AP Chemistry 2020 2021 Anthony Petras Day 16











- Slides: 25

AP Chemistry 2020 -2021 Anthony Petras

Day 16 • Attendance • Recording On • 3. 1 Intermolecular Forces Review • More on H-Bonding • Intermolecular Forces Practice • 3. 2 Properties of Solids • Properties of Solids Practice • Ionic Solids • Network Solids • Molecular Solids • Metallic Solids • Solids - Review • 3. 3 Solids, Liquid & Gases • 3. 4 Ideal Gas Law • Ideal Gas Law Practice • Homework • Recording Links

3. 1 Intermolecular Forces • London dispersion forces are a result of the Coulombic interactions between temporary, fluctuating dipoles. London dispersion forces are often the strongest net intermolecular force between large molecules. • Dipole-induced dipole interactions are present between a polar and a non-polar molecule. These forces are always attractive. The strength of the force increases with the magnitude of the dipole in the polar molecule and the polarizability of the non-polar molecule. • Dipole-dipole interactions are present between polar molecules. The interaction strength depends on the magnitudes of the dipoles and their relative orientation. Interactions between polar molecules are typically greater than those between nonpolar molecules of comparable size because these interactions act in addition to London dispersion forces. • Ion-dipole forces of attraction are present between ions and polar molecules. These tend to be stronger than dipole-dipole forces. • Hydrogen bonding is a strong type of intermolecular interaction that exists when hydrogen atoms covalently bonded to the highly electronegative atoms (N, O, and F) are attracted to the negative end of a dipole formed by the electronegative atom (N, O, and F) in a different molecule, or a different part of the same molecule.

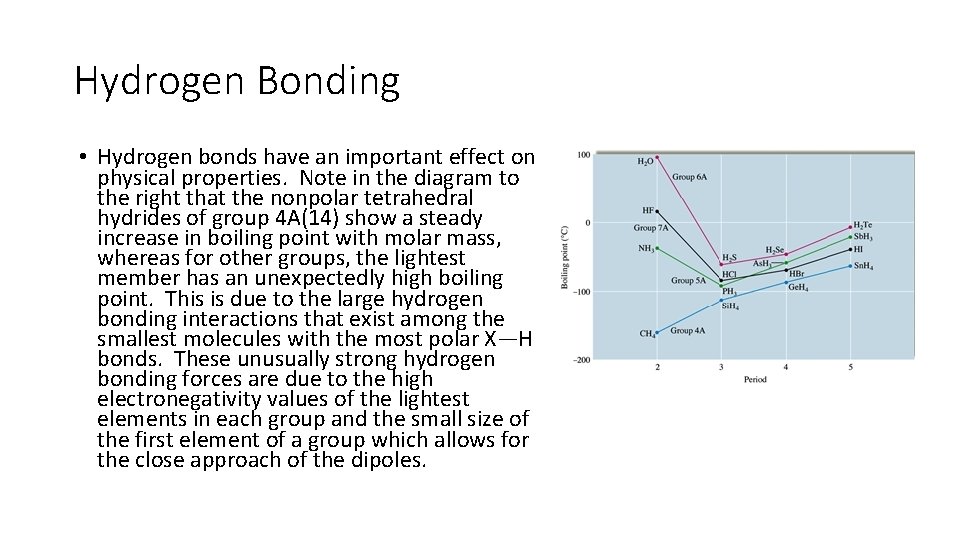
Hydrogen Bonding • Hydrogen bonds have an important effect on physical properties. Note in the diagram to the right that the nonpolar tetrahedral hydrides of group 4 A(14) show a steady increase in boiling point with molar mass, whereas for other groups, the lightest member has an unexpectedly high boiling point. This is due to the large hydrogen bonding interactions that exist among the smallest molecules with the most polar X—H bonds. These unusually strong hydrogen bonding forces are due to the high electronegativity values of the lightest elements in each group and the small size of the first element of a group which allows for the close approach of the dipoles.

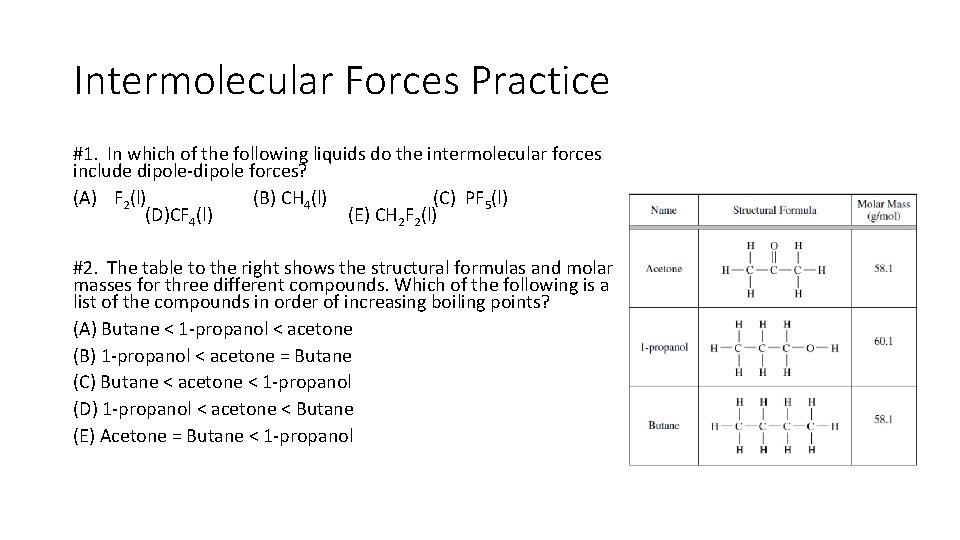
Intermolecular Forces Practice #1. In which of the following liquids do the intermolecular forces include dipole-dipole forces? (A) F 2(l) (B) CH 4(l) (C) PF 5(l) (D)CF 4(l) (E) CH 2 F 2(l) #2. The table to the right shows the structural formulas and molar masses for three different compounds. Which of the following is a list of the compounds in order of increasing boiling points? (A) Butane < 1 -propanol < acetone (B) 1 -propanol < acetone = Butane (C) Butane < acetone < 1 -propanol (D) 1 -propanol < acetone < Butane (E) Acetone = Butane < 1 -propanol

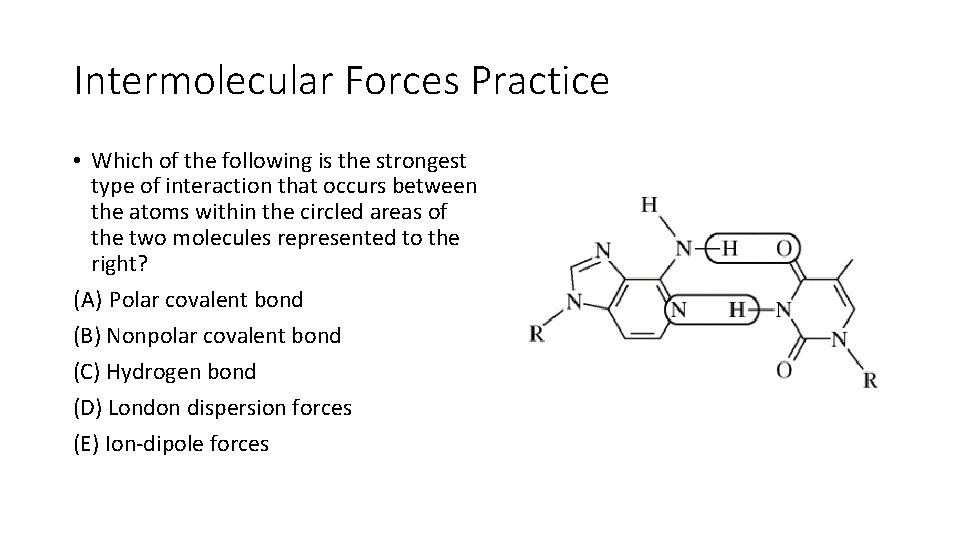
Intermolecular Forces Practice • Which of the following is the strongest type of interaction that occurs between the atoms within the circled areas of the two molecules represented to the right? (A) Polar covalent bond (B) Nonpolar covalent bond (C) Hydrogen bond (D) London dispersion forces (E) Ion-dipole forces

3. 2 Properties of Solids • Many properties of liquids and solids are determined by the strengths and types of intermolecular forces present. Because intermolecular interactions are broken when a substance vaporizes, the vapor pressure and boiling point are directly related to the strength of those interactions. Melting points also tend to correlate with interaction strength, but because the interactions are only rearranged, in melting, the relations can be more subtle.

Properties of Solids – MC Practice (A) CH 4 (B) CH 3 (C) CH 3 COCH 3 (D) CH 3 CH 2 OH (E) HOCH 2 OH #1. Which substance above has the greatest vapor pressure? #2. Which substance above has the greatest viscosity? #3. Which substance above has the greatest enthalpy of fusion? #4. Which of the following has the highest freezing point? (A) H 2 O (B) CH 4 (C) NH 3 (D) Na. Cl (E) C 8 H 18

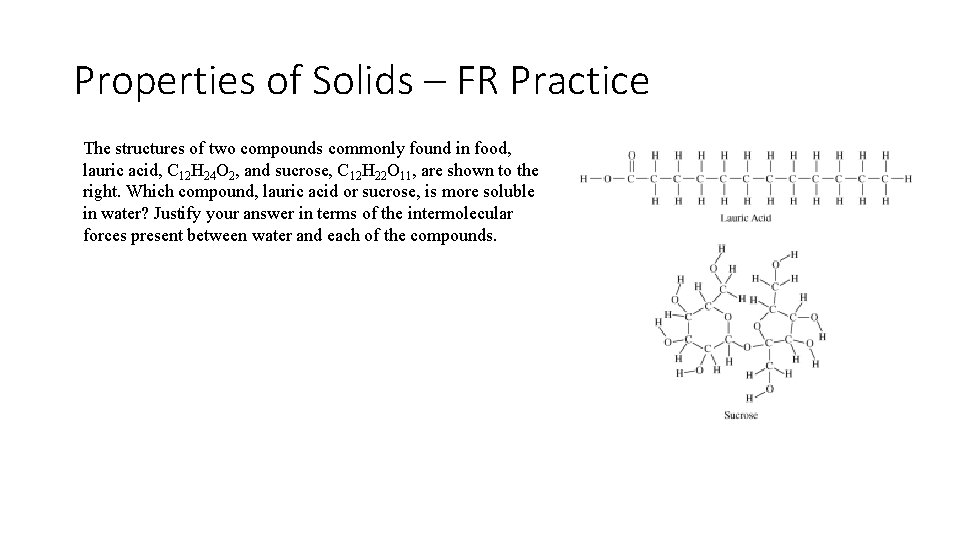
Properties of Solids – FR Practice The structures of two compounds commonly found in food, lauric acid, C 12 H 24 O 2, and sucrose, C 12 H 22 O 11, are shown to the right. Which compound, lauric acid or sucrose, is more soluble in water? Justify your answer in terms of the intermolecular forces present between water and each of the compounds.

Properties of Solids – Ionic Solids • Due to strong interactions between ions, ionic solids tend to have low vapor pressures, high melting points, and high boiling points. They tend to be brittle due to the repulsion of like charges caused when one layer slides across another layer. They conduct electricity only when the ions are mobile, as when the ionic solid is melted or dissolved in water or another solvent.

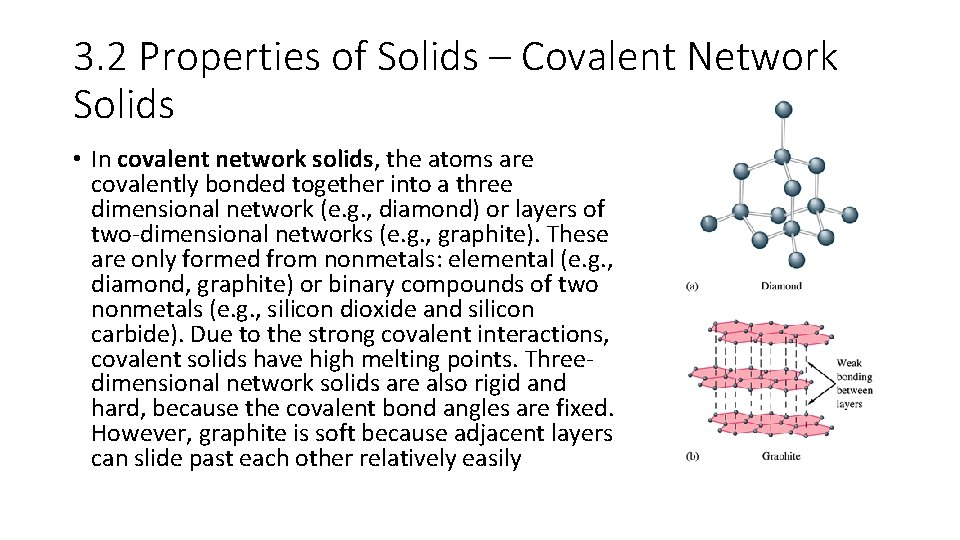
3. 2 Properties of Solids – Covalent Network Solids • In covalent network solids, the atoms are covalently bonded together into a three dimensional network (e. g. , diamond) or layers of two-dimensional networks (e. g. , graphite). These are only formed from nonmetals: elemental (e. g. , diamond, graphite) or binary compounds of two nonmetals (e. g. , silicon dioxide and silicon carbide). Due to the strong covalent interactions, covalent solids have high melting points. Threedimensional network solids are also rigid and hard, because the covalent bond angles are fixed. However, graphite is soft because adjacent layers can slide past each other relatively easily

3. 2 Properties of Solids – Molecular Solids • Molecular solids are composed of distinct, individual units of covalently-bonded molecules attracted to each other through relatively weak intermolecular forces. Molecular solids generally have a low melting point because of the relatively weak intermolecular forces present between the molecules. They do not conduct electricity because their valence electrons are tightly held within the covalent bonds and the lone pairs of each constituent molecule. Molecular solids are sometimes composed of very large molecules or polymers.

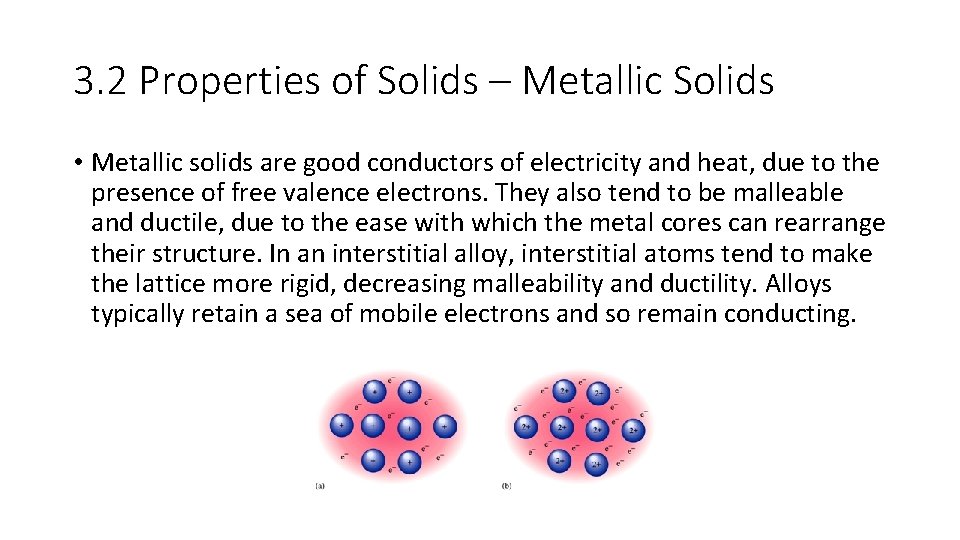
3. 2 Properties of Solids – Metallic Solids • Metallic solids are good conductors of electricity and heat, due to the presence of free valence electrons. They also tend to be malleable and ductile, due to the ease with which the metal cores can rearrange their structure. In an interstitial alloy, interstitial atoms tend to make the lattice more rigid, decreasing malleability and ductility. Alloys typically retain a sea of mobile electrons and so remain conducting.

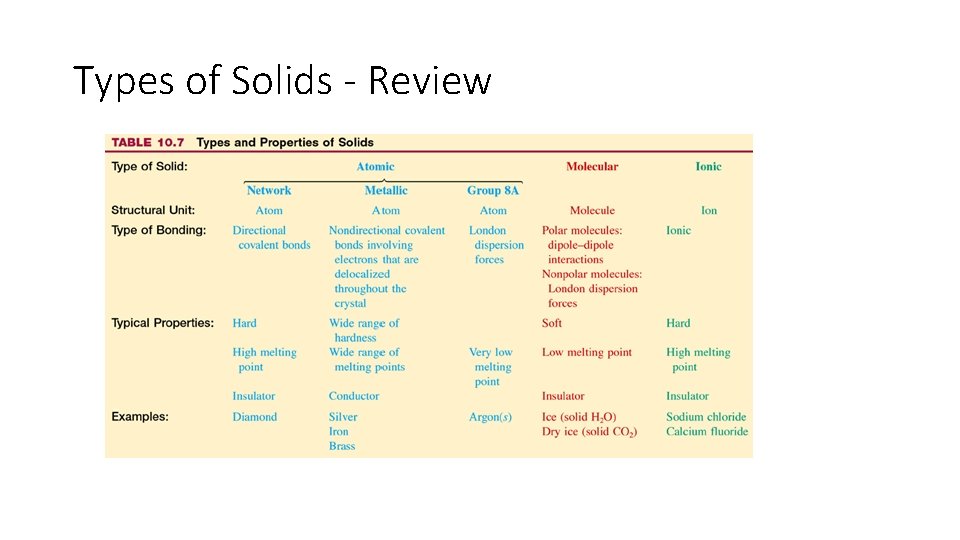
Types of Solids - Review

Types of Solids - Review

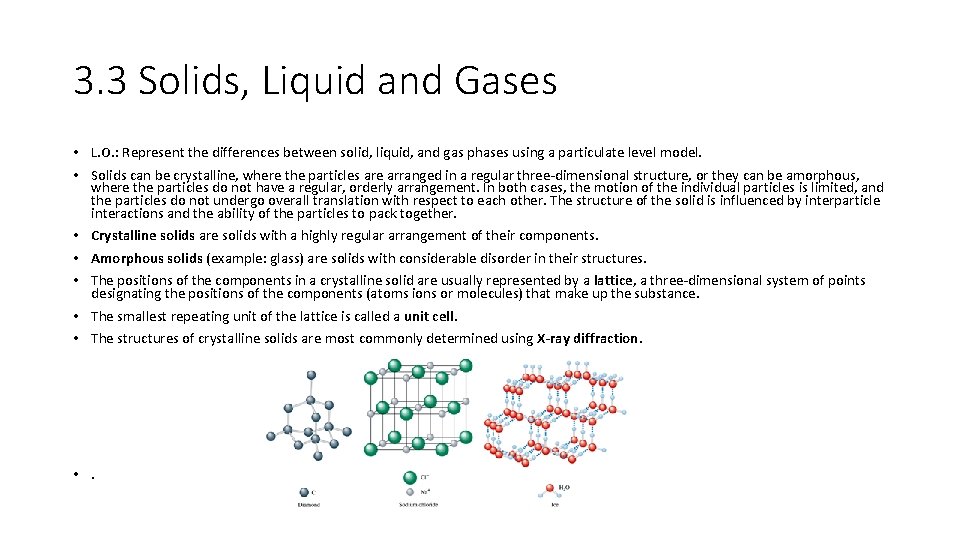
3. 3 Solids, Liquid and Gases • L. O. : Represent the differences between solid, liquid, and gas phases using a particulate level model. • Solids can be crystalline, where the particles are arranged in a regular three-dimensional structure, or they can be amorphous, where the particles do not have a regular, orderly arrangement. In both cases, the motion of the individual particles is limited, and the particles do not undergo overall translation with respect to each other. The structure of the solid is influenced by interparticle interactions and the ability of the particles to pack together. • Crystalline solids are solids with a highly regular arrangement of their components. • Amorphous solids (example: glass) are solids with considerable disorder in their structures. • The positions of the components in a crystalline solid are usually represented by a lattice, a three-dimensional system of points designating the positions of the components (atoms ions or molecules) that make up the substance. • The smallest repeating unit of the lattice is called a unit cell. • The structures of crystalline solids are most commonly determined using X-ray diffraction. • .

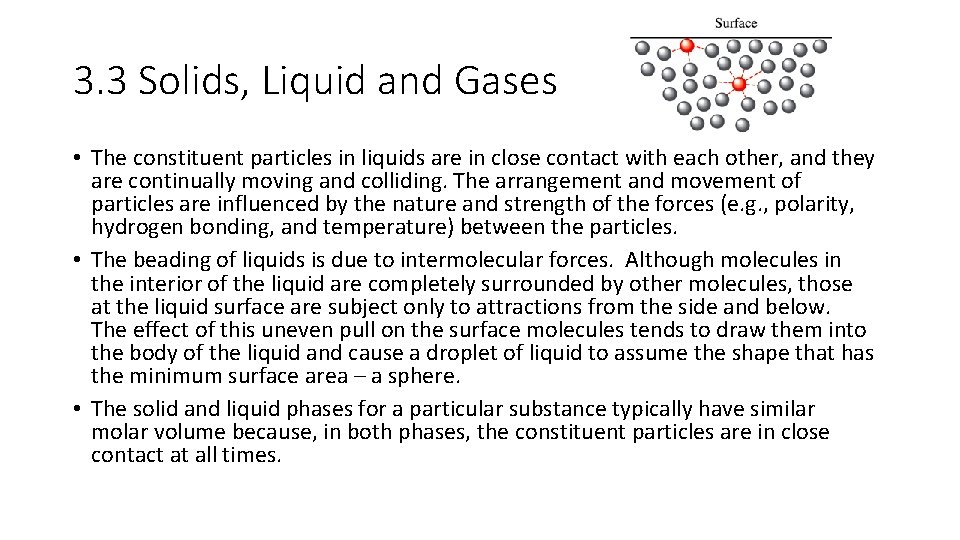
3. 3 Solids, Liquid and Gases • The constituent particles in liquids are in close contact with each other, and they are continually moving and colliding. The arrangement and movement of particles are influenced by the nature and strength of the forces (e. g. , polarity, hydrogen bonding, and temperature) between the particles. • The beading of liquids is due to intermolecular forces. Although molecules in the interior of the liquid are completely surrounded by other molecules, those at the liquid surface are subject only to attractions from the side and below. The effect of this uneven pull on the surface molecules tends to draw them into the body of the liquid and cause a droplet of liquid to assume the shape that has the minimum surface area – a sphere. • The solid and liquid phases for a particular substance typically have similar molar volume because, in both phases, the constituent particles are in close contact at all times.

3. 3 Solids, Liquid and Gases • The resistance of a liquid to increase in its surface area is called the surface tension of a liquid. Liquids with strong intermolecular forces, such as those with polar molecules, tend to have relatively high surface tensions. • Polar liquids exhibit capillary action, the spontaneous rising of a liquid in a narrow tube. Two different forces are responsible for this behavior: cohesive forces, the intermolecular forces among the molecules of the liquid, and adhesive forces, the forces between the liquid molecules and their container. • Adhesive forces occur when a container is made of a substance that has polar bonds. Glass surfaces contain many oxygen atoms with partial negative charges that are attractive to the positive end of a polar molecule such as water. • Because water has strong cohesive (intermolecular) forces and strong adhesive forces to glass, it pulls itself up a glass capillary tube to a height where the weight of the column of water just balances the water’s tendency to be attracted to the glass surface. • The concave shape of the meniscus shows water’s adhesive forces toward glass are stronger than its cohesive forces. A nonpolar liquid such as mercury shows a convex meniscus. This behavior is characteristic of a liquid in which the cohesive forces are stronger than the adhesive forces toward glass. • Viscosity is a measure of a liquid’s resistance to flow. Liquids with large intermolecular forces tend to be highly viscous.

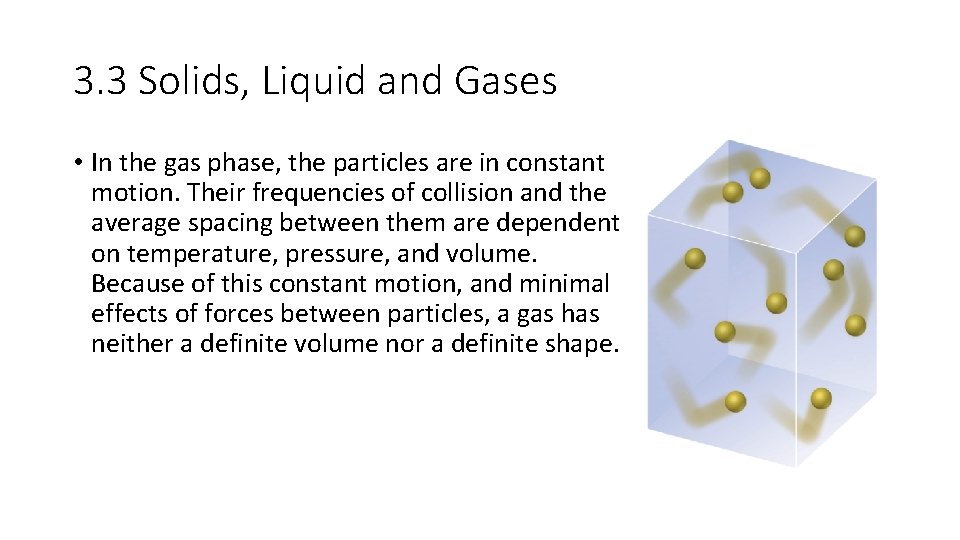
3. 3 Solids, Liquid and Gases • In the gas phase, the particles are in constant motion. Their frequencies of collision and the average spacing between them are dependent on temperature, pressure, and volume. Because of this constant motion, and minimal effects of forces between particles, a gas has neither a definite volume nor a definite shape.

3. 4 Ideal Gas Law • L. O. : Explain the relationship between the macroscopic properties of a sample of gas or mixture of gases using the ideal gas law. • The macroscopic properties of ideal gases are related through the ideal gas law: EQN: PV = n. RT. P = pressure, V = volume, n = number of moles, R= ideal gas law constant (0. 08206 L atm/mol K), T = temperature (in Kelvin) • Explain the relationship between variables within an equation when one variable changes. • The ideal gas law is a limiting law – it expresses behavior that real gases approach at low pressures and high temperatures. • Ideal gases are hypothetical substances; though most gases obey the ideal gas law equation closely enough at pressures below 1 atm.

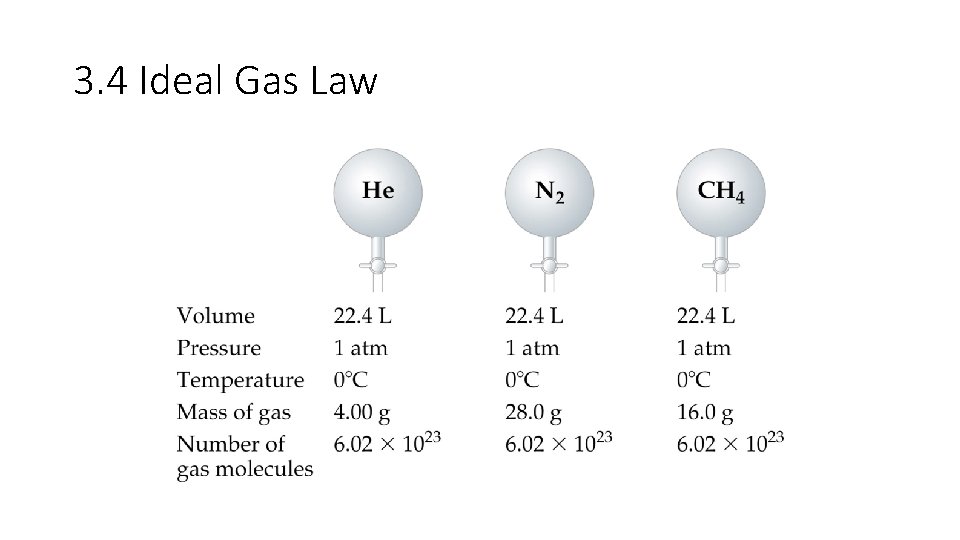
3. 4 Ideal Gas Law • In a sample containing a mixture of ideal gases, the pressure exerted by each component (the partial pressure) is independent of the other components. Therefore, the total pressure of the sample is the sum of the partial pressures. • In 1803 Dalton proposed that for a mixture of gases in a container, the total pressure exerted is a sum of the pressure that each gas would exert if it were alone. • Assuming each gas to behave ideally, the partial pressure of each gas can be: P 1 = n 1 RT/V, P 2 = n 2 RT/V, etc. • The total pressure of a mixture can be represented as Ptotal = P 1 + P 2 + P 3 …. . Etc • Mole fraction: the ratio of the number of moles of a given component in a mixture to the total number of moles in the mixture. • The formula used in determining mole fractions is: X 1 = n 1/ntotal = P 1/Ptotal (use either moles or pressure). Mole fraction has no units.

3. 4 Ideal Gas Law

Ideal Gas Law Practice 1. A sample of 1. 00 mole of hydrogen gas is mixed with 5. 00 mole of helium gas. If the total pressure of the system is 3. 00 atmospheres, the partial pressure of the helium gas is: (A) 0. 500 atm (B) 1. 00 atm (C) 1. 50 atm (D) 2. 00 atm (E) 2. 50 atm 2. A sample of an ideal gas is cooled from 50. 0° C to 25. 0°C in a sealed container of constant volume. Which of the following values for the gas will decrease? I. The density of the gas. II. The average distance between the molecules III. The average speed of the molecules. (A) I only (B) II only (D) I and III (C) III only (E) II and III 3. A mixture of nitrogen and helium gases containing 4. 00 grams of helium, exerts a total pressure of 800. mm Hg. If the partial pressure of the nitrogen gas is 480 mm of Hg, what is the mass of the nitrogen gas in the mixture? (A) 21. 0 g (B) 42. 0 g (C) 56. 0 g (D) 28. 0 g (E) 6. 00 g 4. Hydrogen gas is collected over water at 24°C. The total pressure of the sample is 755 millimeters of mercury. At 24°C, the vapor pressure of water is 22 millimeters of mercury. What is the partial pressure of the hydrogen gas? (A) 22 mm Hg (B) 733 mm Hg (C) 755 mm Hg (D) 760 mm Hg (E) 777 mm Hg 5. Equal masses of He and Ne are placed in a sealed container. What is the partial pressure of He if the total pressure in the container is 6 atm? (A) 1 atm (B) 2 atm (C) 3 atm (D) 4 atm (E) 5 atm 6. What is the total pressure after 2. 00 moles of H 2(g), 1. 00 mole of O 2(g), 2. 00 moles of N 2(g) and 1. 00 mole CO 2(g) are injected into a rigid 22. 4 L container 273 K? (A) 760 mm. Hg (B) 2280 mm. Hg (C) 4560 mm. Hg (D) 9120 mm. Hg (E) 63, 500 mm. Hg

Homework – Day 17 • Review this Power. Point • 3. 1, 3. 2 due 11/9/2020 (REQUIRED) • Lockdown Unit 2, 3. 3, 3. 4, 3. 5 & 3. 6 due 11/16/2020 (REQUIRED) • Available Resources: (NOT REQUIRED) • Sartep AP chapter 10 worksheets

Recording Links – New Quarter – New Links • Period 1: • 17. https: //us. bbcollab. com/recording/cd 877 eb 7702145 bda 62 cfea 20 f 6 ad 4 eb • 16. https: //us. bbcollab. com/recording/ae 785 f 0803644 cd 38558 d 22 eced 5 e 785
 Anthony petras
Anthony petras Anthony petras
Anthony petras Day 1 day 2 day 3 day 4
Day 1 day 2 day 3 day 4 Day 1 day 2 day 817
Day 1 day 2 day 817 Petras tehachapi
Petras tehachapi Petras dovydaitis
Petras dovydaitis Negalima gyventi be duonos
Negalima gyventi be duonos Petras cvirka biografija
Petras cvirka biografija Jeremy petras
Jeremy petras Dr petras kisielius
Dr petras kisielius Vivien petras
Vivien petras Petras banys
Petras banys Petras kudaras
Petras kudaras Teacher goals for 2020-2021
Teacher goals for 2020-2021 Nfhs volleyball uniform rules 2020-2021
Nfhs volleyball uniform rules 2020-2021 Elearning utmspace
Elearning utmspace Bachillerato internacional ramiro maeztu
Bachillerato internacional ramiro maeztu Edu.ro programe scolare 2020-2021
Edu.ro programe scolare 2020-2021 Fort bend course selection guide
Fort bend course selection guide Nomenclatorul documentelor scolare 2019
Nomenclatorul documentelor scolare 2019 Horizon 2020 2021
Horizon 2020 2021 Bayonne school calendar 2020-2021
Bayonne school calendar 2020-2021 Convocatoria cobaeh 2020-2021
Convocatoria cobaeh 2020-2021 Ppms 2020 2021
Ppms 2020 2021 Masht planprogramet 2020
Masht planprogramet 2020 Dokumen 1 ktsp sma tahun 2020 2021
Dokumen 1 ktsp sma tahun 2020 2021