is graphed and the equivalence point volume is read off the graph! ? ? Spike in p. H indicates the equivalence point

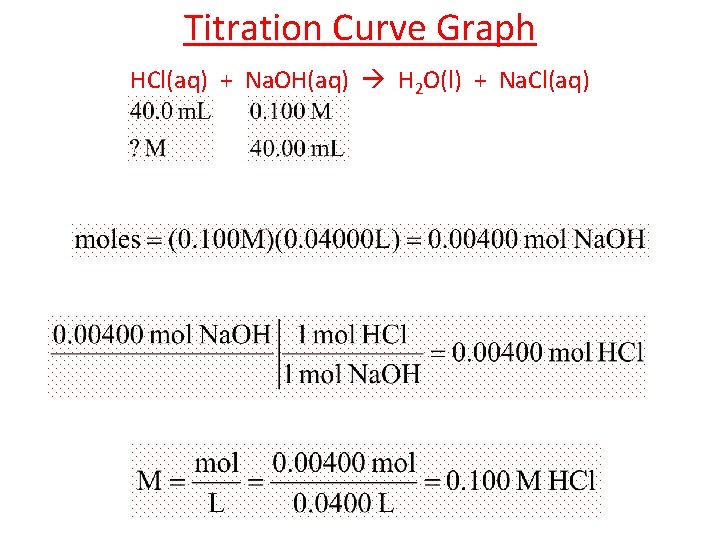
Titration Curve Graph ? ? HCl(aq) + Na. OH(aq) H 2 O(l) + Na. Cl(aq)

Titration Curve Graph HCl(aq) + Na. OH(aq) H 2 O(l) + Na. Cl(aq)

If 56. 0 m. L of sodium hydroxide neutralize 19. 0 m. L of 0. 235 M nitric acid, find the concentration of the base. mol H+ = mol OH– HNO 3 0. 235 M Na. OH H+ + NO 3– 0. 235 M Na+ + OH– XM XM [ H+ ] VA = [ OH– ] VB 0. 235 M (19. 0 m. L) = [ OH– ] (56. 0 m. L) [ OH– ] = 4. 465 M(ml) 56. 0 ml = MNa. OH = 0. 0797 M Na. OH

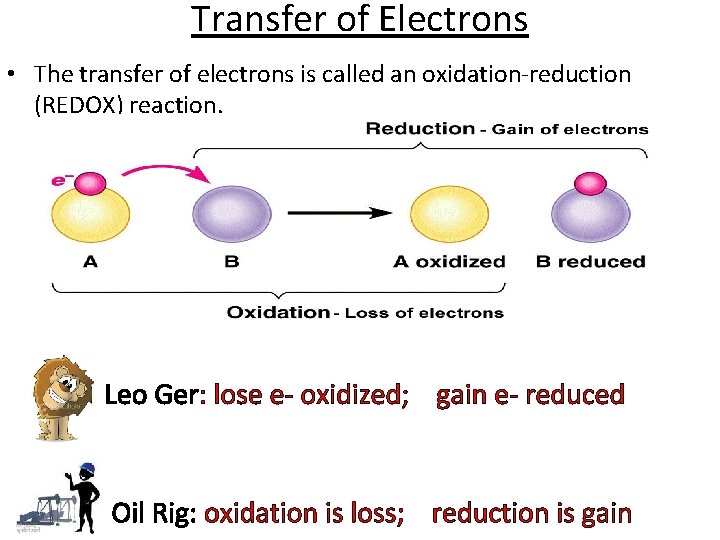
Oxidation-Reduction (a. k. a. , Redox) Reactions In redox reactions, electrons are transferred between species. Oxidation is the loss of electrons, so when a substance is oxidized, its charge… increases. Think about what O wants to do when it gets near stuff. Reduction is the gain of electrons, so when a substance is reduced, its charge… decreases.

Transfer of Electrons • The transfer of electrons is called an oxidation-reduction (REDOX) reaction. Leo Ger: lose e- oxidized; gain e- reduced Oil Rig: oxidation is loss; reduction is gain

Types of Redox Reactions • Many different types of reactions are also redox – Synthesis – Decomposition – Single Replacement – Combustion • Some reactions are NOT redox – Double replacement

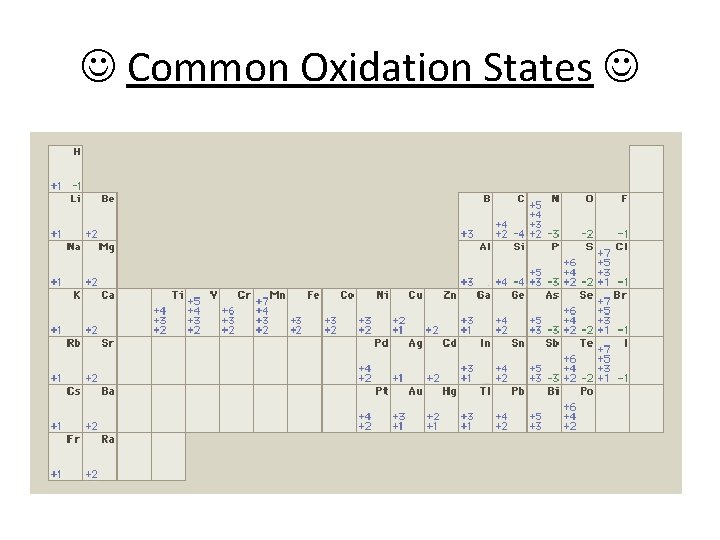
Common Oxidation States

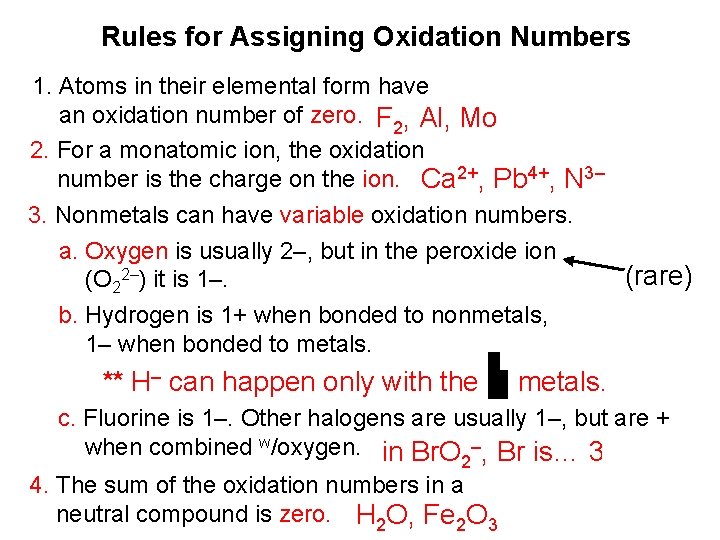
Rules for Assigning Oxidation Numbers 1. Atoms in their elemental form have an oxidation number of zero. F 2, Al, Mo 2. For a monatomic ion, the oxidation number is the charge on the ion. Ca 2+, Pb 4+, N 3– 3. Nonmetals can have variable oxidation numbers. a. Oxygen is usually 2–, but in the peroxide ion (rare) (O 22–) it is 1–. b. Hydrogen is 1+ when bonded to nonmetals, 1– when bonded to metals. ** H– can happen only with the metals. c. Fluorine is 1–. Other halogens are usually 1–, but are + when combined w/oxygen. in Br. O –, Br is… 3+ 2 4. The sum of the oxidation numbers in a neutral compound is zero. H 2 O, Fe 2 O 3

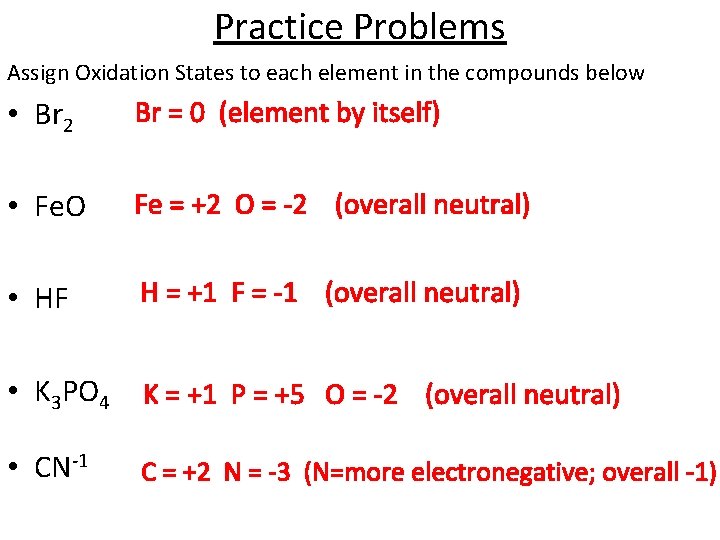
Practice Problems Assign Oxidation States to each element in the compounds below • Br 2 Br = 0 (element by itself) • Fe. O Fe = +2 O = -2 (overall neutral) • HF H = +1 F = -1 (overall neutral) • K 3 PO 4 K = +1 P = +5 O = -2 (overall neutral) • CN-1 C = +2 N = -3 (N=more electronegative; overall -1)

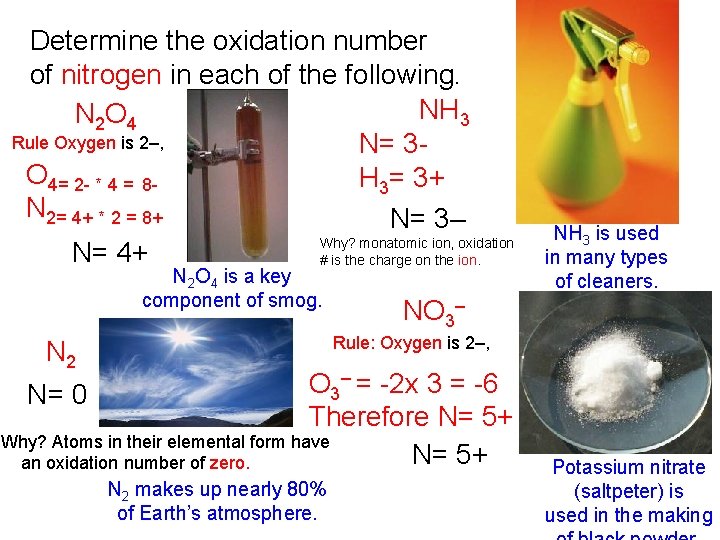
Determine the oxidation number of nitrogen in each of the following. NH 3 N 2 O 4 Rule Oxygen is 2–, N= 3 O 4= 2 - * 4 = 8 H 3= 3+ N 2= 4+ * 2 = 8+ N= 3– Why? monatomic ion, oxidation N= 4+ # is the charge on the ion. N 2 O 4 is a key component of smog. NH 3 is used in many types of cleaners. NO 3– Rule: Oxygen is 2–, N 2 – = -2 x 3 = -6 O 3 N= 0 Therefore N= 5+ Why? Atoms in their elemental form have N= 5+ an oxidation number of zero. N 2 makes up nearly 80% of Earth’s atmosphere. Potassium nitrate (saltpeter) is used in the making

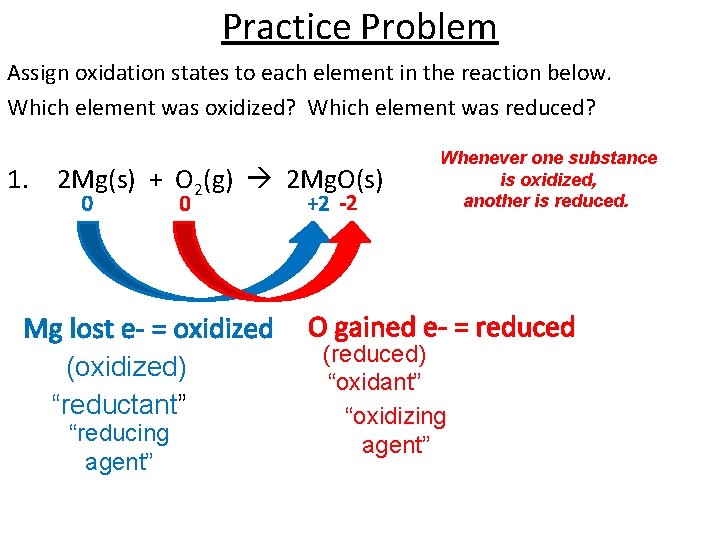
Practice Problem Assign oxidation states to each element in the reaction below. Which element was oxidized? Which element was reduced? 1. 2 Mg(s) + O 2(g) 2 Mg. O(s) 0 0 Mg lost e- = oxidized (oxidized) “reductant” “reducing agent” +2 -2 Whenever one substance is oxidized, another is reduced. O gained e- = reduced (reduced) “oxidant” “oxidizing agent”

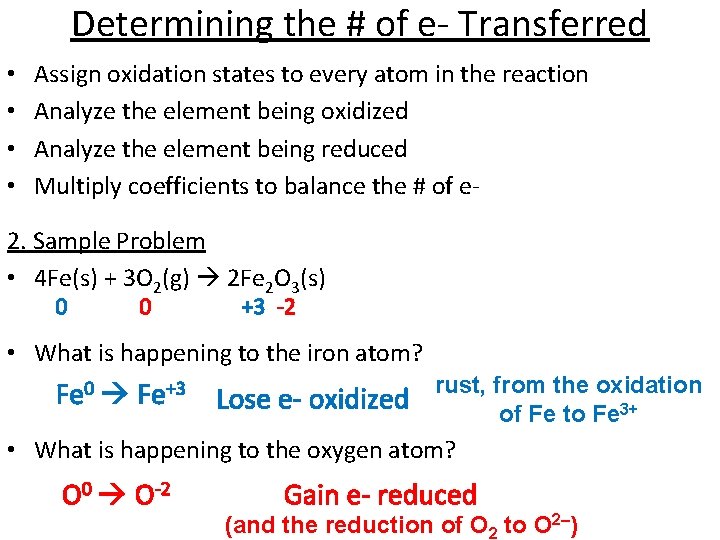
Determining the # of e- Transferred • • Assign oxidation states to every atom in the reaction Analyze the element being oxidized Analyze the element being reduced Multiply coefficients to balance the # of e- 2. Sample Problem • 4 Fe(s) + 3 O 2(g) 2 Fe 2 O 3(s) 0 0 +3 -2 • What is happening to the iron atom? Fe 0 Fe+3 Lose e- oxidized rust, from the oxidation of Fe to Fe 3+ • What is happening to the oxygen atom? O 0 O-2 Gain e- reduced (and the reduction of O 2 to O 2–)

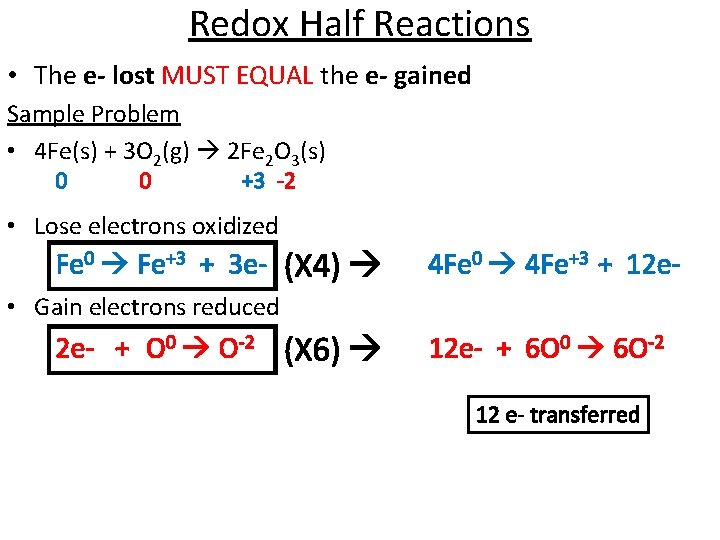
Redox Half Reactions • The e- lost MUST EQUAL the e- gained Sample Problem • 4 Fe(s) + 3 O 2(g) 2 Fe 2 O 3(s) 0 0 +3 -2 • Lose electrons oxidized Fe 0 Fe+3 + 3 e- (X 4) 4 Fe 0 4 Fe+3 + 12 e- • Gain electrons reduced 2 e- + O 0 O-2 (X 6) 12 e- + 6 O 0 6 O-2 12 e- transferred

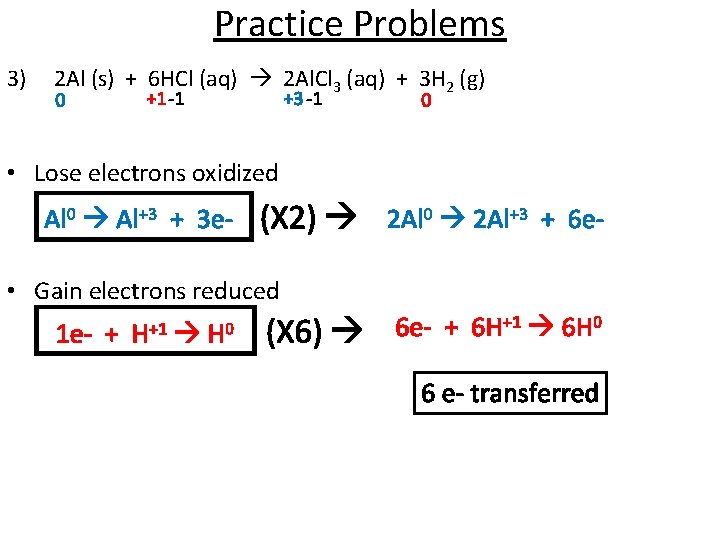
Practice Problems 3) 2 Al (s) + 6 HCl (aq) 2 Al. Cl 3 (aq) + 3 H 2 (g) 0 +1 -1 +3 -1 0 • Lose electrons oxidized Al 0 Al+3 + 3 e- (X 2) 2 Al 0 2 Al+3 + 6 e- • Gain electrons reduced 1 e- + H+1 H 0 (X 6) 6 e- + 6 H+1 6 H 0 6 e- transferred

Balancing Redox Reactions • In order to balance redox reactions we need to remember the following: 1) Conservation of Mass: the amount of each element present at the beginning of the reaction must be present at the end. 2) Conservation of Charge: electrons are not lost in a chemical reaction. They are transferred from one reactant to another. • Half-reactions are a convenient way of separating oxidation and reduction reactions. Let’s look at an easy example…

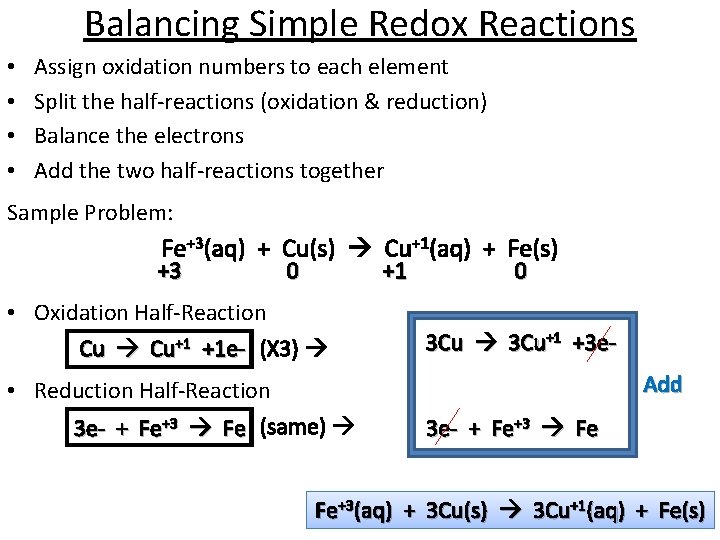
Balancing Simple Redox Reactions • • Assign oxidation numbers to each element Split the half-reactions (oxidation & reduction) Balance the electrons Add the two half-reactions together Sample Problem: Fe+3(aq) + Cu(s) Cu+1(aq) + Fe(s) +3 0 +1 • Oxidation Half-Reaction Cu Cu+1 +1 e- (X 3) • Reduction Half-Reaction 3 e- + Fe+3 Fe (same) 0 3 Cu+1 +3 e. Add 3 e- + Fe+3 Fe Fe+3(aq) + 3 Cu(s) 3 Cu+1(aq) + Fe(s)

Balancing Acidic Redox Solutions Rules for Balancing Acidic Half-Reactions 1. Divide the equation into two half-reactions 2. Balance each half-reaction separately by following these steps in order: A. Balance all the elements (except H and O) B. Balance oxygen (use H 2 O) C. Balance hydrogen (use H+) D. Balance charge (use e-) 3. Multiply each half-reaction, if necessary, so that the e- are balanced 4. Add the half-reactions together and simplify if possible

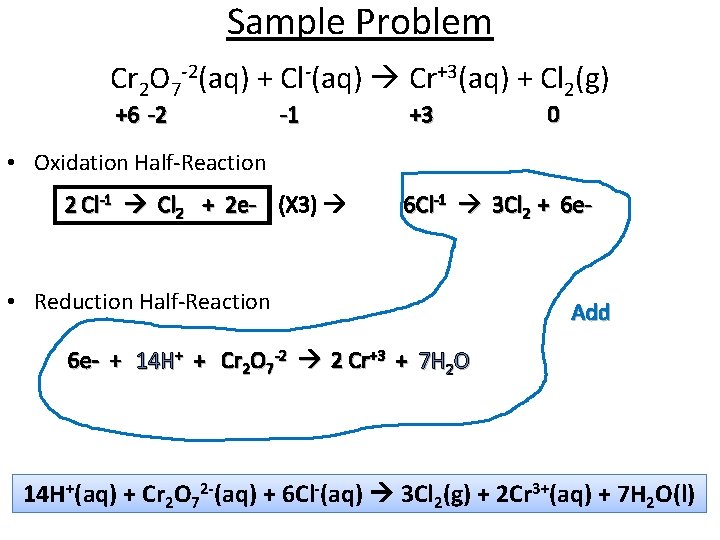
Sample Problem Cr 2 O 7 -2(aq) + Cl-(aq) Cr+3(aq) + Cl 2(g) +6 -2 -1 +3 0 • Oxidation Half-Reaction 2 Cl-1 Cl 2 + 2 e- (X 3) 6 Cl-1 3 Cl 2 + 6 e- • Reduction Half-Reaction Add 6 e- + 14 H+ + Cr 2 O 7 -2 2 Cr+3 + 7 H 2 O 14 H+(aq) + Cr 2 O 72 -(aq) + 6 Cl-(aq) 3 Cl 2(g) + 2 Cr 3+(aq) + 7 H 2 O(l)

Practice Problem • Balance the following redox reaction that takes place in acidic solution. I 2(aq) + Cl 2(aq) IO 3 -1(aq) + HCl(g) 0 0 +5 -2 +1 -1 • Oxidation Half-Reaction 6 H 2 O + I 2 2 IO 3 -1 + 12 H+ + 10 e- • Reduction Half-Reaction Add & Simplify 2 e- + 2 H+ + Cl 2 2 HCl (X 5) 10 e- + 10 H+ + 5 Cl 2 10 HCl 6 H 2 O(l) + I 2(aq) + 5 Cl 2(aq) 10 HCl(g) + 2 IO 3 -1(aq) + 2 H+(aq)

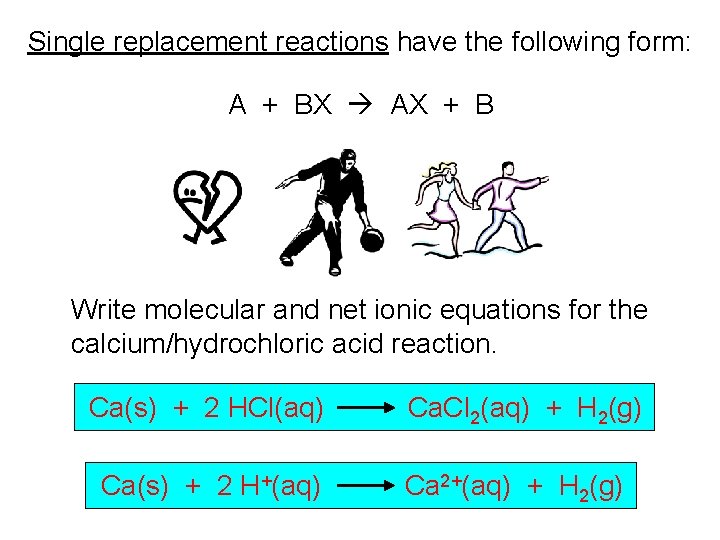
Single replacement reactions have the following form: A + BX AX + B Write molecular and net ionic equations for the calcium/hydrochloric acid reaction. Ca(s) + 2 HCl(aq) Ca(s) + 2 H+(aq) Ca. Cl 2(aq) + H 2(g) Ca 2+(aq) + H 2(g)

Activity Series A method for predicting reactions via single replacement. • It can be used to predict products of chemical reactions

The activity series is a list of metals. easily lose e– At the top of the list are the highlydon’t like losing reactive metals. electrons At the bottom are the not-so-reactive metals. The activity series is readily Available in standard references. Which of the following metals (i. e. , …will change Pb 2+ to Pb? ) will reduce Pb. CO 3? Ag Mg Hg Magnesium metal is more reactive than lead, silver and mercury

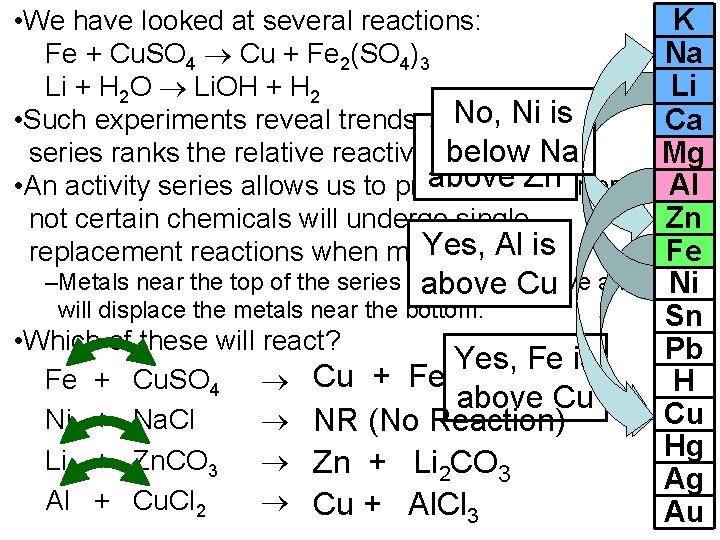
• We have looked at several reactions: Fe + Cu. SO 4 Cu + Fe 2(SO 4)3 Li + H 2 O Li. OH + H 2 No, activity Ni is • Such experiments reveal trends. The Li is below Na series ranks the relative reactivity. Yes, of metals. above Zn or • An activity series allows us to predict whether not certain chemicals will undergo single Yes, Al is replacement reactions when mixed. –Metals near the top of the series are most reactive above Cu and will displace the metals near the bottom. • Which of these will react? Yes, Fe is Fe + Cu. SO 4 Cu + Fe 2(SO 4)3 above Cu Ni + Na. Cl NR (No Reaction) Li + Zn. CO 3 Zn + Li 2 CO 3 Al + Cu. Cl 2 Cu + Al. Cl 3 K Na Li Ca Mg Al Zn Fe Ni Sn Pb H Cu Hg Ag Au

• Hydrogen is the only nonmetal listed. H 2 may be displaced in S. R. reactions. • It can also be given off when a metal reacts with H 2 O (producing H 2 + metal hydroxide). • A reaction with H 2 O depends on metal reactivity & water temperature. • Will Mg react with H 2 O? Only if the H 2 O is hot/steam. Mg + H 2 O H 2 + Mg(OH)2 • Will Zn replace H? Zn + HCl H 2 + Zn. Cl 2 cold H 2 O hot H 2 O Complete these reactions: Al + H 2 O(steam)H 2 + Al(OH)3 NR (No Reaction) Cu + H 2 O H 2 + Ca. SO 4 Ca + H 2 SO 4 H 2 + Na. OH steam K Na Li Ca Mg Al Zn Fe Ni Sn Pb H Cu Hg Ag Au

Metals: React with Oxygen • The following metals react with oxygen, forming oxides • Sb • Bi • Cu

Metals: Fairly Unreactive • The following metals do not react with water, steam, acids, or oxygen (directly) • Hg • Ag • Pt • Au

Generalizations • An element exposed to an aqueous compound will replace any element below it in the activity series • Larger intervals between elements in the activity series make a reaction more plausible • The more active a metal the stronger it bonds to oxygen, making decomposition more difficult • Elements at the top of the series are never found in nature as uncombined elements • Elements at the bottom of the series are often found in nature as uncombined elements

ACTIVITY SERIES PROBLEMS • • • Cr(s) + H 2 O(l) NO! Water must be steam! Pb(s) + HNO 3(aq) YES! Pb(NO 3)2 + H 2(g) Ba. O + Δ NO! Barium has strong bonds with oxygen. • Mg(s) + H 2 O(g) • YES! Mg(OH)2(aq) + H 2(g)

Balance equation Types of chem reaction Stoichiometry and moles Net ionic equation acid/base titration oxidation and reduction